I. FOREWORDS
Polycyclic aromatic hydrocarbons (PAHs) are a class of environmental contaminants that has long been of interest in the fields of organic chemistry, theoretical chemistry, physical chemistry, environmental science, toxicology, cancer research, and energy sciences. Concerning environmental science and cancer research, majority of the research has focused on the occurrence, environmental fate, degradation/remediation, chemical transformation, genotoxicity, metabolism and metabolic activation, DNA adduct formation, mutagenesis, and carcinogenesis. Although many books and reviews on these subjects have been published, PAH photochemistry and phototoxicity have received much less attention. Therefore, it is intended for this article to provide an up-to-date source of photochemical reaction, photo-transformation, and phototoxicity of PAHs and their oxygenated, nitrated, halogenated, and amino substituted derivatives on a molecular basis. A perspective for future work is also discussed.
II. INTRODUCTION
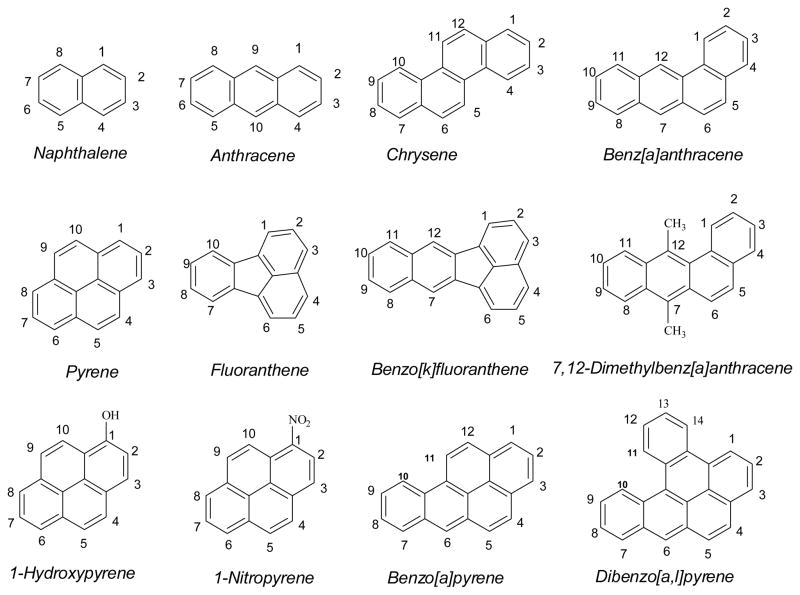
PAHs are a class of widely spread mutagenic and tumorigenic environmental contaminants (1–7). They are produced mainly from incomplete combustion of organic materials during both natural events and human activities. These include wild fires and volcanic eruptions, and burning of fossil fuels and petroleum products during industrial production, food processing, operation of machinery including automobiles, airplanes and ships (1–3, 5, 6, 8–12). PAHs are carbon compounds with two or more annelated aromatic rings. The nomenclatures and numbering systems of the representative PAHs are shown in Figure 1. Being ubiquitous in the environment, PAHs are thought to induce cancer tumors, primarily in the lungs, bladder and in the skin (2, 6, 13–17). The International Agency for Research on Cancer and the United States Environmental Protection Agency have classified some of these compounds as probable human carcinogens (5, 8). Complex mixtures of airborne contaminants may play an important role in human cancer incidences. For example, it has been reported that long-term exposure to air pollution in some of America’s biggest metropolitan areas significantly raises the risk of lung cancer deaths, and PAHs in the polluted ambient air may be one of the contributing factors (16). Numerous books and review articles have reported the occurrence and distribution of PAHs in the environment (1, 2, 4–6, 9, 10, 13, 18–20).
Figure 1.
Chemical structures and ring numbering systems for representative PAHs
PAHs themselves are relatively non-reactive chemicals toward biological macromolecules under physiological conditions. Rather, they require metabolic activation in order to exert genotoxicity, including mutagenicity and tumorigenicity (2, 4, 8, 13, 14, 18, 21–23). Upon entering the body, the cellular defense system attempts to “remove” these foreign substances by metabolism. PAH metabolism in a mammalian system is principally in the liver and is catalyzed mainly by the cytochrome P450 enzymes, although other metabolizing enzymes are also involved. Upon metabolism, PAHs become more polar and water-soluble to be excreted out of the body, thus completing the removal or the biological “detoxification” process. However, metabolism of some PAHs also generates reactive intermediates that are capable of forming covalent adducts with nucleic acids, leading to genotoxicity.
A. Mammalian Metabolic Activation of PAHs
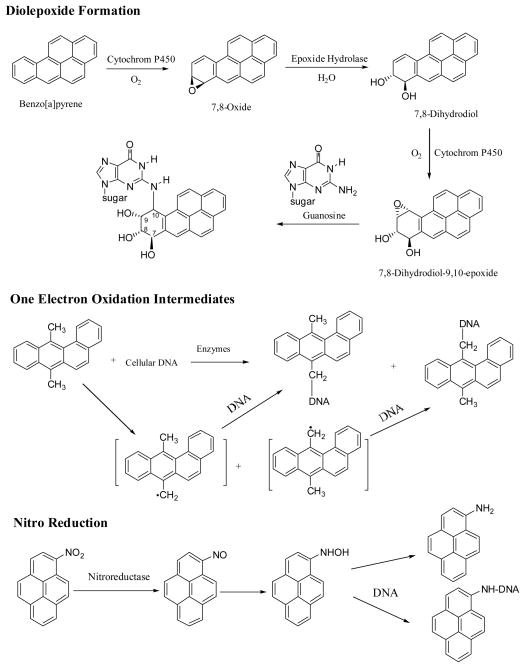
It is generally accepted that formation of diolepoxide-derived DNA adducts is the principal metabolic activation pathway leading to cancer initiation (13, 15, 24–26) (Figure 2). The diolepoxide metabolites, particularly the bay-region diolepoxides, can react with DNA to form covalently bound DNA adducts (2–4, 13–15). The diolepoxide-derived DNA adducts have been identified from metabolism of a number of PAHs in vitro and in vivo and their structures have been well characterized (27).
Figure 2.
Metabolic activation pathways for PAHs and Nitro-PAHs
Metabolism of PAHs through one electron oxidation resulting in reactive free radical intermediates that can react with DNA to form DNA covalent adducts has also been reported (28–32) (Figure 2). Due to differences in mechanisms of metabolic activation, the ultimate carcinogenic metabolites or intermediates as well as the types of DNA adducts formed from the diolepoxide formation or from the free radical generation are different.
The carcinogenesis of nitro-PAHs has been intensively studied since the early 1980s. Because of bearing a nitro functional group, the metabolic activation of nitro-PAHs involves multiple activation pathways including ring oxidation and nitro-reduction. The principal activation pathway involves reduction of the nitro group to the N-hydroxyamino-PAH intermediates that are capable of binding to DNA leading DNA adduct formation (18, 22, 33–35) (Figure 2).
B. Light-Induced Toxicity and PAH Activation by Light
With extended aromatic ring systems, PAHs can absorb light in the UVA (320 – 400 nm) and visible (> 400 nm) region. Usually, PAHs with three or four aromatic rings can absorb UVA light and those with five or more aromatic rings as well as the hydroxy, amino, nitro-substituted PAHs three or four aromatic rings can absorb visible light (12). Upon absorbing light energy, PAHs can be excited to upper energy states (singlet or triplet) that can undergo electron or energy transfer to molecular oxygen, solvents, or biological molecules in the cell to generate reactive species. The excited state PAHs can also react with oxygen or other molecules to generate reactive intermediates. These reactive species or intermediates can damage cellular constituents such as cell membrane, nucleic acids, or proteins. Thus, PAHs can be “activated” by light irradiation to cause cellular damages and exert toxicity including carcinogenicity. This activation pathway is similar to the enzymatic activation pathway in terms of converting relatively inert PAHs to reactive species. There are evidences that PAH mixtures and individual PAHs are phototoxic toward microorganisms, plants, cells, and animals (36–46). It was found that PAHs are generally more toxic when they are exposed to light than if they are kept in the dark. The increase in toxicity can exceed well over 100 times (42, 45).
III. PAH PHOTOCHEMISTRY
The photochemistry of PAHs and related compounds has been studied in organic solvents and on solid surfaces (soil or sediments), but only sparingly in aqueous solutions due to their solubility problems (47). Several review articles have covered the photodegradation of PAHs and discussed their photo-oxidation products (48–50). The topics covered include degradation rates, effects of coexisting ions on the photochemical reaction quantum yield and rates, and identification of some of the photodegradation products. The main photochemical reaction for PAHs is the reaction with molecular oxygen leading to more water-soluble oxygenated compounds or into smaller degraded organic molecules. This oxidative process is one of the chief degradation pathways for PAHs in the natural aquatic environment. Photodegradation products of the following PAHs are characterized: naphthalene in vapor (51–53), phenanthrene in silica-air inter-phase (54, 55), anthracene in aqueous and organic solvents (56–60), pyrene in soil surface or on carbon (61–64), benzo[a]pyrene in aqueous media (65, 66), and some heterocyclic compounds in solutions or on solid surfaces. Since then, characterization of photoproducts of some PAH in aqueous or water/organic solvent mixtures are available: naphthalene (53), pyrene (67, 68), benz[a]anthracene (57, 69, 70), methyl substituted BAs (69), 1-hydroxypyrene (71), and 1-aminopyrene (AP). Therefore, a summary of these newly characterized photoproducts as well as the results on the photolysis of nitro and amino-PAHs that have not been included in any of the previous reviews.
Photolysis of naphthalene in aqueous solutions yields 7-hydroxy-1,4-naphthoquinone (I), 2-formylcinnamaldehyde (II), and 2-carboxycinnamaldehyde (III) (53) (Figure 3). The authors suggested that compound I be formed via the 1,4-naphthoquinone intermediate, and compounds II and III be from the breaking of the C1-C2 bond in naphthalene.
Figure 3.
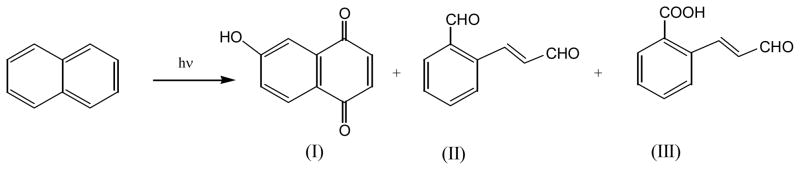
Photochemical reaction of naphthalene in aqueous solution
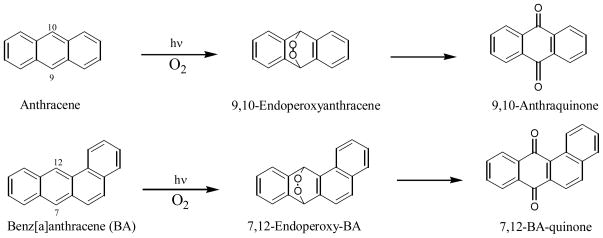
The photochemical reaction of anthracene and benz[a]anthracene (BA) follows the same pattern because they both have two most reactive positions in the molecule: 9 and 10 positions in anthracene and 7 and 12 positions in BA. Molecular orbital calculations show that the 7 and 12 positions in BA and methyl substituted BAs have the highest electron density (69). Upon light irradiation, both anthracene and BA react with oxygen to form endoperoxides. Rearrangement and further oxidation of the endoperoxides lead to the formation of quinones (58, 59, 69, 72) (Figure 4).
Figure 4.
Photo-oxidation of anthrancene and benz[a]anthracene in aqueous solution
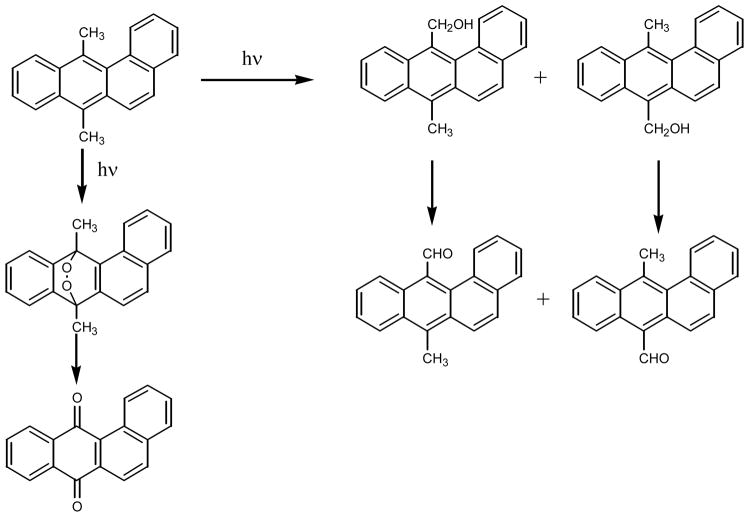
Irradiation of 7,12-dimethyl-BA in aqueous solutions leads to the formation of 7,12-endoperoxide and subsequently to 7,12-BA-quinone (70) (Figure 5). In addition, the methyl groups can also be oxidized during the photolysis, yielding first hydroxymethyl followed by further oxidation to formyl groups.
Figure 5.
Photo-oxidation of 7,12-dimethylbenz[a]anthracene in aqueous solution
Photolysis of pyrene yields 1,6- and 1,8-pyrenequinones as stable products whether in aqueous solution or in surfactant media (67) (Figure 6). If pyrene is irradiated by light on soil, eight photoproducts are detected with five of them identified structurally. They include the two pyrenequinones detected in the aqueous solution photolysis, two pyrenediols, 1,6- and 1,8-pyrenediol, and a pyrene dimer, 1,1′-bipyrene are also identified (62). More than 20 photoproducts are detected if pyrene is irradiated by sunlight on filter paper (73). The authors separated the oxygenated and the acidic fractions of the photoproducts and found that both fractions are more mutagenic toward Salmonella typhimurium bacteria with or without S-9 activation. The same test with the pure 1,6- or 1,8-pyrenequinone indicates that the mutagenicity of the mixture is not from the quinoes, but from other unknown oxygenated photoproducts.
Figure 6.
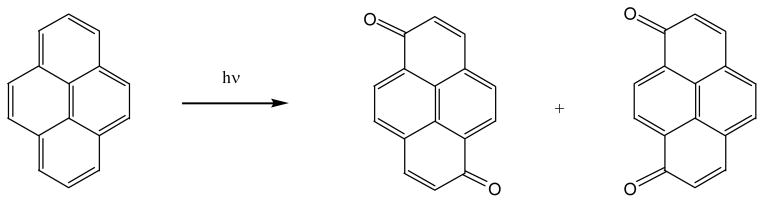
Photo-oxidation of pyrene in solution
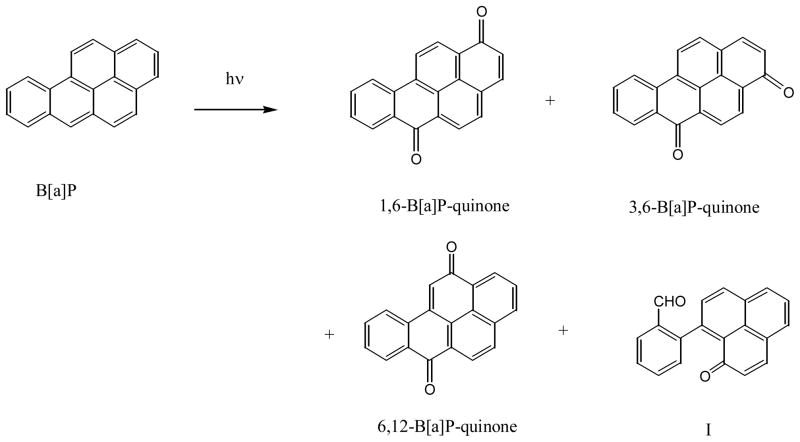
The most widely studied PAH is benzo[a]pyrene (B[a]P). Photolysis of B[a]P in aqueous solution yields 1,6-, 3,6-, and 6,12-B[a]P-quinones (66) and in benzene yields a ring open product (I) in addition to those three quinones (65) (Figure 7). 6-Oxy-B[a]P radical is observed if B[a]P is irradiated (74). It is suggested that further oxidation of the 6-oxy-B[a]P yields the quinones. It is proposed that the oxy-radical of B[a]P is responsible for DNA damage caused by the combination of UV light and B[a]P (75).
Figure 7.
Photo-oxidation of benzo[a]pyrene in aqueous solution or benzene
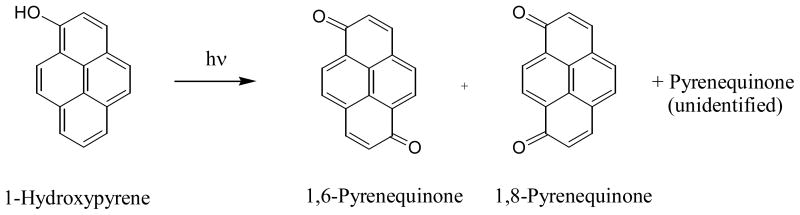
Hydroxy-PAHs are the oxidative metabolites commonly detected in the urine of animals or humans exposed to PAHs and are often used as biomarkers (11, 76). Among them, 1-hydroxypyrene (HP) is the most widely used biomarker for PAH exposure (17, 76–81). Upon light irradiation in aqueous solutions, HP is converted to three pyrenequinones: 1,6-pyrenquinone, 1,8-pyrenequinones, and an unidentified quinone (71) (Figure 8). The 1,6 and 1,8-pyrenequinones are also among the photolysis products for pyrene in aqueous solutions (67). The unidentified photoproduct has the correct molecular weight for a quinone, but the positions of the oxygen atoms are not determined.
Figure 8.
Photo-oxidation of 1-hydroxypyrene in aqueous solution
The photodegradation and photochemistry of nitro- and amino-PAHs have also been studied. Nitro-PAHs are a class of carcinogens detected in the atmosphere and some nitro-PAHs are more carcinogenic than their parent PAHs (18, 22, 82, 83). Amino-PAHs are usually the metabolite of nitro-PAHs. The photochemical degradation of nitro-PAHs mainly leads to photo-oxidation products. The photochemical reaction rate of nitro-PAHs depends on the position of the nitro-substitution (82, 84–87). Nitro groups peri to two hydrogen atoms is forced to be in a perpendicular orientation to the aromatic ring due to steric hindrance while nitro groups peri only to one hydrogen atom stay in a parallel orientation to the aromatic ring (18, 22, 33, 83) (Figure 9). Thus, nitro-PAHs can be divided into two categories: those with the perpendicular and others with the parallel nitro groups relative to the aromatic ring. Photochemically, the former nitro-PAHs react faster than the latter due to a light activated nitrite rearrangement mechanism as will be discussed later (87). It has long been recognized by Fu et al (18, 22, 83) that nitro-PAHs having perpendicular nitro groups are less mutagenic than nitro-PAHs having the nitro group parallel to the aromatic rings. This toxicity difference is attributed to their inability to be metabolized into reactive intermediates (N-hydroxyamino-PAH) that can form covalent DNA adducts.
Figure 9.
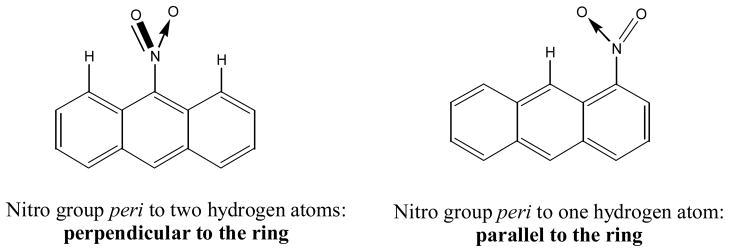
Nitro group orientation relative to the aromatic ring
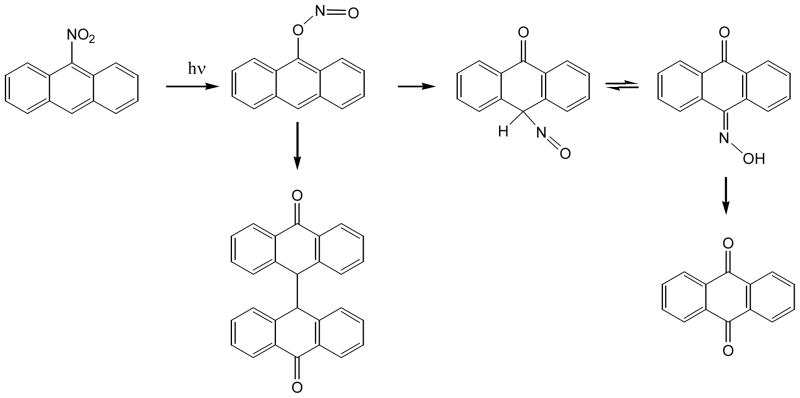
9-Nitroanthracene is the first nitro-PAH whose photochemistry is studied. Photolysis of 9-nitroanthracene, whose nitro group is peri to two hydrogen atoms, yields mainly 9,10-anthraquinone (56, 88) (Figure 10). The authors proposed that the formation of the 9,10-anthraquinone is via the rearrangement of a nitrite intermediate. Upon absorbing light energy, the nitro group rearranges to become a nitrite. The nitrite in turn rearranges to place a nitroso group on the opposite side of the six-member ring bearing the nitrite. This position is one of the two most reactive sites in anthracene. Further oxidation leads to 9,10-anthraquinone. Some nitrites lead to dimer formation via free radical intermediates.
Figure 10.
Photo-transformation of 9-nitroanthracene
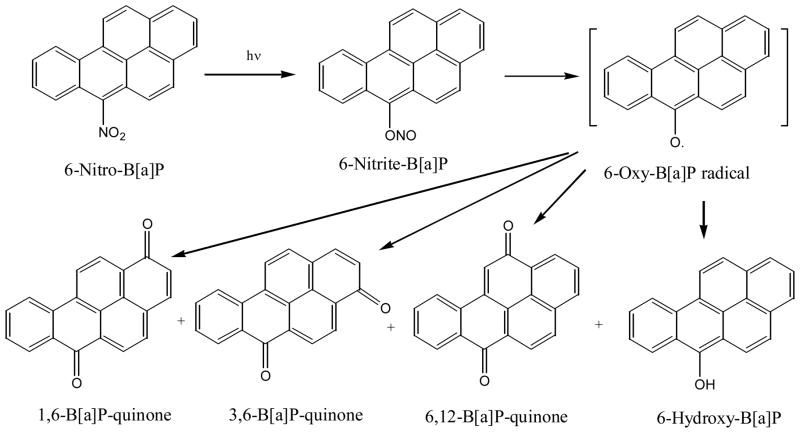
Photolysis of 6-nitro-B[a]P, another nitro-PAH with the nitro group peri to two hydrogen atoms, yields 1,6-, 3,6-, and 6,12-B[a]P-quinones and 6-hydroxy-B[a]P, also via the nitrite intermediate (82, 83, 87–89) (Figure 11). The three quinones are the same as the photolysis products of B[a]P (66).
Figure 11.
Photo-transformation of 6-nitrobenzo[a]pyrene
Unlike 9-nitroanthracene, the rearrangement of the nitrite does not form the nitroso substituted compound because the nitroso group does not have a reactive carbon in 6-nitro-B[a]P as it does in 9-nitro-anthracene to bind to (56). Thus nitric oxide is released into the solution by the photolysis of 6-nitro-B[a]P. The authors claim that the nitric oxide released can cause DNA single strand cleavage (89). Also, photolysis of 1- or 3-nitro-B[a]P does not result in the same intermediates, although their photoproducts have not been characterized. In these two compounds, the nitro group is peri only to one hydrogen atom.
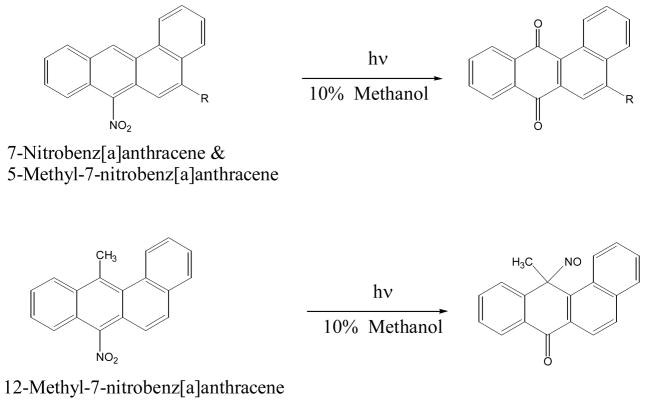
Irradiation of a 10% methanolic solution of 7-nitro-BA or 5-methyl-7-nitro-BA yields mainly the respective 7,12-BA-quinone (Figure 12). Irradiation of 12-methyl-7-nitro-BA does not produce the 7,12-BA-quinone. The main photoproduct isolated matches the molecular mass for 12-methyl-12-nitrosobenz[a]anthracen-7-one (90), the rearrangement intermediate for a 9-nitroanthracene-type reaction discussed above (56) (Figure 12). The difference is that the presence of the 12-methyl group prevented further oxidation to 7,12-BA-quinone.
Figure 12.
Photo-transformation of 7-nitrobenz[a]anthracene and its methyl derivatives
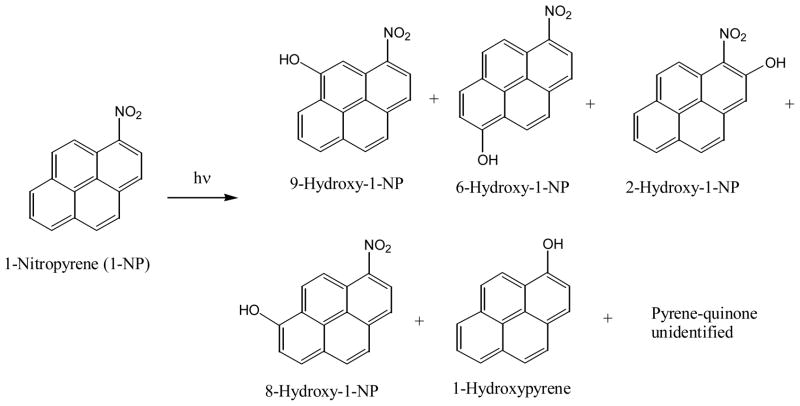
Unlike the nitro-PAHs that have the perpendicular nitro substituent, nitro-PAHs with a parallel nitro substituent are comparatively more stable under light irradiation. The photochemical reaction of these nitro-PAHs is complex due to the formation of complex mixture of photoproducts. This is probably why most of these photodegradation products have not been isolated and characterized, although the photodegradation of some of this type of nitro-PAHs have been studied (82, 84–86, 91–93). To have a look of the difficulty of studying the photochemical reaction of this type of nitro-PAHs, one can look at the nine photodecomposition products for 1-nitropyrene (85, 86, 93). Several publications contributed to the identification of some of the nine photoproducts: pyrene quinone (no information about the position of the oxygen) (86), 1-hydroxypyrene (86), and several monohydroxy-1-nitropyrenes (85, 86, 92, 93) (Figure 13).
Figure 13.
Photo-transformation of 1-nitropyrene
The photodegradation of other nitro-PAHs that have been studied includes 2-nitroanthracene, 2- and 4-nitropyrene, 1,8-dinitropyrene, 3-nitrofluoranthene, 1- and 3-nitro-B[a]P, 9-nitrodibenz[a,c]anthrancene, and 7-nitrodibenz[a,h]anthracene. But their photoproducts have not been identified.
It was found that photolysis of amino-PAHs generates direct acting mutagens (94–101). Photolysis of 2-aminofluorene converts it into 2-nitrosofluorene, 2-nitrofluorene, and 2-amino-9-fluorenone (94, 96, 100, 101) (Figure 14). Both the nitro and nitrosofluorenes are direct acting mutagens.
Figure 14.
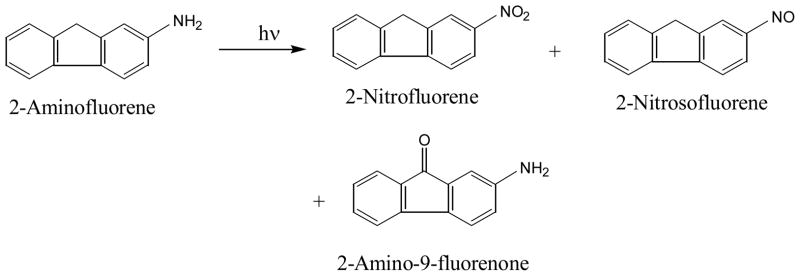
Photo-oxidation of 2-aminofluorene in aqueous solution
Photolysis of 1-aminopyrene (AP) was first reported to produce the direct acting mutagen 1-nitropyrene and several other unidentified photoproducts (95, 99). A more careful examination of the photochemical reaction products reveals that 1-nitrosopyrene, 1-hydroxyaminopyrene and some pyrene-quinone dimers are also formed in addition to 1-nitropyrene (102). Therefore, a progressive photo-oxidation mechanism is proposed. In this mechanism, the formation of 1-hydroxyaminopyrene is of particular interest because this is also the reactive intermediate that can form DNA covalent adducts from the enzymatic reduction of nitro-PAHs (18, 22, 33–35). Indeed, irradiation of AP together with calf thymus DNA, some AP-DNA covalent adducts are detected (102) (Figure 15).
Figure 15.
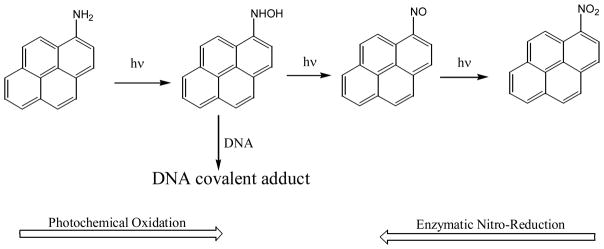
Photo-oxidation of 1-aminopyrene in aqueous solution
Halogenated PAHs (chloro and bromo-PAHs) are also detected in the environment and are found to be carcinogenic (19). So far there has been neither phototoxicity study nor study of their photochemical reaction appeared in the literature.
In conclusion, the way the photochemical reaction of various PAHs proceeds depends on the structure of the PAHs themselves, the number of fused rings, the arrangement of the rings, the substituent type and position, as well as the coexisting molecules and solvents.
IV. PAH MOLECULAR PHOTOTOXICITY
Light-induced toxicity of PAHs has been recognized some seventy years ago (103). Some of the early studies focused on the impact of light and PAH on the development of cancer (104–107). Later on, more work have been directed toward the study of the molecular mechanism of phototoxicity of PAHs (40, 73, 95, 97, 101, 108–118). A review article on the effects of near ultraviolet radiation on the toxicity of PAHs in animals and plants appeared in 1996 (46).
When referring to phototoxicity, it includes both the acute toxicity that ultimately leads to cell death and the genotoxicity that ultimately leads to tumor formation and other genotoxic diseases such as teratology. The source of phototoxicity should come from damages to cellular components caused by reactive intermediates due to photo-excitation of PAHs. Therefore, the study of phototoxicity mechanism on the molecular level is here referred to as molecular phototoxicity.
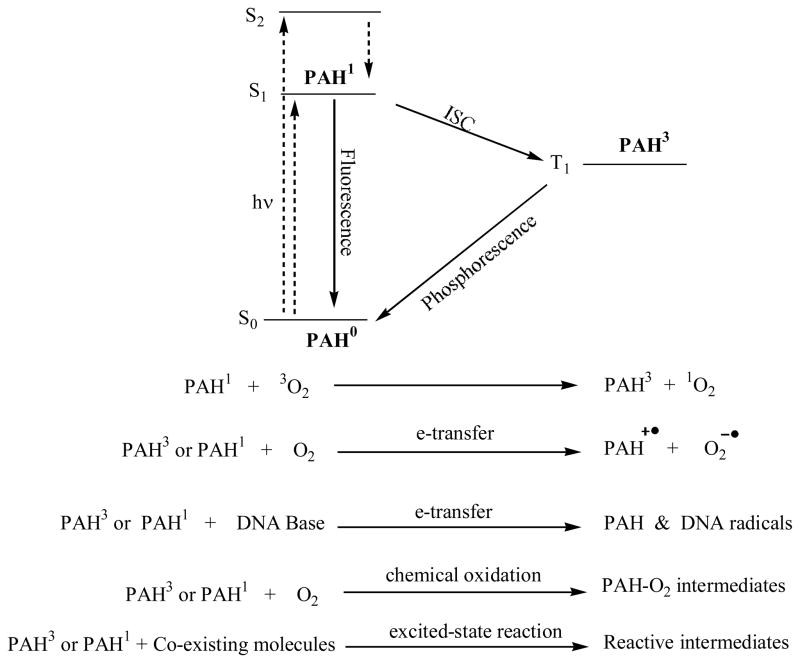
In principle, PAHs can absorb light energy and be excited to the first and upper excited singlet states (S1, S2, …). Molecules in the upper singlet excited electronic states relax quickly to the S1 state by giving up heat energy. PAH molecules in the excited singlet state can lose its energy by emitting a photon and return to the ground state (fluorescence) or intersystem crossing to the triplet state T1 (ISC), which can in turn loses its energy by emitting a photon and return to ground state (phosphorescence). There are other pathways that can lead to energy loss of the excited state molecule such as internal conversion, electron or energy transfer to other molecules, etc. All these pathways except electron or energy transfer should be harmless to cells. Energy transfer to other molecules may invoke a series of excited state reactions of that molecule it transfers energy to. Transfer of an electron to other molecules can produce free radical species that are usually reactive toward biological macromolecules. In addition to these pathways, excited state PAH molecules (both in singlet and triplet states) can react with other co-existing molecules in a variety of ways. The most common co-existing molecules are oxygen, solvent, and other biologically important molecules such as proteins, nucleic acids, cell membrane components, ions, amino acids and coenzymes in the cell. Several examples of the excited-state reactions are given in Figure 16. As a result of the reaction, singlet oxygen, superoxide radical, PAH radical, DNA radical, oxygenated PAH reactive intermediates, or other reactive intermediates are formed and can damage cellular constituents, resulting in either acute toxicity or genotoxicity. Depending on the degree of the damages and to which biological macromolecule is the damage, the damage can (i) be repaired and becomes harmless, (ii) lead to cell death since the damage is too severe to be repaired or (iii) lead to mutation if the damage is to the genetic code DNA.
Figure 16.
Excited state photophysics and photochemistry of PAHs
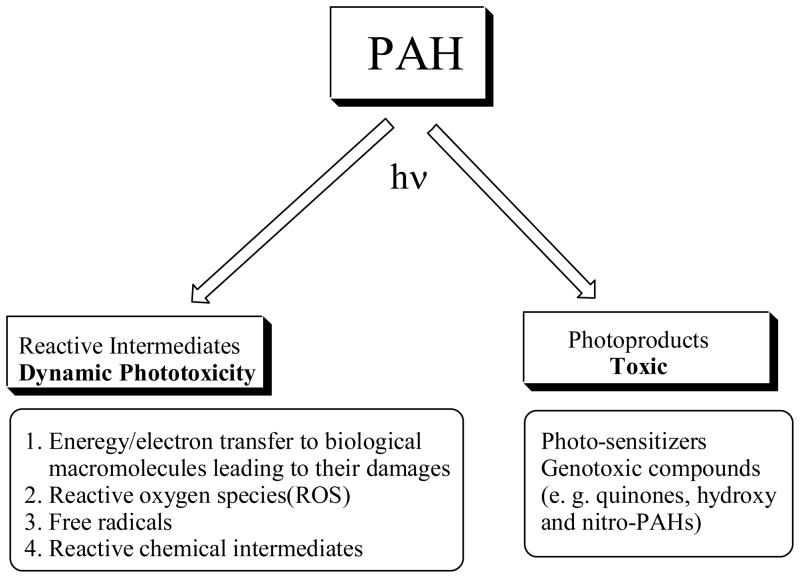
From a mechanistic point of view, there are two pathways resulting in phototoxicity: (i) dynamic phototoxicity: damages to cells during photo-transformation of chemical species. This includes excited state energy transfer to biological macromolecules resulting in high energy biological molecules, electron transfer to produce both a PAH and a biological molecule free radical, production of short-lived reactive intermediates such as singlet oxygen, superoxide free radical, and chemically reactive species; (ii) toxic photoproducts: during photolysis, some relatively light-stable compounds such as quinones, nitro-PAHs are produced and they may be toxic both with or without metabolic or light activation. These two pathways are summarized in Figure 17.
Figure 17.
Dynamic phototoxicity and formation of toxic photoproducts
Light-induced damages to cell membranes, proteins, and DNA by many other molecules have been observed (119–121). Irradiation of a PAH molecule, anthracene, together with human serum albumin resulted in covalent binding of anthracene to protein and caused protein-protein cross-links, and to cell membrane via lipid peroxidation (115). Evidences for cell membrane damages by irradiation of B[a]P, anthracene, or 1-nitropyrene mixed with cells were also reported (111, 112, 116). As it is summarized by Arfsten et al in their review of PAH phototoxicity (46), DNA is the chief target for photo-induced damages by PAHs. Although proteins and cell membranes can be important photo-damage targets for PAHs, much work have concentrated on DNA damages. This is possibly due to the carcinogenic nature of PAH molecules, which have usually been associated with DNA covalent adduct formation after metabolic activation.
Possible DNA damages by light and a chemical are: (i) single strand cleavage, (ii) double strand cleavage, (iii) deletion of a base (depurination/depyrimidation), (iv) oxidation of guanine to 8-hydroxy or 8-oxoguanine, (v) thymine-thymine dimer formation, (vi) DNA covalent adduct, (vii) DNA-DNA cross-links, and (viii) DNA-protein cross-links. Most of these damages can be repaired without leading to mutation. To date, several types of DNA damages by the combination of PAHs and light have been studied: formation of PAH-DNA covalent adducts, DNA single strand cleavage, depurination/depyrimidation, and formation of oxidative product 8-hydroxyguanine.
A. Light-Induced PAH-DNA Covalent Adduct Formation
It was reported in 1964 that PAH-DNA covalent adduct can be formed through metabolic activation (122). In the same year, Ts’O and Lu first reported that irradiation of radioactive 3H-labeled B[a]P by light (>300 nm or > 340 nm) can induce the formation of covalent B[a]P-DNA adduct (123). A follow-up experiment on studying chemical linkage of B[a]P with DNA induced by light irradiation in the presence of various chemicals also confirmed that irradiation on the mixture of dibenz[a,c]anthracene or dibenz[a,h]anthracene with DNA in aqueous solutions can form covalent DNA adducts (124). The use of light of >300 nm confirms that PAH, not DNA, is excited to the excited state to facilitate the photochemical reaction that produces PAH-DNA covalently linked products. In 1975, covalent linked anthracene-DNA adduct was reported if human skin or monkey kidney epithelial cells and anthracene are irradiated together (125). In 1983, Sinha and Chignell (115) reported that covalent anthracene-DNA adduct is detected if anthracene and human serum albumin are irradiated together. The amount of anthracene bound to DNA depends on the time of irradiation, but not on the presence of oxygen.
In the 1970s, two groups attempted to study PAH-DNA adduct structure using model photochemical reaction systems by irradiating a free DNA base with a PAH molecule in organic solvents (126, 127). Irradiation of B[a]P with 1-methylcytosine in methanol-acetone mixture produced 6-(1-methylcytos-5-yl)-B[a]P (127), a product by joining the most reactive carbon (C-6) of B[a]P with 1-methylcytosine’s C-5 carbon as shown in Figure 18.
Figure 18.
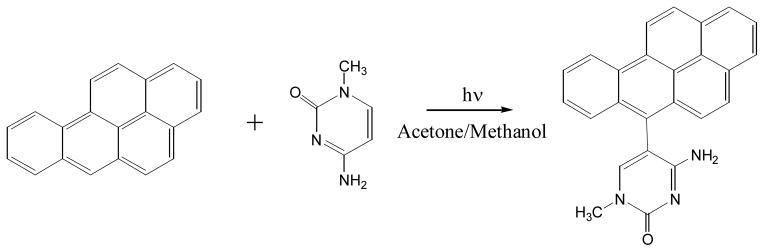
Photoreaction of benzo[a]pyrene with 1-methylcytosine
Irradiation of anthracene with 1-methylcytosine produced two products, with one being 5-(anthracen-9-yl)-1-methylcytosine and the other unidentified (126) (Figure 19).
Figure 19.
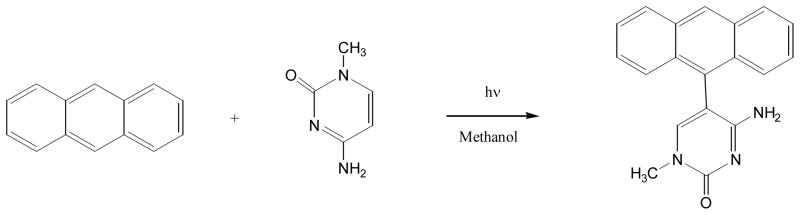
Photoreaction of anthracene with 1-methylcytosine
Irradiation of B[a]P with thymine gives 1-(benzo[a]pyren-6-yl)thymine by linking the C-6 of B[a]P with thymine’s N-1 nitrogen (126) (Figure 20). However, whether these structures of anthrancene with 1-methylcytosine or B[a]P with thymine/1-methylcytosine are related to their photo-induced DNA adduct derived from double helical DNA remains to be seen.
Figure 20.
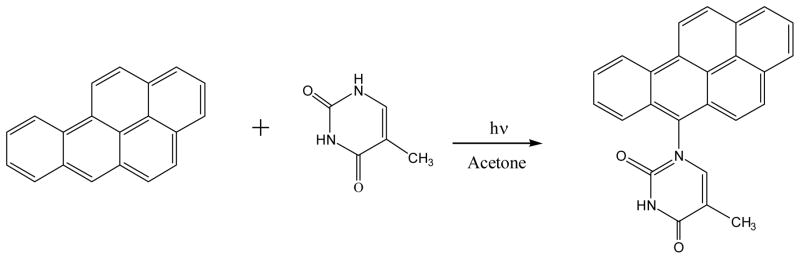
Photoreaction of benzo[a]pyrene with thymine
It was attempted to compare the photo-induced PAH-DNA adduct with the metabolic PAH-DNA adduct. Direct isolation of PAH-DNA adducts from the cell or even from oligomer DNA is difficult. Therefore, no light-indcued PAH-DNA adducts have been isolated and characterized so far. Hoard et al (128) and Strniste et al (75) have attempted to compare B[a]P-DNA adduct from both enzymatic and light-activation pathways. Upon irradiation of 14C-labeled B[a]P with single strand DNA, the DNA was enzyme digested and the adducts were compared using HPLC with the microsome-metabolized B[a]P adducts. Although these adducts elute at the same region on HPLC, they can potentially be very different in terms of chemical structure. Another observation is that the light-induced B[a]P-DNA adducts appear to be oxygen dependent.
Recently, another method to analyze PAH-DNA photo-adducts was developed using dialysis and UV absorption spectra (129). 1-Hydroxypyrene (HP) and calf thymus DNA were irradiation together for a period of time before the mixture was dialyzed in semi-permeable membrane tubes that allow small molecules (unbound HP or its photoproducts and other DNA fragments) to diffuse into the solution and only the DNA bound HP remains in the solution within the dialysis tube. The absorption spectra of the light-irradiated or non-irradiated (dark control) solutions in the dialysis tubes are recorded. If HP-DNA covalent adduct is formed, the light-irradiated solution should have the absorption features for both HP and DNA. Indeed, after irradiation and dialysis, about 30% of the original HP absorption remained in the dialysis tube together with DNA (129). One restriction for this method is if the PAH chromophore in the PAH-DNA adduct does not have a distinct absorption at wavelengths >280 nm, its absorption will be interfered by the absorption of DNA bases and cannot be easily identified. This method also requires a high photochemical reaction yield since there must be a high enough concentration for PAH-DNA adducts to be detected by the spectrophotometric instrument (usually in μM range). In comparison to the conventional method for detecting DNA adducts via metabolism, the 32P-postlabeling technique, this is a much less sensitive technique.
In an effort to study the HP-DNA covalent adduct, HP and an eight-base pair duplex oligodeoxynucleotide, d(5′-GCTAGGGC-3′)/d(5′-GCCCTAGC-3′), were irradiated together by UVA light. It produced three products shown on the HPLC chromatogram that carry both the absorbance of HP and DNA (129). However, where and how HP is bound to DNA remains unsolved.
In addition to HP, DNA covalent adducts of 1-hydroxy-B[a]P, 1-aminopyrene, 6-aminochrysene, and 3-aminofluoranthene are detected if they are irradiated together with DNA by UVA light (102, 130). However, none of these covalent adducts have been characterized structurally. Mutagenesis study of the light-induced PAH-DNA covalent adducts and structural comparison of these adducts with the PAH-DNA adducts from metabolic activation would be interesting.
In vivo, the formation of PAH-DNA covalent adducts by light irradiation and metabolic activation could be competing factors if light is available for PAHs that are in the cell. In this case, it should be simultaneously subject to enzymatic oxidation and undergo light-induced degradation. Actually, it is found that irradiation of a PAH, B[a]P or 7,12-dimethyl-BA while it is in the cells, tends to lower the amount of DNA covalent adducts formed by enzymatic activation (75, 116, 117, 131). The authors attributed this decrease of the amount of enzymatic PAH-DNA adducts mainly to the photodegradation of the PAHs. However, it is possible that formation of light-induced DNA covalent adducts, which should be different from the enzyme-activated DNA covalent adducts, competes with the enzyme-activated formation of DNA covalent adducts.
B. Light-Induced DNA Single Strand Cleavage by PAHs
Another form of DNA damage by the combination of light and PAHs is DNA single strand cleavage. Strniste et al first observed that irradiation of light and B[a]P mixed with PM2 DNA caused it to convert from the supercoiled form to the relaxed circular form (75). Saito et al discovered that irradiation of 1-nitropyrene and related compounds with Chinese hamster cells caused DNA strand cleavage (132) and Utsumi et al found that the combination of 7,12-dimethyl-BA and near UV light caused more single strand cleavage of the DNA in the cell than without near UV light irradiation (133). Later, Kagan et al discovered that two different compounds, anthracene (116) and 1-nitropyrene (91), can cause pBR322 DNA to convert from supercoiled form to relaxed circular form upon irradiation. The photo-induced nicking of supercoiled DNA by 1-nitropyrene or anthracene can occur under either aerobic or anaerobic conditions, with the former being stronger in causing DNA single strand cleavage (91). The authors suggested that hemolysis of various cells due to the combination of light irradiation and the presence of a PAH could also be due to DNA damages in addition to damages to the cell membrane. DNA covalently bound B[a]P diolepoxide adduct can also cause laser light (355 nm) induced DNA cleavage (134). In fact, laser light-induced DNA single strand cleavage by covalently linked B[a]P diolepoxide has been used for mapping where B[a]P diolepoxide is bound to the DNA chain (135–138).
A systematic light-induced DNA single strand cleavage study has been carried out for various PAHs using UVA light and ΦX174 plasmid DNA (69, 129, 139–142). In these experiments, the amount of DNA single strand cleavage depends on both light and PAH doses. Therefore, by fixing the light dose at 170 J/cm2 (1 hour irradiation), a relative DNA photocleavage efficiency indicator, C25, the concentration at which 25% of the original supercoiled DNA is converted into relaxed, open circular DNA upon the combination of a PAH and light irradiation is determined. The C25 can be used for comparing relative DNA photocleavage efficiency for various PAHs. The smaller the C25 values, the more efficient is a PAH toward DNA single strand cleavage.
As shown in Table 1, the DNA photocleavage efficiency (C25) depends on both (i) the structure of the PAHs and (ii) the ring size and arrangement of the rings. A larger ring does not necessarily mean higher DNA photocleavage efficiency. The three-ring anthrancene is a stronger DNA photocleaver than the four-ring pyrene, but is similar to the four-ring chrysene and BA and the five ring B[a]P (140). The presence of a substituent such as hydroxy, amino, bromo, and nitro, generally increases the DNA photocleavage efficiency for anthracene, pyrene, and B[a]P, but not for chrysene. Metabolic products of B[a]P are all more efficient in causing DNA photocleavage (142). In other words, metabolic oxidation of PAHs into phenolic derivatives may not be a detoxification process, as one would think. Rather, the metabolites can be more phototoxic than the original PAH molecules themselves. Methyl substitution on BA has an interesting effect on their DNA photocleavage efficiency. Methyl substitution at 4, 5, 6, 8, 9, 10 positions does not affect the photocleavage efficiency for BA, but methyl substitution at other positions, especially at 7 and 12 positions, decreases the photocleavage efficiency for BA. This structure-photocleavage efficiency relationship match the HOMO-LUMO gap of these methyl substituted BAs (69).
Table 1.
Relative DNA single strand cleavage efficiencies (C25) for selected PAHs in aqueous solutions (10 mM sodium phosphate at pH 7 in 4% dimethylformamide).
| PAH | Number of Rings | C25 (μM) | Reference |
|---|---|---|---|
| Naphtahlene | 2 Rings | n. d. | (140) |
| 2-Hydroxycarbazole | ~100 | (140) | |
| Anthracene | 3 Rings | 8.3 | (140) |
| 9-Nitroanthracene | 0.83 | (140) | |
| Pyrene | 4 Rings | 51 | (140) |
| 1-Aminopyrene | 4.2 | (140) | |
| 1-Hydroxypyrene | 0.36 | (129) | |
| 1-Nitropyrene | 9.9 | (140) | |
| 1-Bromopyrene | 4.0 | (140) | |
| Chrysene | 5.2 | (140) | |
| 6-Aminochrysene | 60 | (140) | |
| 1,4-Chrysenequinone | 5.8 | (140) | |
| 3-Aminofluoranthene | 32 | (140) | |
| Benz[a]anthracene (BA) | 18 | (69) | |
| 1-Methyl-BA | 60 | (69) | |
| 2-Methyl-BA | 74 | (69) | |
| 3-Methyl -BA | 34 | (69) | |
| 4-Methyl-BA | 12 | (69) | |
| 5-Methyl-BA | 13 | (69) | |
| 6-Methyl-BA | 20 | (69) | |
| 7-Methyl-BA | ~100 | (69) | |
| 7-Hydroxymethyl-BA | ~100 | ||
| 8-Methyl-BA | 18 | (69) | |
| 9-Methyl-BA | 17 | (69) | |
| 10-Methyl-BA | 12 | (69) | |
| 11-Methyl-BA | 42 | (69) | |
| 12-Methyl-BA | 93 | (69) | |
| 12-Hydroxymethyl-BA | ~100 | ||
| 7,12-Dimethyl-BA | ~100 | (69) | |
| BA-7,12-dione | 11.8 | (69) | |
| Benzo[a]pyrene (B[a]P) | 5 Rings | 6.0 | (140) |
| 1-Hydroxy-B[a]P | 0.6 | (142) | |
| 3-Hydroxy-B[a]P | 2.5 | (142) | |
| 6-Acetoxy-B[a]P | 1.3 | (142) | |
| 7-Hydroxy-B[a]P | 0.1 | (142) | |
| 9-Hydroxy-B[a]P | 1.3 | (142) | |
| B[a]P-3,6-quinone | 3.9 | ||
| B[a]P-1,6-quinone | 2.5 | ||
| B[a]P-7,8-dihydrodiol | 1.1 |
From knowing the photochemical reaction of these PAHs as discussed earlier, one can imagine that the DNA photocleavage efficiency has to do with how the photoreaction of these PAHs proceeds in aqueous solutions. Compounds that can easily be oxidized to quinones, such as anthracene, BA, B[a]P, and chrysene, have lower C25 (high efficiency) than those that are more difficult to be oxidized. Also, the presence of a hydroxy, bromo, nitro, or amino substituent in PAHs usually enhances their DNA photocleavage efficiency, possibly due to enhanced photochemical reaction of the substituted PAHs.
For example, methyl-substitution at the 7 or 12 position of BA blocks the formation of 7,12-BA-quinone and thus greatly reduces the photocleavage efficiency of BA (69). Presence of a 9-nitro group in anthracene facilitates the nitrite-mediated rearrangement reaction to form anthraquinone (56), thus enhances the DNA photocleavage of anthracene. Presence of the 1-hydroxy or 1-amino group in pyrene greatly enhances their photodegradation reaction in comparison to pyrene and these two compounbds are also more efficient in causing DNA photocleavage than pyrene (102, 129, 130).
Nitro-PAHs can also induce DNA single strand cleavage upon irradiation. Table 2 lists some of the recent results. Presence of a nitro group peri to two hydrogen atoms in a PAH enhances its ability to cause DNA photocleavage. 9-Nitroanthracene, 7-nitro-BA, 5-methyl-7-nitro-BA, 12-methyl-7-nitro-BA are better DNA photocleavers than their respective parent PAHs anthracene, BA, 5-methyl-BA, 12-methyl-BA, respectively under the same conditions. Mono nitro-substituted pyrenes, whose nitro group peri to one hydrogen atom, are worse DNA photocleavers than the parent PAH pyrene. As it is discussed earlier, orientation of the nitro group governs the photochemical reaction rate and the formation of intermediates, and photoproducts. The DNA cleavage data clearly indicates a strong linkage between the photochemical reaction activity of the nitro-PAHs and their DNA photocleavage efficiency. This is possibly due to the nitro rearrangement reaction discussed above for nitro-PAHs with the nitro group perpendicular to the aromatic ring (56, 89, 90). Combining with the observation that PAHs that can easily convert to quinones are strong DNA photocleavers, DNA photocleavage by PAHs in general is closely related to their photochemical reaction rate, photochemical reactivity, and formation of reactive intermediates.
Table 2.
Relative DNA single strand cleavage efficiencies (C25) for selected nitro-PAHs in aqueous solutions (10 mM sodium phosphate at pH 7 in 10% dimethylformamide).
| PAH | Nitro-orientation | C25 (μM) | Reference |
|---|---|---|---|
| 9-Nitroanthracene | Perpendicular | 0.83 | (140) |
| Anthracene | 8.3 | (140) | |
| 7-Nitro-BA | Perpendicular | 2.8 | (90) |
| Benz[a]anthracene | 18 | (90) | |
| 5-Methyl-7-Nitro-BA | Perpendicular | 5.2 | (90) |
| 5-Methyl-BA | 13 | (90) | |
| 12-Methyl-7-Nitro-BA | Perpendicular | 5.2 | (90) |
| 12-Methyl-BA | 85 | (90) | |
| 1-Nitropyrene | Parallel | 78 | (90) |
| 2-Nitropyrene | Parallel | >100 | (90) |
| 4-Nitropyrene | Parallel | >100 | (90) |
| Pyrene | 60 | (143) | |
| 1-Nitro-B[a]P-t-7,8-dihydrodiol | Parallel | >100 | (90) |
| 3-Nitro-B[a]P-t-7,8-dihydrodiol | Parallel | >100 | (90) |
Solvents and coexisting chemicals can play an important role for light-induced DNA cleavage by PAHs. Since the solubility for PAHs in water is generally not good (47), the presence of an organic solvent can increase the solubility of almost all PAHs and thus increase their effective concentration. As a result, it may increase the efficiency of PAHs causing DNA single strand photocleavage. Actually, increasing the amount of dimethylformamide, dimethylsulfoxide, or methanol up to 20% in water increases the amount of DNA single strand photocleavage by pyrene. This is presumably due to better pyrene solubility with increasing organic solvent concentration. However, it generally decreases the DNA cleavage efficiency for the water-soluble HP and AP (under working concentrations) with the increasing amount of the organic solvent used (141, 143). It is believed that these organic solvents can act both as a quencher for DNA photocleavage and a solvent to help to dissolve the PAH. Co-existing chemicals such as biologically important ions (141, 143), other added quenchers (69, 129, 139–142, 144), and amino acids (139) can either enhance or inhibit the light-induced DNA cleavage by PAHs. Therefore, how the chemical present affect the DNA photocleavage by PAHs depends on how they affect the photochemical process of a particular PAH.
Mechanistic studies reveal that singlet oxygen, superoxide and other radicals intermediates, including possible PAH radicals, are involved in causing DNA single strand photocleavage by PAHs (69, 91, 129, 140). Both an oxygen-dependent and an independent pathway cause the DNA photocleavage. From PAH to PAH, the involvement of one or the other reactive species in DNA photocleavage can be different. In summary, the efficiency of light-induced DNA cleavage by PAHs is closely related to the photochemical reaction of the PAHs and the present of chemicals or solvents that affect the photochemical reaction or the production of reactive intermediates.
C. Other DNA Damages by the Combination of Light and PAHs
The third type of DNA damage induced by the combination of light and PAHs is the oxidation of guanine into 8-hydroxy or 8-oxoguanine. Exposure of mammalian cells to B[a]P and fluorescent light induces 3–10 fold more 8-hydroxyguanine formation than it is carried out with B[a]P but without light (113). A similar observation by UVA light irradiation of B[a]P mixture with calf thymus DNA or with cultured human epidermoid carcinoma cells was reported by another group (145). These studies also points to the involvement of superoxide anion during the oxidation of guanine.
There are also evidences that DNA base deletion is a DNA damage induced by the combination of PAHs and light. Alkaline treatment enhances the amount of single strand cleavages in some cases (75). However, there has been no quantitative or qualitative analysis for this type of damage.
V. PERSPECTIVES
PAHs are a class of environmental contaminants that are present in the air, aquatic systems, and sentiments. Understanding of the environmental fate and their toxic mechanism to the ecological systems is important. Environmental fate of these compounds are linked to degradation/transformation by light, microorganisms, and other chemical reactions in the solid-state (sentiment) or solution. Light-transformation of these compounds can lead to either detoxification by degradation to small and less harmful molecules or to more toxic species by transformation into oxidation products that can be better photosensitizers (quinones) or direct acting mutagens (amino to nitro-PAHs).
Photo-oxidation of the basic PAH moieties: 2-ring: naphthalene (53), 3-ring: anthracene (57–60, 69, 72), phenanthrene (54, 55), 4-ring: BA (69, 70), pyrene (67, 68), and 5-ring: B[a]P (65, 66), can produce respective quinones, ring opening products, or hydroxy-substituted products. Photo-oxidation products of other basic PAH molecules such as azulene (2-ring), fluoranthene (4-ring), chrysene (4-ring), and larger ring PAHs (>5-ring) has not been reported, although they are also found as popular contaminants. One speculation is that their photodegradation is more complex due to their special ring arrangement. In azulene, it is a five-carbon ring bridged together with a seven-carbon ring and in fluoranthene three benzene rings are connected together by a five-carbon ring. This kind of ring connectivity does not allow the formation of quinones that are usually more stable photochemically that enables isolation and characterization. Larger ring PAHs are relatively rare and their photoproducts can be more complex due to greater complexity of the rings. Photo-oxidation of some nitro (56, 82, 84, 86, 88–90, 146), amino (94–96, 102), and hydroxy (71) PAHs also lead to quinones. In addition to quinones, hydroxy (53, 65, 67, 93), carboxy (53), hydroxymethyl and formyl-PAHs for methyl substituted PAHs (70) are also detected among the photolysis products. Quinones are known reactive oxygen species sensitizers (28, 31, 147, 148). On the other hand, quinones are relatively stable compounds against photo-oxidation. There is no appreciable degradation if 7,12-BA-quinone is irradiated with UVA light (170 J/cm2/h) for eight hours. During the irradiation, 7,12-BA-quinone can cause DNA strand cleavage (69). Therefore, these quinones are potentially more phototoxic.
Another aspect of the PAH photoproducts, whether quinones or hydroxy-substituted derivatives, is that they are more water-soluble. The concentration of these compounds in the aquatic systems can be much higher than the PAHs themselves in the aqueous media. Therefore, they are potentially more toxic toward aquatic organisms because of the higher concentrations.
PAH molecules entering the cell usually undergo metabolism to form more water-soluble species before they are excreted. The metabolic products, usually the hydroxy-derivatives of the parent PAHs, are thought to be less mutagenic than the parent compound. However, 1-hydroxypyrene, the metabolic product of pyrene, is found to be very strong DNA photocleavers (129) and more phototoxic (144). In addition, the hydroxy derivatives of B[a]P are also more efficient in causing DNA photocleavage (142). Therefore, metabolism of PAHs into hydroxy-PAHs may not be a detoxification process.
During the photodegradation of the PAHs, whether in aquatic or cellular environment, free radical intermediates and reactive oxygen species are produced and they can damage cellular constituents. Although this review focuses on the damage to DNA, damages to proteins or cell membranes may also be contributing factors to phototoxicity. Especially, the light-induced formation of PAH-DNA covalent adducts deserves special attention, e. g., the high yield of the photo-induced PAH-DNA covalent adduct formation. More than 30% of HP is covalently linked to calf thymus DNA upon UVA irradiation (129). Early studies also indicate that similar amount of amino-PAHs can be covalently linked to DNA upon UVA light irradiation (130). Using the much more sensitive 32P-post-labeling technique, an early result indicates that 7,12-dimethyl-BA can form DNA-covalent adducts (unpublished results). It indicates that light-induced PAH-DNA covalent adducts could be a popular phenomena for all the PAH molecules, although the degree of photobinding can be different significantly. So far there is no structural data available yet for the photo-induced PAH-DNA covalent adduct. However, one may imagine that the light-induced PAH-DNA covalent adducts must be different from those metabolic PAH-DNA adducts due to different reaction mechanisms. Because of the structural differences, they may have a different mutagenicity activity. Nonetheless, photo-transformation of PAHs is another activation pathway in addition to the known metabolic activation pathway of PAHs that transforms relatively harmless inert PAH molecules into monstrous reactive intermediates.
PAHs are environmental contaminants exist in food, air, water, soil and sediment (1–7). Some commercial medicines contain PAHs as well. For example, coal tar, a complex mixture of PAHs, is widely used in creams, ointments, lotions, and shampoos and for the treatment of psoriasis (6, 149). Topical application of coal tar on the skin followed by ultraviolet light radiation, known as the Goeckerman therapy for psoriasis, has an increased risk of developing cancer (149). It has been shown that UVB radiation and coal tar have an additive effect on inducing metabolizing enzyme activities and DNA adduct formation in the mouse skin (43). Roofers and highway asphalt workers also have a high risk to be exposed to both PAHs and light at the same time (150). It is known that PAHs can induce skin cancer (6, 151, 152). Since human skin is exposed to light, it is of particular importance and significance to investigate human health risks posed by exposure to the combination of PAHs and light.
Acknowledgments
The author wishes to thank the National Institutes of Health through generous grants: NIH-MBRS SCORE S06GM08047 and NIH-RCMI 5G12RR13459 and the US Army Research Office: DAAD 19-01-1-0733 to JSU.
References
- 1.Baum E. Occurrence and surveillance of polycyclic aromatic hydrocarbons. In: Gelboin H, Ts’O T, editors. Polycyclic Aromatic Hydrocarbons and Cancer. Vol. 1. Academic Press; New York: 1978. pp. 45–70. [Google Scholar]
- 2.Connell DW, Hawker DW, Warne MJ, Vowles PP. Polycyclic aromatic hydrocarbons (PAHs) In: McCombs K, Starkweather AW, editors. Introduction into Environmental Chemistry. CRC Press LLC; Boca Raton, FL: 1997. pp. 205–217. [Google Scholar]
- 3.Shaw GR, Connell DW. Prediction and monitoring of the carcinogenicity of polycyclic aromatic compounds (PACs) Rev Environ Contam Toxic. 1994;135:1–62. doi: 10.1007/978-1-4612-2634-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Harvey RG. Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenicity. Cambridge University Press; London: 1991. [Google Scholar]
- 5.IARC. Part I: Chemical, Environmental and Experimental Data. International Agency for Research on Cancer; Lyon: 1983. Polynuclear aromatic compounds. [PubMed] [Google Scholar]
- 6.National Toxicology Program, P. H. S. US Department of Health and Human Services. 8th Report on Carcinogens. Integrated Laboratory Systems, Inc; Research Triangle Park, NC: 1998. pp. 178–181. [Google Scholar]
- 7.Menzie CA, Potocki BB, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environ Sci Technol. 1992;26:1278–1284. [Google Scholar]
- 8.U. S. Department of Health and Human Services, P. H. S. ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) Atlanta: 1995. [Google Scholar]
- 9.Angerer J, Mannschreck C, Gundel J. Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 1997;70:365–377. doi: 10.1007/s004200050231. [DOI] [PubMed] [Google Scholar]
- 10.Angerer J, Mannschreck C, Gundel J. Occupational exposure to polycyclic aromatic hydrocarbons in a graphite-electrode producing plant: biological monitoring of 1-hydroxypyrene and monohydroxylated metabolites of phenanthrene. Int Arch Occup Environ Health. 1997;69:323–331. doi: 10.1007/s004200050155. [DOI] [PubMed] [Google Scholar]
- 11.Jongeneelen FJ. Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons: 1-hydroxypyrene in urine of people. Toxicol Lett. 1994;72:205–211. doi: 10.1016/0378-4274(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 12.Dabestani R, Ivanov IN. A comparison of physical, spectroscopic and photophysical properties of polycyclic aromatic hydrocarbons. Photochem Photobiol. 1999;70:10–34. [Google Scholar]
- 13.Dipple A. Polycyclic aromatic hydrocarbons and carcinogenesis. Ameraican Chemical Society; Washington, DC: 1985. [Google Scholar]
- 14.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 15.Lesko SA. Chemical Carcinogenesis: Benzopyrene system. Methods in Enzymology. 1984;105:539–550. doi: 10.1016/s0076-6879(84)05074-6. [DOI] [PubMed] [Google Scholar]
- 16.Warshawsky D. Polycyclic aromatic hydrocarbons in carcinogenesis. Environ Health Perspect. 1999;107:317–320. doi: 10.1289/ehp.99107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talaska G, Underwood P, Maier A, Lewtas J, Rothman N, Jaeger M. Polycyclic aromatic hydrocarnons (PAHs), nitro-PAHs and related environmental compounds: Biological markers of exposure and effects. Envirn Health Perspect. 1996;104:701–908. doi: 10.1289/ehp.96104s5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu PP, Herrero-Saenz D. Nitro-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. Environ Carcinog & Ecotox Rev. 1999;C17:1–43. [Google Scholar]
- 19.Fu PP, von Tungeln LS, Chiu LH, Own ZY. Halogenated-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. Environ Carcinog & Ecotox Rev. 1999;C17:71–109. [Google Scholar]
- 20.Harvey RG. Polycyclic Aromatic Hydrocarbons. Wiley-VCH; New York: 1996. [Google Scholar]
- 21.Gelbroin HV. Benzo[a]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 22.Fu PP. Metabolism of nitro-polycyclic aromatic hydrocarbons. Drug Metabolism Rev. 1990;22:209–268. doi: 10.3109/03602539009041085. [DOI] [PubMed] [Google Scholar]
- 23.Lesko SA, Lorentzen RJ, Ts’O POP. Benzo[a]pyrene metabolism: One-electron pathways and the role of nuclear enzymes. In: Gelboin H, Ts’O T, editors. Polycyclic Hydrocarbons and Cancer. Vol. 1. Academic Press; New York: 1978. pp. 261–269. [Google Scholar]
- 24.Hemminki K, Koskinen M, Rajaniemi H, Zhao C. DNA adducts, mutations, and cancer 2000. Regul Toxicol Pharmacol. 2000;32:264–275. doi: 10.1006/rtph.2000.1431. [DOI] [PubMed] [Google Scholar]
- 25.Chen SY, Wang LY, Lunn RM, Tsai WY, Lee PH, Lee CS, Ahsan H, Zhang YJ, Chen CJ, Santella RM. Polycyclic aromatic hydrocarbon-DNA adducts in liver tissues of hepatocellular carcinoma patients and controls. Int J Cancer. 2002;99:14–21. doi: 10.1002/ijc.10291. [DOI] [PubMed] [Google Scholar]
- 26.Hemminski K, Grzybowska E, Chorazy M, Twardowska-Saucha K, Sroczynski JW, Putman KL, Randrath K, Phillips DH, Hewer A, Santella RM, Perera FP. DNA adducts in humans related to occupational exposure to aromatic compounds. In: Vainio H, Sorsa M, McMichael AJ, editors. Complex Mixtures and Cancer Risk. Vol. 104. IARC Scientific Publisher; Lyon, France: 1990. pp. 181–192. [PubMed] [Google Scholar]
- 27.Geacintov NE, Cosman M, Hingerty BE, Amin S, Broyde S, Patel DJ. NMR solution structure of stereoisomeric covalent polycyclic aromatic carcinogen-DNA adducts: Principles, patterns, and diversity. Chem Res Toxicol. 1997;10:111–146. doi: 10.1021/tx9601418. [DOI] [PubMed] [Google Scholar]
- 28.Flowers L, Ohinishi ST, Penning TM. DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: role of reactive oxygen species, Cu(II)/Cu(I) redox cycling, and o-semiquinone anion radicals. Biochemistry. 1997;36:8640–8648. doi: 10.1021/bi970367p. [DOI] [PubMed] [Google Scholar]
- 29.Lorentzen RJ, Caspary WJ, Lesko SA, Ts’o POP. The autoxidation of 6-hydroxybenzo[a]pyrene and 6-oxobenzo[a]pyrene radical, reactive metabolites of benzo[a]pyrene. Biochemistry. 1975;14:3970–3977. [Google Scholar]
- 30.RamaKrishna NVS, Devanesan PD, Rogan EG, Cavalieri EL, Jeong H, Jankowiak R, Small GJ. Mechanism of metabolic activation of the potent carcinogen 7:12-dimethylbenz[a]anthracene. Chem Res Toxicol. 1992;5:220–226. doi: 10.1021/tx00026a011. [DOI] [PubMed] [Google Scholar]
- 31.Penning TM, Burczynski ME, Hung CF, McCoull KD, Palackal NT, Tsuruda LS. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: Generation of reactive and redox active O-quinones. Chem Res Toxicol. 1999;12:1–18. doi: 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- 32.Rogan EG, Devanesan PD, RamaKrishna NVS, Higginbotham S, Padmavathi NS, Chapman K, Cavalieri EL, Jeong H, Jankowiak R, Small GJ. Identification and quantitation of benzo[a]pyrene-DNA adducts formed in mouse skin. Chem Res Toxicol. 1993;6:356–363. doi: 10.1021/tx00033a017. [DOI] [PubMed] [Google Scholar]
- 33.Fu PP, Qui FY, Jung H, von Tungeln LS, Zhan DJ, Lee MJ, Wu YS, Heflich RH. Metabolism of isomeric nitrobenzo[a]pyrenes leading to DNA adducts and mutagenesis. Mutat Res. 1997;376:43–51. doi: 10.1016/s0027-5107(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 34.Howard PC, Beland FA, Cerniglia CE. Reduction of the carcinogen 1-nitropyrene to 1-aminopyrene by rat intestinal bacteria. Carcinogenesis. 1983;4:985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- 35.Silvers KJ, Couch LH, Rorke EA, Howard PC. Role of nitroreductases but not cytochroms P450 in the metabolic activation of 1-nitropyrene in the HepG2 human hepatoblastoma cell line. Biochem Pharmacol. 1997;54:927–936. doi: 10.1016/s0006-2952(97)00268-2. [DOI] [PubMed] [Google Scholar]
- 36.Larson RA, Berenbaum MR. Environmental phototoxicity. Environ Sci Technol. 1988;22:354–360. [Google Scholar]
- 37.Utesch D, Eray K, Diehl E. Phototoxicity testing of polycyclic aromatic hydrocarbons (PAH) in mammalian cells in vitro. Polycycl Arom Compd. 1996;10:117–121. [Google Scholar]
- 38.Huang XD, Krylov SN, Ren L, McKonkey BJ, Dixon DG, Greenberg BM. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: II. An empirical model for the toxicity of 16 polycyclic aromatic hydrocarbons to the duckweek lemna gibba L. G-3. Environ Toxicol Chem. 1997;16:2296–2303. [Google Scholar]
- 39.Krylov SN, Huang XD, Zeiler LF, Dixon DG, Greenberg BM. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: I. physical model based on chemical kinetics in a two-compartment system. Environ Toxicol Chem. 1997;16:2283–2295. [Google Scholar]
- 40.Landrum PF, Giesy JP, Oris JT, Allred PM. Photoinduced toxicity of polycyclic aromatic hydrocarbons to aqautic organisms. In: Vandermeulen JH, Hrudey SE, editors. Symposium on Oil Pollution of Freshwater. Pergamon; Ann Arbor: 1987. pp. 304–318. [Google Scholar]
- 41.Mekeyan OG, Ankley GT, Veith GD, Call DJ. QSARs for photoinduced toxicity: I. Acute lethality of polycyclic aromatic hydrocarbons to Daphnia magna. Chemosphere. 1994;28:567–582. [Google Scholar]
- 42.Pelletier MC, Burgess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrae lavae and juveniles. Envin Toxicol Chem. 1997;16:2190–2199. [Google Scholar]
- 43.Mukhtar H, del Tito BJ, Matgouranis PM, Das M, Asokan P, Bickers DR. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: Possible relevance to the tumorigenicity of the Goeckerman Regimen. J Invest Dermatol. 1986;87:348–353. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- 44.Schirmer K, Herbrick JS, Greenberg BM, Dixon DG, Bols NC. Use of fish gill cells in culture to evaluate the cytotoxicity and photocytotoxicity of intact and photomodified creasote. Environ Toxicol Chem. 1998;18:1277–1288. [Google Scholar]
- 45.Swartz RC, Ferraro SP, Lamberson JO, Cole FA, Ozretich RJ, Boese BL, Schults DW, Behrenfeld M, Ankley GT. Photoactivation and toxicity of mixtures of polycyclic aromatic hydrocarbon compounds in marine sediment. Environ Toxicol Chem. 1997;16:2151–2157. [Google Scholar]
- 46.Arfsten DP, Schaeffer DJ, Mulveny DC. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: A review. Ecotoxicol Environ Safety. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- 47.Mackay D, Shiu WY. Aqueous solubility of polynuclear aromatic hydrocarbons. J Chem Eng Data. 1977;22:399–402. [Google Scholar]
- 48.Pagni RM, Sigman ME. The photochemistry of PAHs and PCBs in water and on solids. In: Boule P, editor. Environmental Photochemistry. Vol. 2. Springer-Verlag; Berlin-Heidelberg: 1999. pp. 139–180. [Google Scholar]
- 49.Payne JR, Phillips CR. Photochemistry of petroleum in water. Environ Sci Technol. 1985;19:569–579. doi: 10.1021/es00137a602. [DOI] [PubMed] [Google Scholar]
- 50.Kochany J, Maguire RJ. Abiotic transformations of polynuclear aromatic hydrocarbons and polynuclear aromatic nitrogen heterocycles in aquatic environments. Sci Total Environ. 1994;144:17–31. [Google Scholar]
- 51.Bunce NJ, Zhu J. Products from photochemical reactions of naphthalene in air. Polycycl Arom Compd. 1994;5:123–130. [Google Scholar]
- 52.Lane DA, Tang H. Photochemical degradation of polycyclic aromatic compounds. I. Naphthalene. Polycycl Arom Compd. 1994;5:131–138. [Google Scholar]
- 53.Vialaton D, Richard C, Baglio D, Paya-Perez AB. Mechanism of the photochemical transformation of naphthalene in water. J Photochem Photobiol A. 1999;123:15–19. [Google Scholar]
- 54.Barbas JT, Sigman ME, Dabestani R. Photochemical oxidation of phenanthrene sorbed on silica gel. Environ Sci Technol. 1996;30:1776–1780. [Google Scholar]
- 55.Wen S, Zhao J, Sheng G, Fu J, Peng P. Photocatalytic reactions of phenanthrene at TiO2/water interfaces. Chemosphere. 2002;46:871–877. doi: 10.1016/s0045-6535(01)00149-7. [DOI] [PubMed] [Google Scholar]
- 56.Chapman OL, Heckert DC, Reasoner JW, Thackaberry SP. Photochemical studies on 9-nitroanthracene. J Am Chem Soc. 1966;88:5550–5554. [Google Scholar]
- 57.Konig J, Balfanz E, Funcke W, Raomanowski T. Structure-activity relationships for the photooxidation of anthracene and its anellated homologues. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Compounds: Mechanisms, Methods, and Metabolism. Bartelle Press; Columbus: 1984. pp. 739–748. [Google Scholar]
- 58.Schmidt R, Schaffner K, Trost W, Brauer HD. Wavelength dependent and dual photochemistry of the endoperoxides of anthracene and 9:10-dimethylanthracene. J Phys Chem. 1984;88:956–958. [Google Scholar]
- 59.Sigman ME, Zingg SP, Pagni RM, Burns JH. Photochemistry of anthracene in water. Tetrahedron Lett. 1991;32:5737–5740. [Google Scholar]
- 60.Sugiyama N, Iwata M, Yoshioka M, Yamada K, Aoyama H. Photooxidation of anthracene. Bull Chem Soc Jpn. 1969;42:1377–1379. [Google Scholar]
- 61.Taskar PK, Solomon JJ, Daisey JM. Rates and products of reaction of pyrene adsorbed on carbon, silica and alumina. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: Mechansims, Methods and Metabolism. Bartelle Press; Columbus: 1984. pp. 1285–1298. [Google Scholar]
- 62.Fatiadi AJ. Effects of temperature and of ultraviolet radiation on pyrene absorbed on garden soil. Environ Sci Technol. 1967;1:570–572. doi: 10.1021/es60007a003. [DOI] [PubMed] [Google Scholar]
- 63.Mao Y, Thomas JK. Photochemical reactions of pyrene on surfaces of .gamma-alumina and silica-alumina. Langmuir. 1992;8:2501–2508. [Google Scholar]
- 64.Liu X, Lu K-K, Mao Y, Thomas JK. Photoinduced reactions on clay and model systems. In: Helz GR, Zepp RG, Crosby DG, editors. Aquatic Surface Photochemistry. Lewis; Ann Harbor: 1994. pp. 187–699. [Google Scholar]
- 65.Lee-Ruff E, Kazarians-Moghaddam H, Katz M. Controlled oxidation studies of benzo[a]pyrene. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: A Decade of Progress. Bartelle Press; Columbus: 1988. pp. 519–534. [Google Scholar]
- 66.Katz M, Chan C, Tosine H, Sakuma T. Relative rates of photochemical and biological oxidation (in vitro) of polynuclear aromatic hydrocarbons. In: Jones P, Leber P, editors. Polynuclear Aromatic Hydrocarbons Third International Symposium on Chemistry and Biology -Carcinogenesis and Mutagenesis. Ann Arbor Science; Ann Arbor: 1979. pp. 171–190. [Google Scholar]
- 67.Sigman ME, Schuler PF, Ghosh MM, Dabestani R. Mechanism of pyrene photochemical oxidation in aqueous and surfactant solutions. Environ Sci Technol. 1998;32:3980–3885. [Google Scholar]
- 68.Ya-Ping S, Ma B, Lawson GE, Bunker CE, Rollins HW. Effects of photochemical reactions of pyrene in alcohol and aqueous solvent systems on spectroscopic analyses. Anal Chim Acta. 1996;319:379–386. [Google Scholar]
- 69.Dong S, Fu PP, Shirsat RN, Hwang HM, Leszczynski J, Yu H. UVA light-induced DNA cleavage by isomeric methylbenz[a]anthracenes. Chem Res Toxicol. 2002;15:400–409. doi: 10.1021/tx015567n. [DOI] [PubMed] [Google Scholar]
- 70.Wood JL, Barker CL, Grubbs CJ. Photooxidation products of 7,12-dimethylbenz[a]anthracene. Chem-Biol Interact. 1979;26:339–347. doi: 10.1016/0009-2797(79)90036-x. [DOI] [PubMed] [Google Scholar]
- 71.Zeng K, Hwang H-M, Fu PP, Yu H. Identification of 1-hydroxypyrene photoproducts and study of the effect by humic substances on its photolysis. Polycycl Arom Compd. 2002 In press. [Google Scholar]
- 72.Fox MA, Olive S. Photooxidation of anthracene on atmospheric particulate matter. Science. 1979;205:582–583. doi: 10.1126/science.205.4406.582. [DOI] [PubMed] [Google Scholar]
- 73.Takeda N, Teranishi K, Hamada K. Mutagenicity of the sunlight-exposed sample of pyrene in Salmonella typhimurium TA98. Bull Envirn Contam Toxicol. 1984;33:410–417. doi: 10.1007/BF01625563. [DOI] [PubMed] [Google Scholar]
- 74.Inomata M, Nagata C. Photoinduced phenoxy radical of 3,4-benzopyrene. Gann. 1972;63:119–130. [PubMed] [Google Scholar]
- 75.Strniste GF, Martinez E, Martinez AM, Brake RJ. Photo-induced reactions of benzo[a]pyrene with DNA in vitro. Cancer Res. 1980;40:245–252. [PubMed] [Google Scholar]
- 76.Dor F, Dab W, Empereur-Bissonnet P, Zmirou D. Validity of biomarkers in environmental health studies: The case of PAHs and benzenes. Crit Rev Toxicol. 1999;29:129–168. doi: 10.1080/10408449991349195. [DOI] [PubMed] [Google Scholar]
- 77.Merlo F, Anderson A, Weston A, Pan CF, Haugen A, Valerio F, Reggiardo G, Fontana V, Garte S, Puntoni R, Abbondandolo A. Urinary excretion of 1-hydroxypyrene as a marker for exposure to urban air levels of polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomark Prev. 1998;7:147–155. [PubMed] [Google Scholar]
- 78.Ramasamy SM, Hurtubise RJ, Weston A. Detection of 1-hydroxypyrene as a urine biomarker of human PAH exposure determined by fluorescence and solid-matrix luminescence spectroscopy. Applied Spec. 1997;51:1377–1383. [Google Scholar]
- 79.Roggi C, Minoia C, Sciarra GF, Apostoli P, Maccarini L, Magnaghi S, Cenni A, Fonte A, Nidasio GF, Micoli G. Urinary 1-hydroxypyrene as a marker of exposure to pyrene: an epidemiological survey on a general population group. Sci Total Environ. 1997;199:247–254. doi: 10.1016/s0048-9697(97)05458-2. [DOI] [PubMed] [Google Scholar]
- 80.Siwinska E, Mielzynska D, Kwapulinski J. Evaluation of intra and inter individual variation of urinary 1-hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons. Sci Toal Envirn. 1998;217:175–183. doi: 10.1016/s0048-9697(98)00186-7. [DOI] [PubMed] [Google Scholar]
- 81.Strickland P, Kang D, Sithisarakul P. Polycyclic aromatic hydrocarbon metabolite in urine as biomarkers of exposure and effect. Environ Health Perspect. 1996;104:927–935. doi: 10.1289/ehp.96104s5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pitts JN, Grosjean D, Schmid JP, Fitz DR, Belser WL, Knudson GB, Hynds PM. Atmospheric reactions of polycyclic aromatic hydrocarbons: Facile formation of mutagenic nitro derivatives. Science. 1978;202:515–519. doi: 10.1126/science.705341. [DOI] [PubMed] [Google Scholar]
- 83.Fu PP, Chou MW, Beland FA. Effects of nitro substitution on the in vitro metabolic activation of polycyclic aromatic hydrocarbons. In: Yang SK, Silverman BD, editors. Polycyclic Aromatic Hydrocarbon Carcinogensis: Structure -Activity Relationships. II. CRC Press; Boca Raton, Florida: 1988. pp. 37–65. [Google Scholar]
- 84.Stark G, Stauff J, Miltenburger HG, Stumm-Fischer I. Photodecomposition of 1-nitropyrene and other direct-acting mutagens extracted from diesel-exhaust particulates. Mutat Res. 1985;155:27–33. doi: 10.1016/0165-1218(85)90021-7. [DOI] [PubMed] [Google Scholar]
- 85.Yang DTC, Chou A, Chen E, Chiu LH, Ni Y. Photodecomposition of environmental nitro-polycyclic aromatic hydrocarbons. Polycycl Arom Compd. 1994;5:201–208. [Google Scholar]
- 86.Holloway MP, Biaglow MC, McCoy EC, Anders M, Rosenkranz HS, Howard PC. Photochemical instability of 1-nitropyrene, 3-nitrofluoranthene, 1,8-dinitropyrene and their parent polycyclic aromatic hydrocarbons. Mutat Res. 1987;187:199–207. doi: 10.1016/0165-1218(87)90037-1. [DOI] [PubMed] [Google Scholar]
- 87.Pitts JN. Formation and fate of gaseous and particulate mutagens and carcinogens in real and simulated atmospheres. Environ Health Perspect. 1983;47:115–140. doi: 10.1289/ehp.8347115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ioki Y. Aryloxy radicals by photorearrangement of nitro-compounds. J Chem Soc Perkin II. 1977:1240–1242. [Google Scholar]
- 89.Fukuhara K, Kurihara M, Miyata N. Photochemical generation of nitric oxide from 6-nitrobenzo[a]pyrene. J Am Chem Soc. 2001;123:8662–8666. doi: 10.1021/ja0109038. [DOI] [PubMed] [Google Scholar]
- 90.Moncrief A, Jiao Y, Dong S, Hwang H-M, Yu H. Recent Advances in Environmental Health Disparities, Toxicology and Carcinogenesis. Jackson State University; Jackson, MS: 2002. Study of the photochemical degradation of nitro-substituted polycyclic aromatic hydrocarbons and light-induced damages to DNA; p. 59. [Google Scholar]
- 91.Kagan J, Wang TP, Benight AS, Tuveson RW, Wang GR, Fu PP. The phototoxicity of nitro polycyclic aromatic hydrocarbons of environmental importance. Chemosphere. 1990;20:453–466. [Google Scholar]
- 92.Yasuhara A, Huwa K. Formation of 1-nitro-2-hydroxypyrene from 1-nitropyrene by photolysis. Chem Lett (Japan) 1983:347–348. [Google Scholar]
- 93.Koizumi A, Suzuki T, Saitoh N, Kamiyama S. A novel compound, 9-hydroxy-1-nitropyrene, is a major photodegraded compound of 1-nitropyrene in the environment. Arch Environ Health. 1994;49:87–92. doi: 10.1080/00039896.1994.9937459. [DOI] [PubMed] [Google Scholar]
- 94.Okinaka RT, Nickols JW, Whaley TW, Strniste GF. Phototransformation of 2-aminofluorene into N-oxidized mutagens. Carcinogenesis. 1984;5:1741–1743. doi: 10.1093/carcin/5.12.1741. [DOI] [PubMed] [Google Scholar]
- 95.Okinawa RT, Nickols JW, Whaley TW, Strniste GF. 1-Nitropyrene: a mutagenic product induced by the action of near ultraviolet light on 1-aminopyrene. Mutat Res. 1986;173:93–98. doi: 10.1016/0165-7992(86)90083-7. [DOI] [PubMed] [Google Scholar]
- 96.Strniste GF, Nickols JW, Okinaka RT. Photochemical oxidation of 2-aminofluorene: Correlation between the induction of direct-acting mutagenicity and the formation of nitro and nitroso aromatics. Mutat Res. 1985;151:15–24. doi: 10.1016/0027-5107(85)90177-0. [DOI] [PubMed] [Google Scholar]
- 97.McCoy EC, Hyman J, Rosenkranz HS. Conversion of environmental pollutant to mutagens by visible light. Biochem Biophys Res Comm. 1979;89:729–734. doi: 10.1016/0006-291x(79)90690-9. [DOI] [PubMed] [Google Scholar]
- 98.De Flora S, Camoirano A, Izzotti A, D’Anostini F, Bennicelli C. Photoactivation of mutagens. Carcinogenesis. 1989;10:1089–1097. doi: 10.1093/carcin/10.6.1089. [DOI] [PubMed] [Google Scholar]
- 99.Okinaka RT, Nickols JW, Strniste GF, Whaley TW. Photochemical transformation of primary aromatic amines into “direct-acting” mutagens. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: Chemistry, Characterization and Carcinogenesis. Bartelle Press; Columbus: 1986. pp. 717–728. [Google Scholar]
- 100.Okinaka RT, Nickols JW, Whaley TW, Strniste GF. Phototransformation of polycyclic aromatic hydrocarbons into stable, mutagenic components. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: Mechanisms, Methods and Metabolism. Bartelle Press; Columbus: 1984. pp. 961–971. [Google Scholar]
- 101.Okinaka RT, Whaley TW, Hollstein U, Nickols JW, Strniste GF. Identification of a promutagenic compound fomed by the action of near-ultraviolet light on a primary aromatic amine. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: A Decade of Progress. Bartelle; Columbus: 1988. pp. 661–671. [Google Scholar]
- 102.Zeng K, Dong S, Hwang H-M, Yu H. Recent Advances in Environmental Health Disparities, Toxicology and Carcinogenesis. Jackson State University; Jackson, MS: 2002. Study of photochemical transformation of 1-aminopyrene and light-induced DNA damages; p. 49. [Google Scholar]
- 103.Kohn-Speyer AC. Effect of ultra-violet radiation on the incidence of tar cancer in mice. Lancet. 1929;217:1305–1306. [Google Scholar]
- 104.Morton JJ, Luce-Clausen EM, Mahoney EB. Visible light and skin tumors induced with benzpyrene in mice. Cancer Res. 1942;2:256–260. [Google Scholar]
- 105.Morton JJ, Luce-Clausen EM, Mahoney EB. The effect of visible light on the development of tumors induced by benzpyrene in the skin of mice. Am J Roent Rad Ther. 1940;43:896–898. [Google Scholar]
- 106.Rusch HP, Kline BE, Baumann CA. The nonadditive effect of ultraviolet light and other carcinogenic procedures. Cancer Res. 1942;2:183–188. [Google Scholar]
- 107.Santamaria L, Giordano GG, Alfisi M, Cascione F. Effects of light on 3,4-benzpyrene carcinogenesis. Nature. 1966;210:824–825. doi: 10.1038/210824a0. [DOI] [PubMed] [Google Scholar]
- 108.Camalier RF, Gantt R, Price FM, Stephens EV, Baeck AE, Taylor WG, Sanford KK. Effect of visible light on benzo(a)pyrene binding to DNA of cultured human skin epithelial cells. Cancer Res. 1981;41:1789–1793. [PubMed] [Google Scholar]
- 109.Fernandez M, L’Haridon J. Influence of lighting conditions on toxicity and genotoxicity of various PAH in the newt in vivo. Mutat Res. 1992;298:31–41. doi: 10.1016/0165-1218(92)90026-v. [DOI] [PubMed] [Google Scholar]
- 110.Kagan J, Kagan E. The toxicity of benzo[a]pyrene and pyrene in the mosquito Aedes aegypti in the dark and in the presence of ultraviolet light. Chemosphere. 1986;15:243–251. [Google Scholar]
- 111.Kagan J, Stokes A, Gong HH, Tuveson RW. Light-dependent cytotoxicity of fluoranthene: Oxygen-dependent membrane damage. Chemosphere. 1987;16:2417–2422. [Google Scholar]
- 112.Kagan J, Tuveson RW, Gong HH. The light-dependent cytotoxicity of benzo[a]pyrene: Effect of human erythrocytes, Escherichia coli cells, and haemophilus influenzae transforming DNA. Mutat Res. 1989;216:231–242. doi: 10.1016/0165-1161(89)90048-4. [DOI] [PubMed] [Google Scholar]
- 113.Mauthe RJ, Cook VM, Coffing SL, Baird WM. Exposure of mammalian cell cultures to benzo[a]pyrene and light results in oxidative DNA damage as measured by 8-hydroxydeoxyguanosine formation. Carcinogenesis. 1995;16:133–137. doi: 10.1093/carcin/16.1.133. [DOI] [PubMed] [Google Scholar]
- 114.Pfaum M, Boiteux S, Epe B. Visible light generates oxidative DNA base modification in high excess of strand breaks in mammalian cells. Carcinogenesis. 1994;15:297–300. doi: 10.1093/carcin/15.2.297. [DOI] [PubMed] [Google Scholar]
- 115.Sinha BK, Chignell CF. Binding of anthracene to cellular macromolecules in the presence of light. Photochem Photobiol. 1983;37:33–37. doi: 10.1111/j.1751-1097.1983.tb04430.x. [DOI] [PubMed] [Google Scholar]
- 116.Tuveson RW, Wang GR, Wang TP, Kagan J. Light-dependent cytootoxic reactions of anthracene. Photochem Photobiol. 1990;52:993–1002. doi: 10.1111/j.1751-1097.1990.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 117.Utsumi H, Elkind MM. Photodynamic cytotoxicity of mammalian cells exposed to sunlight-sumulated near ultraviolet light in the presence of the carcinogen 7,12-dimethylbenz[a]anthracene. Photochem Photobiol. 1979;30:271–278. doi: 10.1111/j.1751-1097.1979.tb07146.x. [DOI] [PubMed] [Google Scholar]
- 118.White GL, Fu PP, Heflich RH. Effect of nitro substitution on the light-mediated mutagenicity of polycyclic aromatic hydrocarbons in Samonella typhimurium TA 98. Mutat Res. 1985;144:1–7. doi: 10.1016/0165-7992(85)90115-0. [DOI] [PubMed] [Google Scholar]
- 119.Kochevar KE. Phototoxicity mechanisms: chlorpromazine photosensitized damage to DNA and cell membranes. J Invest Dermatol. 1981;77:59–64. doi: 10.1111/1523-1747.ep12479244. [DOI] [PubMed] [Google Scholar]
- 120.Davies MJ, Truscott RJ. Photo-oxidation of proteins and its role in cataractogenesis. J Photochem Photobiol B. 2001;63:114–125. doi: 10.1016/s1011-1344(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 121.Bagheri H, Lhiaubet V, Montastruc JL, Chouini-Lalanne N. Photosensitivity to ketoprofen: mechanisms and pharmacoepidemiological data. Drug Safety. 2000;22:339–349. doi: 10.2165/00002018-200022050-00002. [DOI] [PubMed] [Google Scholar]
- 122.Brooks P, Lawley PD. Evidence for the binding of polynuclear aromatic hydrocarbons to the nucleic acids of mouse skin: Relation between carcinogenic power of hydrocarbons and their binding to deoxyribonucleic acid. Nature. 1964;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- 123.Ts’o POP, Lu P. Interaction of nucleic acids, II. Chemical linkage of the carcinogenic 3,4-benzpyrene to DNA induced by photoradiation. Proc Nat Acad Sci USA. 1964;51:272–280. doi: 10.1073/pnas.51.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lesko SA, Ts’o POP, Umans RS. Interaction of nucleic acids. V, Chemical linkage of 3,4-benzpyrene to deoxyribonucleic acids in aqueous solution. Biochemistry. 1969;8:2291–2298. doi: 10.1021/bi00834a009. [DOI] [PubMed] [Google Scholar]
- 125.Blackburn GM, Taussig PE. The photocarcinogenicity of anthracene: Photochemical binding to deoxyribonucleic acid in tissue culture. Biochem J. 1975;149:289–291. doi: 10.1042/bj1490289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blackburn GM, Fenwick RG, Lockwood G, Williams GM. Photoproducts from DNA pyrimidine bases and polycyclic aromatic hydrocarbons. Nucleic Acids Res. 1977;4:2487–2494. doi: 10.1093/nar/4.7.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cavalieri E, Calvin M. Photochemical coupling of benzo[a]pyrene with 1-methylcytosine: Photoenhancement of carcinogebicity. Photochem Photobiol. 1971;14:641–653. doi: 10.1111/j.1751-1097.1971.tb06202.x. [DOI] [PubMed] [Google Scholar]
- 128.Hoard DE, Ratliff RL, Bingham JM, Strniste GF. Reaction induced in vitro between model DNA and benzo[a]pyrene by near-ultraviolet radiation. Chem-Biol Interact. 1981;33:179–194. doi: 10.1016/0009-2797(81)90039-9. [DOI] [PubMed] [Google Scholar]
- 129.Dong S, Hwang HM, Shi X, Holloway L, Yu H. UVA-induced DNA single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem Res Toxicol. 2000;13:585–593. doi: 10.1021/tx990199x. [DOI] [PubMed] [Google Scholar]
- 130.Wilson K, Green J, Dong S, Hwang H-M, Yu H. Light-induced DNA cleavage and formation of covalent adduct with amino substituted polycyclic aromatic hydrocarbons. American Chemical Society 222nd National Meeting; 2001; ACS, Chicago. p. CHED 201. [Google Scholar]
- 131.Baird WM. Effect of light on the hydrocarbon-DNA adducts formed in hamster embryo cells. Int J Cancer. 1978;22:292–297. doi: 10.1002/ijc.2910220312. [DOI] [PubMed] [Google Scholar]
- 132.Saito K, Mita S, Kamataki T, Kato R. DNA single strand breaks by nitropyrenes and related compounds in Chinese hamster V79 cells. Cancer Lett. 1984;24:121–127. doi: 10.1016/0304-3835(84)90127-7. [DOI] [PubMed] [Google Scholar]
- 133.Utsumi H, Kitani H, MCLC, Elkind MM. 7,12-Dimethylbenz[a]anthracene plus near-u.v. light initiates DNA damage and repair in Chinese hamster cells. Carcinogenesis. 1987;8:1439–1444. doi: 10.1093/carcin/8.10.1439. [DOI] [PubMed] [Google Scholar]
- 134.Li B, Mao B, Liu TM, Xu J, Dourandin A, Amin S, Geacintov NE. Laser pulse-induced photochemical strand cleavage of site-specifically and covalently modified (+)-anti-benzo[a]pyrene diol epoxide-oligonucleotide adducts. Chem Res Toxicol. 1995;8:396–402. doi: 10.1021/tx00045a011. [DOI] [PubMed] [Google Scholar]
- 135.Dittrich KA, Krugh TR. Analysis of site-specific binding of anti-benzo[a]pyrene diol epoxide to restriction fragments of pBR322 DNA via photochemical mapping. Chem Res Toxicol. 1991;4:270–276. doi: 10.1021/tx00021a002. [DOI] [PubMed] [Google Scholar]
- 136.Dittrich KA, Krugh TR. Mapping of anti-benzo[a]pyrene diol epoxide adducts to human c-Ha-ras1 protooncogene. Chem Res Toxicol. 1991;4:277–281. doi: 10.1021/tx00021a003. [DOI] [PubMed] [Google Scholar]
- 137.Boles TC, Hogan ME. High-resolution mapping of carcinogen binding sites on DNA. Biochemistry. 1986;25:3039–3043. doi: 10.1021/bi00358a045. [DOI] [PubMed] [Google Scholar]
- 138.Boles TC, Hogan ME. Site-specific carcinogen binding to DNA. Proc Nat Acad Sci USA. 1984;81:5623–5627. doi: 10.1073/pnas.81.18.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dong S, Fu PP, Hwang H-M, Yu H. Effects of histidine on light-induced DNA single strand cleavage by selected polycyclic aromatic hydrocarbons. Polycycl Arom Compd. 2002 In press. [Google Scholar]
- 140.Dong S, Hwang HM, Harrison C, Holloway L, Shi X, Yu H. UVA light-induced DNA cleavage by selected polycyclic aromatic hydrocarbons. Bull Environ Contam Toxicol. 2000;64:467–474. doi: 10.1007/s001280000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong S, Wang S, Stewart G, Hwang H-M, Yu H. Effect of solvents and biologically relevant ions on the light-induced DNA cleavage by polycyclic aromatic hydrocarbons. Int J Mol Sci. 2002 In press. [Google Scholar]
- 142.Yu H, Dong S, Fu PP, Hwang H-M. UVA light-induced DNA single strand cleavage by hydroxybenzo[a]pyrenes. Polycycl Arom Compd. 2002 In press. [Google Scholar]
- 143.Wang S, Dong S, Hwang H-M, Fu PP, Yu H. Recent Advances in Environmental Health Disparities, Toxicology and Carcinogenesis. Jackson State University; Jackson, MS: 2002. Solvent effect on the light-induced DNA cleaavge by selected polycyclic aromatic hydrocarbons; p. 51. [Google Scholar]
- 144.Hwang HM, Shi X, Ero I, Jayasinghe A, Dong S, Yu H. Microbial ecotoxicity and mutagenicity of 1-hydroxypyrene and its photoproducts. Chemosphere. 2001;45:445–451. doi: 10.1016/s0045-6535(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 145.Liu Z, Lu Y, Rosenstein B, Lebwohl M, Wei H. Benzo[a]pyrene enhances the formation of 8-hydroxy-2′-deoxyguanosine by ultraviolet A radiation in calf thymus DNA and human epidermoid carcinoma. Biochemistry. 1998;37:10307–10312. doi: 10.1021/bi980606o. [DOI] [PubMed] [Google Scholar]
- 146.Benson JM, Brooks AL, Cheng YS, Henderson TR, White JE. Environmental transformation of 1-nitropyrene on glass surfaces. Atmos Environ. 1985;19:1169. [Google Scholar]
- 147.Amitage B. Photocleavage of nucleic acids. Chem Rev. 1998;98:1171–1200. doi: 10.1021/cr960428+. [DOI] [PubMed] [Google Scholar]
- 148.Henderson PT, Armitage B, Schuster GB. Selective photocleavage of DNA by anthraquinone derivatives: Targeting the single-strand region of hairpin structure. Biochemistry. 1998;37:2991–3000. doi: 10.1021/bi972419g. [DOI] [PubMed] [Google Scholar]
- 149.Stern RS, Zierler S, Parrish JA. Skin carcinoma in patients with psoriasis treated with topical tar and artificial ultraviolet radiation. Lancet. 1980;2:732–733. doi: 10.1016/s0140-6736(80)91231-3. [DOI] [PubMed] [Google Scholar]
- 150.Reed LD, Liss GM. PAH exposure among pitch and asphalt roofing workers. In: Cooke M, Dennis AJ, editors. Polynuclear Aromatic Hydrocarbons: Mechanisms, Methods, and Metabolism. Bartelle Press; Columbus: 1985. pp. 1089–1095. [Google Scholar]
- 151.Mastrangelo G, Fadda E, Marzia V. Polycyclic aromatic hydrocarbons and cancers in man. Environ Health Perspect. 1996;104:1166–1170. doi: 10.1289/ehp.961041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Godschalk RWL, Ostertag JU, Moonen EJC, Neumann HAM, Kleinjans JCS, van Schooten FJ. Aromatic DNA adducts in human white blood cells and skin after dermal application of coal tar. Cancer Epidemiol Biomark Prev. 1998;7:767–773. [PubMed] [Google Scholar]