Abstract
Background:
Current clinical outcome measurements may overestimate the long term success of anterior cruciate ligament reconstruction (ACLR). There is a need to understand biomechanics of the knee joint during daily activities. This systematic review provides a comprehensive overview of the literature related to gait in patients following ACLR. The purpose of this systematic review was to investigate the available literature and provide a comprehensive overview of kinematic and kinetic variables that present during gait in patients after ACLR.
Methods:
A literature search was performed in AMED, CINAHL, EMBASE, Medline and Scopus between January 2000 and October 2012. Inclusion criteria included articles written in English, German or Dutch, and those reporting on gait analysis in patients after ACLR. Kinematic and/or kinetic data of the uninjured and ACLR knee and healthy controls (CTRL) were outcome measurements of interest. Each study's methodological quality was assessed using the Critical Appraisal Skills Programme critical appraisal tool.
Results:
Twenty two studies fulfilled the inclusion criteria. A total of 479 patients with a mean age of 27.3 were examined. Time between the injury and surgery and ranged from 3 weeks to 5.7 years. Gait analysis was done at a mean of 29.3 months after surgery. Gait was found to be altered in the sagittal, frontal and transverse planes after ACLR and may take months or years to normalize, if normalization occurs at all.
Conclusion:
Patients after ACLR have altered gait patterns that can persist for up to five years after surgery. It is imperative that rehabilitation techniques are examined in order to minimize changes in knee biomechanics during gait, as they have the potential to impact on the development of osteoarthritis.
Level of evidence:
3a
Keywords: Anterior cruciate ligament, biomechanics, gait
INTRODUCTION
Although an anterior cruciate ligament (ACL) injury is relatively uncommon in relation to exposure, the result is a debilitating injury to those afflicted. Those, who would like to stay active in cutting type sports are often counseled to undergo an ACL reconstruction (ACLR).1 Despite restoration of nearly normal anterior translation of the knee, patients often face residual impairments including pain, strength, swelling or stiffness for years after surgery.2 Logically, the focus on outcome measures has been directed towards impairments. Recent research indicates that greater emphasis should be placed on sports participation outcomes rather than on impairment-based outcomes.3 For example, return to pre-injury level of sports is often not achieved in the first post-operative year, although 90% of the patients have a nearly normal knee according to impairment based outcomes. It appears that the current impairment measures may overestimate the success of ACLR. Hence, additional experiments are needed to provide clinically relevant, functional information in order to bridge the gap between basic science and patient relevant outcomes.4 Gait is such an activity that has been studied in patients after ACLR showing that gait alterations are frequently encountered in this patient population. Of particular interest is altered loading of the knee in terms of higher adduction moments during gait, which has been suggested as a causative factor that may be related to the early onset of osteoarthritis (OA).5 Although up to 80% of patients after ACLR may develop OA within 10-15 years after surgery if there was concomitant meniscal, medial collateral ligament and chondral injury,6 there is no evidence to suggest that high adduction moments of the knee are a sole cause of OA after ACLR. Activities that have a repetitive nature have been suggested to be a causative factor for the development of OA.7 Recently, a review on gait in patients with ACL deficient (ACLD) and ACLR knees was published but the authors reported only on sagittal plane moments.8 To best of the authors' knowledge, a comprehensive overview of gait patterns after ACLR is still unavailable. The purpose of this systematic review was to investigate the available literature and provide a comprehensive overview of kinematic and kinetic variables that present during gait in patients after ACLR.
MATERIAL AND METHODS
PRISMA guidelines that use systematic and explicit methods to identify, select, and critically appraise relevant research were followed. A literature search was performed using AMED, CINAHL, EMBASE, Medline, and Scopus. The search terms in Medline were entered in two groups: Group one; ‘Anterior Cruciate Ligament’ [Mesh], Anterior Cruciate Ligament/surgery*, Knee-Joint' [Mesh]; Group two: ‘Gait’ [Mesh], ‘Walking’ [Mesh]. The search terms in each group were combined with the OR operator, the AND operator was used to combine the results from both groups to obtain the final yield. Comparable search terms were used for the other databases. Only studies that examined human adults and were conducted between January 2000 and October 2012 were assessed for inclusion. The titles and abstracts of the studies were screened for potential relevance. The following inclusion criteria were applied 1) full text published in English, German or Dutch; 2) studies reporting on gait analysis in patients after ACLR; 3) subjects between 18-45 years of age at time of recruitment; 4) studies published between 2000 and 2012 and 5) kinematic and/or kinetic data of the uninjured and ACLR knee and healthy controls (CTRL) were reported in numbers. Exclusion criteria were 1) animal studies; 2) case studies; 3) if no side to side comparison was conducted or lacked comparison with a CTRL group; 4) systematic reviews/meta-analysis and 5) only an abstract was available. In addition, reference lists of relevant articles were also screened to identify potential publications not identified in the formal search strategy. The following variables were of interest during the stance phase: initial contact, midstance, peak and minimum joint angles and moments for the sagittal, frontal and transverse planes. The discrete variables were chosen on the basis of their frequent use in the literature and their relation to possible development of OA.9 Each study's methodological quality was assessed using the Critical Appraisal Skills Programme (CASP, available at http://www.casp-uk.net) critical appraisal tools that have been widely employed in systematic reviews to assess the methodological quality of clinical studies. The appraisal was independently conducted by two authors (AG and AB). Disagreements in appraisal were resolved by discussion.
RESULTS
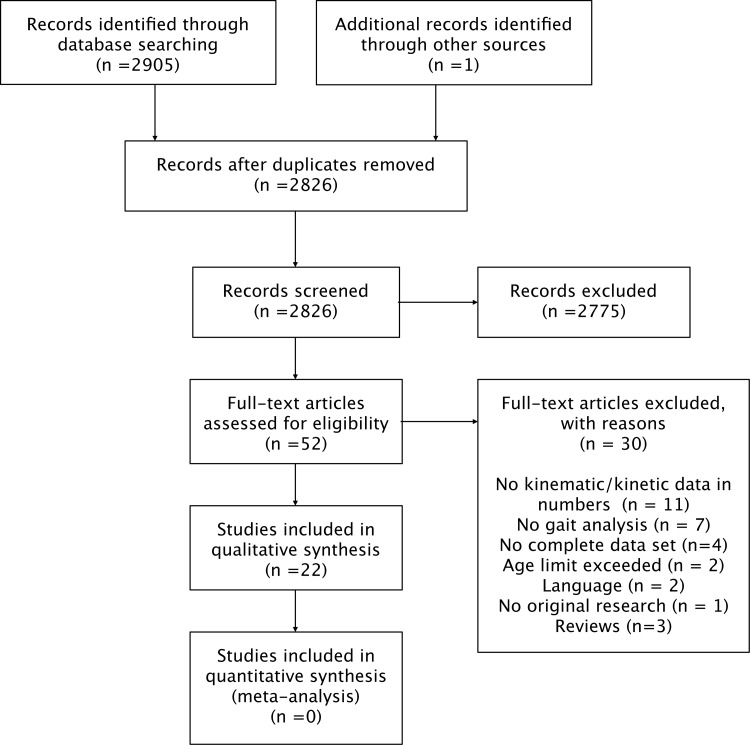
The search terms for the various databases are shown in Table 1 and the total yield after assessment is depicted in Figure 1. A formal meta-analysis was not feasible due the heterogeneous data reported in the included studies. The results of the critical appraisal are presented in Table 2, demonstrating that all the studies clearly defined their research question, utilized an appropriate study design, and clearly defined their population and methods used. However, a major recurrent limitation was that only two studies based their sample size on a power calculation. Of the 52 potential relevant studies, 30 were excluded. Eleven studies did not provide kinematic and/or kinetic data in numbers or of interest for this review.10‐20 Five studies reported incomplete data that precluded side to side comparison.21‐25 The corresponding authors were contacted with the request to provide data. One provided data,22 three did not reply, and one could not be traced. These four studies were therefore excluded.21,23‐25 The results of seven studies did not include gait analysis26‐32 and the age limit was exceeded in two studies.33,34 Two studies were excluded based on language restrictions.35,36 One study was excluded based on including subjects with a confounding factor (extension deficit of 7°).37 Three narrative reviews were excluded.38‐40 Hence, 22 studies2,5,9,22,25,41‐58 fulfilled the inclusion criteria of which several studies reported on the same patient cohort but with different outcomes.44‐46,50‐53 The detailed demographic data of the ACLR and CTRL groups are depicted in Table 3. A total of 479 patients with a mean age of 27.3 years were examined across all studies. There were a total of 203 control subjects with a mean age of 26.7 years.
Table 1.
Search terms for databases.
| MEDLINE | AMED | SCOPUS | CINAHL | EMBASE | |
|---|---|---|---|---|---|
| Search terms | (Anterior Cruciate Ligament[Mesh] OR Anterior Cruciate Ligament/surgery* OR Knee-Joint[Mesh]) AND (Gait[Mesh] OR Walking[Mesh]) | anterior cruciate ligament AND gait (keywords) | anterior cruciate ligament AND gait (keywords) | ((MH “Anterior Cruciate Ligament”) OR (MH “Anterior Cruciate Ligament/SU”) OR (MH “Knee Joint”) AND (MH “Gait”) AND (MH “Gait Analysis”) AND (MH “Walking”)) | anterior cruciate ligament'/exp OR ‘anterior cruciate ligament reconstruction’/exp OR ‘Knee’/exp AND ‘gait’/exp |
Figure 1.
PRISMA flow diagram of search strategy.
Table 2.
Critical Appraisal Skills Programme (CASP) methodological appraisal checklist of included studies.
| Description | The population studied? | The outcomes considered? | Are the aims of the investigation clearly stated? | Was the sample representative of its target population? | Sample based on power calculation | Were the assessors blind to the different groups? | Could selective drop out explain the effect? | Are you confident with the authors' choice and use of statistical methods, if employed (results reported with mean or median, SD or CI and p value) | Were all important outcomes/ results considered? | Accept for further use as Type IV evidence? |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacchini [41] | yes | yes | yes | yes | no | no | n/s | no (only mean and SD, not for individual results) | yes | yes |
| Beard [42] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD, 95%CI & p-value) | yes | yes |
| Butler |5] | yes | yes | yes | yes | yes | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Decker [43] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Ferber [44, 45, 46] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Gao [22] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Georgoulis [47] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Gokeler [48] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Hooper [2] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Hooper [49] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Karanikas [50,51] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Knoll [52,53] | yes | yes | yes | yes | no | no | n/s | no (mean, SD & p-value) | yes | yes |
| Lewek [54] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Roewer [55] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Shin [56] | yes | yes | yes | yes | yes | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Webster [9] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Webster [57] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
| Webster [58] | yes | yes | yes | yes | no | no | n/s | yes (mean, SD & p-value) | yes | yes |
n/s; not stated
Table 3.
Characteristics of the patient groups and control groups.
| Author | Number of included subjects in Patient group (male, female) | Age in years (mean±sd/range) | Time between injury and surgery (mean±sd/range) | Time after surgery (mean±sd/range) | Type of graft | Number included subjects in control group (male, female) | Age in years (meanisd) |
|---|---|---|---|---|---|---|---|
| Bacchini [41] | 8(8) | 28.3(21-33) | 1-2 (N.R.) months | 6.5 months (N.R.) | PT | N.A. | N.A. |
| Beard [42] | 11 (9.2) | 29.7±3.6 | 47±30 months | 6.3 months (5-7) | 5 PT. 6 HS | N.A. | N.A. |
| Butler [5] | 17(3,14) | 23.6±5.8 | N.R. | 5.3±4.4 years | N.R. | 17(14,3) | 23.4±5.7 |
| Decker [43] | 16(N.R) | 27.8±7.4 |
|
6 and 12 weeks (N.R.) | PT | 8 (N.R) | 28.3±4.3 |
| Ferber [44,45,46] | 10(5,5) | 27.7±9.1 | 5.7±5.1 years | 3 months (N.R.) | PT | 10(5,5) | 24.4±3.1 |
| Gao [22] | 14(12,2) | 25.1±5.9 | N.R. | 3-12 months (N.R.) | PT. 1 IS. AT | 15(12,3) | 22.8 (2.6) |
| Georgoulis [47] | 21 (19,2) | 25.0±4.0 | N.R. | 6.9± 3.9 months | PT | 10(8,2) | 24.7±3.7 |
| Gokeler [48] | 14(7,7) | 24.0(21.0-40.0) | N.R. | 5.9 (N.R.) | PT | N.R. | N.R. |
| Hooper [49] |
|
N.R. |
|
6 weeks | PT (Kennedy augmentation) | N.A. | N.A. |
| Hooper [2] |
|
|
N.R. |
|
PT | N.A. | N.A. |
| Karanikas [50,51] |
|
|
N.R. | 3-6 months, 6-12 months,1-2 years (N.R.) | HS, PT | N.R | N.P. |
| Knoll [52,53] |
|
|
N.R. | 6, 16, 32, 52 weeks | PT | 51 (31,20) | 31.7±4.1 |
| Lewek [54] |
|
|
N.R. |
|
HS, PT | 10(8,2) | 32.2±6.6 |
| Roewer [55] | 26(18,8) | 29.6±10.7 | N.R. | 6, 24 months | HS.AL | N.A. | N.A. |
| Shin [56] | 24(9.15) | 33.0±9.2 | 3.6±3.1 years | 2.8±2.8 years | N.R. | N.A. | N.A. |
| Webster [9] |
|
|
|
|
HS, PT | 18(16,2) | 24.7±5.0 |
| Webster [57] |
|
|
|
|
HS | N.A. | N.P. |
| Webster [58] |
|
|
|
|
HS, PT | 17(16,1) | 24.7±5.0 |
PT: Patellar tendon
HS: Hamstrings graft
ACH: Achilles graft
AL: Allograft
AC: Patients with an acute injury of the ACL at the operation date
CH: Patients with a chronic injury of the ACL at the operation date
S.A.: Strong ACL-R; the injured leg 90% or more quadriceps strength compared to the non-involved quadriceps
W.A.: Weak ACL-R: the injured leg 80% or less quadriceps strength compared to the non-involved quadriceps
N.R.: Not reported
PSF: Preferred strike frequency
FDHO: Force driven harmonic oscillator
BIOMECHANICS
The results for the biomechanical outcome measurements are shown in Table 4. A large variance between the time of injury and surgery, and test dates after surgery exists. The time between the injury and surgery ranged from 3 weeks to 5.7 years. Gait analysis was performed at a mean of 29.3 months (range 6 weeks – 5.3 years) after surgery across all studies. It should be noted that gait analysis parameters reported such as flexion at initial contact, peak flexion, walking speed varied considerably. In addition several studies reported only on gait for the ACLR leg without comparison to the uninjured leg whilst others lacked a CTRL group.2,5,9,22,50,51,52,53,54,56,57
Table 4.
Biomechanical outcome measurements.
| Knee angles | Knee external moments | |||||
|---|---|---|---|---|---|---|
| Injured leg ACLR | Non-injured leg ACLR | Control | Injured leg ACLR | Non-injured leg ACLR | Control | |
| Author | °±SD | °±SD | °±SD | Nm/kg | Nm/kg | Nm/kg |
| Baechini [41] | N.R. |
|
|
N.R. | ||
| Beard [42] |
|
|||||
| Butler [5] | Peak adduction 1.9±2.9 | Peak adduction 0.9±2.9 | Peak adduction 0.4±0.1 | Peak adduction 0.3±0.1 | ||
| Decker [43] |
|
Midstance ROM 15.1±3.31 | ||||
| Ferber [44,45,46] | 17.6±4.41 | 18.7±3.91 |
|
9.9±3.31 (extensor angular impulse) | 15.8±4.81 (extensor angular impulse) |
|
| Gao [22] |
|
|
||||
| Georgoulis [47] |
|
|
N.A. | N.A. | ||
| Gokelcr [48] | Difference injured/non-injured flexion-extension 4.9±3.96 | Difference left/right flexion-extension 1.3 | Difference injured, non-injured 0.3± 0.2 | Difference left/right leg 0.1±0.6 | ||
| Hooper [2] |
|
|
N.A |
|
|
N.A. |
| Hooper [49] |
|
|
N.A. | |||
| Karanikas [50,51] |
|
|
N.P | N.P. | ||
| Knoll [52,53] |
|
|
N.R. | N.R. | ||
| Lewek [54] |
|
|
|
Peak extension; 0.7±0.3 | ||
| Rocwer [55] | Peak flexion: 22.3±5.S 6mo: 23.2±6.6 12 mo: 24.4±7.0 | Peak flexion: 25.2±5.4 6mo: 28.1±6.7 12mo;25.4±6.9 | ||||
| Shin [56] |
|
|
||||
| Webster [9] | IR at midstance: HS: 8.7 PT: 8.9 | IR at midstance: HS: 11.5 PT: 14.1 | ||||
| Webster [57] |
|
|
|
|
||
| Webseer [58] | Flexion at 1C:
|
Flexion at IC:
|
||||
PT: Patellar tendon graft
HS: Hamstrings graft
S.A.: Strong ACL-R: the injured leg 90% or more quadriceps strength compared to the non-involved quadriceps
W.A.: Weak ACL-R: the injured leg 80% or less quadriceps strength compared to the non-involved quadriceps
N.R.: Not reported
PSF: Preferred strike frequency
FDHO: Force driven harmonic oscillator
1: ensemble average obtained from 5 intervals during stance
KNEE JOINT KINEMATICS
Flexion and extension
Knee flexion at initial contact for the ACLR leg ranged from -0.1° to 8.1°,2,42,47,49,54,58 and 3° to 4° for the uninjured leg2,49 in the ACLR group and 2.3° to 7.8° for the CTRL group47,54,57
Peak knee flexion during stance ranged from 15.7° to 55.4° for the ACLR leg and 12.1° to 28.1° for the uninjured leg.22,50,52,53,55,57,58 Two studies reported on peak knee extension, one study noted a change for peak extension from 17.9° at six weeks to 5.4° at one year,53 whilst another study found differences related to graft type with 1.5° for patellar tendon and 2.5° hamstring grafts, respectively.58 Midstance ROM in ACLR ranged between 5.3° and 11.0°.2,49,58 As a general trend, ROM did increase over time post ACLR.
Adduction and abduction
Gao et al found an increased adduction of the knee joint of 2-3° in the ACLR leg compared to the healthy leg from the CTRL group.22 Butler et al found the same trend with a mean peak adduction angle of 1.9° for patients after ACLR versus 0.9° for CTRL.5 A difference was found comparing hamstring and patellar tendon grafts.9 The hamstring group had a reduced adduction for the injured leg compared with both the patellar tendon and CTRL, but not when compared with the non-injured knee.
Internal and external rotation
Results regarding internal and external tibial rotation during gait showed mixed outcomes between studies. Gao and Zhang found increased internal rotation in the ACLR leg of patients compared to CTRL subjects.22 Interestingly, Webster and Feller found that 86% of patients after ACLR walk with more external rotation compared to the CTRL group and 42% of patients to have greater than 5° of external rotation of the ACLR leg compared to their non-injured knee.9
KNEE JOINT KINETICS
Knee moments are expressed as external moments for the purpose of this review.
Flexion and extension
Knee extension moments ranged from 0.3 Nm/kg to 0.4 Nm/kg in the ACLR leg2,54 and 0.7 Nm/kg in for CTRL54. Knee flexion moments ranged from 0.1 Nm/kg to 0.3 Nm/kg for both the ACLR and non-injured legs.2,58 Shin et al. reported knee flexion moments by different unit of measure with respective values of 2.9 Nm/%BW/height for the ACLR leg and 3.7 Nm/%BW/height for the non-injured legs.56 Webster et al found that graft type had an influence on the knee biomechanics during gait.58 The external knee flexion moment at midstance was significantly smaller than that in the CTRL legs in 65% of patients in the patellar tendon group and 29% of patients in the hamstring tendon group. In contrast, the external knee extension moment at terminal stance was significantly smaller than in the CTRL legs in 53% of subjects in the hamstring tendon group and 23% of subjects in the patellar tendon group.
Abduction-Adduction
In general, reported values for knee adduction moments ranged from 0.2 Nm/kg to 0.4 Nm/kg for both the ACLR and non-injured knees.2,5,57 Different units of measure were reported with adduction moments of 2.3 Nm/%BW/height and 2.5 Nm/%BW/height for the ACLR and non-injured knees.56 For abduction, they reported 0.6 Nm/%BW/height and 0.7 Nm/%BW/height for the ACLR and non-injured knees respectively.56 Butler and co-workers noted that patients after ACLR had a 21% larger peak knee-abduction moments than the CTRL group.5 In a gender comparison study by Webster et al., females were found to have 23% increased peak knee adduction moments in the ACLR knee compared to the ACLR knee in males.57
Rotation
One study examined rotational moments revealing 1.0 Nm/%BW/height for the peak internal rotation moment for both the ACLR and non-injured knees.56 Peak external rotation moment for the ACLR and non-injured knees were respectively 0.1 Nm/%BW/height and 0.2 Nm/%BW/height.
DISCUSSION
The results of this review clearly show that biomechanical deficits evidenced during gait in ACLR patients are very common. Differences in knee angles and total ROM differences were noted, with the ACLR group showing less knee ROM one year after ACLR. Conflicting results were reported for knee rotation. For the knee moments, clear differences between the ACLR and CTRL groups are mostly demonstrated in the extension moments. These altered kinematic and kinetic patterns remain even up to five years after ACLR. It is therefore apparent that the restoration of a normal gait pattern after ACLR takes a long time and may never be complete. It is unclear whether gait patterns ever return to normal as this review indicated that there is a paucity of both longitudinal and five year follow-up data in this patient population.
The most frequently reported upon gait parameter in this review was the kinematics of knee flexion-extension. Results across studies were consistent for this variable and knee flexion or the total amount of knee range of motion is reduced in patients that have undergone ACLR compared to the non-injured knee or a CTRL group. Chaudhari et al postulated that altered biomechanical loading after ACLD may be the precipitating cause of knee OA as loads shift to areas of cartilage not typically loaded.7 They documented that cartilage appears to be conditioned to load history and that conditioned cartilage likely has different abilities to respond to loads. Hence, altered knee kinematic changes observed in the sagittal plane may also lead to shift of joint load to an infrequently loaded area. Reversing the typical low knee flexion excursion strategy while walking may be advantageous, as joint loads may be distributed over a larger contact area more in line with the conditioned cartilage regions presumably similar to the pre-injury loading pattern.
The more recent studies in the review reported on tibial rotation. The results from these studies were variable, which may in part be attributed to differences in surgical techniques.
The debate on whether single-bundle versus double-bundle ACLR provides better restoration of rotational laxity continues. However, it should be noted that double-bundle reconstruction is not synonymous with “anatomic” ACL reconstruction.59 Anatomic ACLR is defined by placing the ACL graft in the native ACL insertion site area.60 This can be done using a single-bundle, or a double-bundle technique. Using in vivo dynamic stereo X-ray, Tashman et al have been able to show that during running, traditional non-anatomic ACLR resulted in altered knee kinematics especially when it comes to rotational stability of the knee.61
The knee adduction moment is the gait variable most commonly associated with knee osteoarthritis, as a higher moment is indicative of greater medial compartment loading. This review highlights the limited amount of information regarding this variable in patients after ACLR. Although there is currently no evidence that a higher adduction moment is detrimental in a knee with healthy articular cartilage, the higher adduction moment reported by Butler et al5 in this review is one mechanism which has been suggested as a potential contributor to the development of OA in the ACLR knee. Future research is required to investigate such claims.
Pre-operative baseline data were lacking in most studies. Knoll et al examined 25 patients with ACLD prior to and six weeks, four months, eight months and one year after ACLR.53 They found that reestablishment of pre-surgery gait patterns takes at least eight months to occur.53 Others have found persistent gait deficits in patients up to five years after surgery.25 Why do these deficits persist for such an extended period of time? The immediate situation after the surgery may require the patient to move the leg carefully in terms of preventing pain and/or fear of tearing the new ACL. These strategies may be regarded as useful in the acute stage after surgery. Nonetheless, it appears that gait alterations persist for years after surgery, a period during which one could assume that fear of pain or re-injury should have subsided. Decker et al recently presented a new model in terms of nonlinear variability.62 It is based on the premise that a healthy biological system has the ability to adapt to changing environment. Specifically, healthy gait is characterized by optimal movement variability, which allows for flexibility, adaptability, and the ability to respond to unpredictable situations. Hemmerich et al suggested that patients after ACLR may exhibit an “immature stabilization strategy” by utilizing co-contraction in order to increase the joint stiffness and thereby limiting degrees of freedom about the knee joint.29 It appears that altered sensorimotor control may cause changes in movement patterns. Kaprelli et al recently showed that patients with ACLD had diminished activation in several sensorimotor cortical areas.63 This implies that changes in motor control may have occurred after injury to the ACL, which likely continues after ACLR, and may be a factor that explains in part, the altered gait patterns after ACLR.
Rehabilitation procedures after ACLR can influence the recovery of function and long-term clinical results. For example, Decker et al noted that altered gait can be improved by specialized gait retraining programs.43 In a recent randomized controlled trial, patients with ACLD were allocated prior to surgery to a perturbation group performing neuromuscular training and quadriceps strength training, whereas the alternate group performed quadriceps strength training only.34 Despite symmetrical strength achieved by both groups six months after ACLR, the strength group demonstrated decreased knee excursion between legs during mid-stance. On the other hand, patients who had received perturbation training had no differences between legs. This suggests that neuromuscular training prior to surgery is more effective in improving gait after surgery.
Lewek et al reported that patients who have 90% or more quadriceps strength in the injured leg compared to the non-injured leg have more normal joint kinematics, kinetics and muscle activation compared to patients with less than 80% quadriceps strength of the injured leg.54 Webster et al found no relationship between the strength of the hamstrings and quadriceps and joint moments during gait.58 Also Gokeler et al determined that the strength of the quadriceps had no relation with knee joint angles and moments during gait.48 Significantly reduced quadriceps strength compared to the non-injured leg in the first two years after surgery has been reported.51 The results of that study may indicate that improvement of gait and the muscle strength follows different time patterns.
LIMITATIONS OF THE INCLUDED STUDIES
A large variance existed between the time of injury and surgery, as well as time when gait tests were conducted after surgery. This limitation exists because many studies lacked baseline data. Variations in the utilization of 2-D or 3-D methodology were identified with regards to gait analyis parameters. The limitations of marker based motion analysis systems for assessing knee kinematics need to be acknowledged. Of particular relevance is the potential for the movement of markers on the skin and their inability to predict the underlying bone movement. Nonetheless, other techniques which allow for the bone to be directly imaged such as fluoroscopy and stereoradiographic systems cannot be utilized during gait due to limitations of restricted fields of view, quasi-static motion, or one knee being visually blocked by the other. Therefore, skin marker motion analysis is at present the most feasible way to measure knee kinematics during gait.
CONCLUSION
In spite of the different study methods, patients after ACLR have altered gait patterns that can persist for up to five years after surgery. An ACL injury should be regarded as a neurophysiological dysfunction, not simply a musculoskeletal injury. Altered sensorimotor control may be a factor that explains, in part, altered gait after ACL injury and even after subsequent ACLR. Time after injury and reconstruction surgery plays a role in these alterations. These alterations in neuromuscular control might be permanent after ACLR. It is imperative that rehabilitation techniques are examined in order to minimize changes in knee biomechanics during gait, as they have the potential to impact on the development of OA.
REFERENCES
- 1. Marx RG, Jones EC, Angel M, Wickiewicz TL, Warren RF. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy. 2003;19(7):762–770 [DOI] [PubMed] [Google Scholar]
- 2. Hooper DM, Morrissey MC, Drechsler WI, Clark NC, Coutts FJ, McAuliffe TB. Gait analysis 6 and 12 months after anterior cruciate ligament reconstruction surgery. Clin Orthop Relat Res. 2002(403):168–178 [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Taylor NF, Feller JA, Webster KE. Return‐to‐sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40(1):41–48 [DOI] [PubMed] [Google Scholar]
- 4. Yasuda K, Tanabe Y, Kondo E, Kitamura N, Tohyama H. Anatomic double‐bundle anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(9 Suppl):S21–34 [DOI] [PubMed] [Google Scholar]
- 5. Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43(5):366–370 [DOI] [PubMed] [Google Scholar]
- 6. Oiestad BE, Holm I, Aune AK, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow‐up. Am J Sports Med. 2010;38(11):2201–2210 [DOI] [PubMed] [Google Scholar]
- 7. Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222 [DOI] [PubMed] [Google Scholar]
- 8. Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech. 2010;25(4):277–283 [DOI] [PubMed] [Google Scholar]
- 9. Webster KE, Feller JA. Alterations in joint kinematics during walking following hamstring and patellar tendon anterior cruciate ligament reconstruction surgery. Clin Biomech. 2011;26(2):175–180 [DOI] [PubMed] [Google Scholar]
- 10. Coury HJ, Brasileiro JS, Salvini TF, Poletto PR, Carnaz L, Hansson GA. Change in knee kinematics during gait after eccentric isokinetic training for quadriceps in subjects submitted to anterior cruciate ligament reconstruction. Gait Posture. 2006;24(3):370–374 [DOI] [PubMed] [Google Scholar]
- 11. DeVita P, Hortobagyi T. Functional knee brace alters predicted knee muscle and joint forces in people with ACL reconstruction during walking. J Appl Biomech. 2001;17(4):297–311 [Google Scholar]
- 12. Hooper DM, Morrissey MC, Drechsler WI, McDermott M, McAuliffe TB. Validation of the Hughston Clinic subjective knee questionnaire using gait analysis. Med Sci Sports Exerc. 2001;33(9):1456–1462 [DOI] [PubMed] [Google Scholar]
- 13. Kurz MJ, Stergiou N, Buzzi UH, Georgoulis AD. The effect of anterior cruciate ligament reconstruction on lower extremity relative phase dynamics during walking and running. Knee Surg Sports Traumatol Arthrosc. Mar 2005;13(2):107–115 [DOI] [PubMed] [Google Scholar]
- 14. Minning SJ, Myer GD, Mangine RE, Eifert‐Mangine M, Colosimo AJ. Serial assessments to determine normalization of gait following anterior cruciate ligament reconstruction. Scandinavian Journal of Medicine & Science in Sports. 2009;19(4):7p. [DOI] [PubMed] [Google Scholar]
- 15. Moraiti CO, Stergiou N, Ristanis S, et al. The effect of anterior cruciate ligament reconstruction on stride‐to‐stride variability. Arthroscopy. 2009;25(7):742–749 [DOI] [PubMed] [Google Scholar]
- 16. Moraiti CO, Stergiou N, Vasiliadis HS, Motsis E, Georgoulis A. Anterior cruciate ligament reconstruction results in alterations in gait variability. Gait Posture. 2010;32(2):169–175 [DOI] [PubMed] [Google Scholar]
- 17. Rudroff T. Functional capability is enhanced with semitendinosus than patellar tendon ACL repair. Med Sci Sports Exerc. 2003;35(9):1486–1492 [DOI] [PubMed] [Google Scholar]
- 18. Schmalz T, Freiwald J, Greiwing A, Kocker L, Ludwig H, Blumentritt S. Mechanical and electromyographical gait parameters in the course of rehabilitation after anterior cruciate ligament reconstruction. Eur J Sports Traumatol. Relat Res. 2001;23(4):146–151 [Google Scholar]
- 19. Tsivgoulis SD, Tzagarakis GN, Papagelopoulos PJ, et al. Pre‐operative versus post‐operative gait variability in patients with acute anterior cruciate ligament deficiency. J Intl Med Res. 2011;39(2):580–593 [DOI] [PubMed] [Google Scholar]
- 20. Winiarski S. Mechanical energy fluctuations during walking of healthy and ACL‐reconstructed subjects. Acta Bioeng Biomech. 2008;10(2):57–63 [PubMed] [Google Scholar]
- 21. Bush‐Joseph CA, Hurwitz DE, Patel RR, et al. Dynamic function after anterior cruciate ligament reconstruction with autologous patellar tendon. Am.J.Sports Med. 2001;29(1):36–41 [DOI] [PubMed] [Google Scholar]
- 22. Gao B, Zheng NN. Alterations in three‐dimensional joint kinematics of anterior cruciate ligament‐deficient and ‐reconstructed knees during walking. Clin Biomech. 2010;25(3):222–229 [DOI] [PubMed] [Google Scholar]
- 23. Morrissey MC, Hooper DM, Drechsler WI, Hill HJ. Relationship of leg muscle strength and knee function in the early period after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2004;14(6):360–366 [DOI] [PubMed] [Google Scholar]
- 24. Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scanlan SF, Blazek K, Chaudhari AM, Safran MR, Andriacchi TP. Graft orientation influences the knee flexion moment during walking in patients with anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(11):2173–2178 [DOI] [PubMed] [Google Scholar]
- 26. Biernat R, WoÇosewicz M, Tomaszewski W. A protocol of rehabilitation after ACL reconstruction using a hamstring autograft in the first month after surgery ‐ A preliminary report. Orthop Traumatol Rehabil. 2007;9(2):178–86 [PubMed] [Google Scholar]
- 27. Ejerhed L, Kartus J, Sernert N, Kohler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two‐year follow‐up. Am J Sports Med. 2003;31(1):19–25 [DOI] [PubMed] [Google Scholar]
- 28. Georgoulis AD, Ristanis S, Chouliaras V, Moraiti C, Stergiou N. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin Orthop Relat Res. 2007;454:89–94 [DOI] [PubMed] [Google Scholar]
- 29. Hemmerich A, van der Merwe W, Batterham M, Vaughan CL. Knee rotational laxity in a randomized comparison of single‐ versus double‐bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):48–56 [DOI] [PubMed] [Google Scholar]
- 30. Ristanis S, Stergiou N, Patras K, Tsepis E, Moraiti C, Georgoulis AD. Follow‐up evaluation 2 years after ACL reconstruction with bone‐patellar tendon‐bone graft shows that excessive tibial rotation persists. Clin J Sport Med. 2006;16(2):111–116 [DOI] [PubMed] [Google Scholar]
- 31. Ristanis S, Stergiou N, Siarava E, Ntoulia A, Mitsionis G, Georgoulis AD. Effect of femoral tunnel placement for reconstruction of the anterior cruciate ligament on tibial rotation. J Bone Joint Surg Am. 2009;91(9):2151–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Svensson M, Sernert N, Ejerhed L, Karlsson J, Kartus JT. A prospective comparison of bone‐patellar tendon‐bone and hamstring grafts for anterior cruciate ligament reconstruction in female patients. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):278–286 [DOI] [PubMed] [Google Scholar]
- 33. Grant JA, Mohtadi NGH, Maitland ME, Zernicke RF. Comparison of home versus physical therapy‐supervised rehabilitation programs after anterior cruciate ligament reconstruction ‐ A randomized clinical trial. Am J Sports Med. 2005;33(9):1288–1297 [DOI] [PubMed] [Google Scholar]
- 34. Hartigan E, Axe MJ, Snyder‐Mackler L. Perturbation training prior to ACL reconstruction improves gait asymmetries in non‐copers. J Orthop Res. 2009;27(6):724–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bai YH, Zhou J, Liang J. Changes in the gait analysis associated parameters of healthy people and patients with joint disease. J Clin Rehabil Tiss Eng Res. 2007;11(9):1790–1793 [Google Scholar]
- 36. Wang WM, Zhao DW, Cui DP, Li RX, Liu YP, Yang S. [Gait analysis associated with anterior cruciate ligament reconstruction]. Zhonghua Yi Xue Za Zhi. 2009;89(29):2025–2029 [PubMed] [Google Scholar]
- 37. Hunt MA, Di Ciacca SR, Jones IC, Padfield B, Birmingham TB. Effect of Anterior Tibiofemoral Glides on Knee Extension during Gait in Patients with Decreased Range of Motion after Anterior Cruciate Ligament Reconstruction. Physiother Can. 2010;62(3):235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgoulis AD, Ristanis S, Moraiti CO, et al. ACL injury and reconstruction: Clinical related in vivo biomechanics. Orthop Traumatol Surg. Res. 2010;96(8):S119–S128 [DOI] [PubMed] [Google Scholar]
- 39. Georgoulis AD, Ristanis S, Moraiti C, Mitsou A, Bernard M, Stergiou N. Three‐dimensional kinematics of the tibiofemoral joint in ACL‐deficient and reconstructed patients shows increased tibial rotation. Oper Techn Orthop. 2005;15(1):49–56 [Google Scholar]
- 40. Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial Rotation in Anterior Cruciate Ligament (ACL)‐Deficient and ACL‐Reconstructed Knees: A Theoretical Proposition for the Development of Osteoarthritis. Sports Med. 2007;37(7):601–613 [DOI] [PubMed] [Google Scholar]
- 41. Bacchini M, Cademartiri C, Soncini G. Gait analysis in patients undergoing ACL reconstruction according to Kenneth Jones' technique. Acta Biomed. 2009;80(2):140–149 [PubMed] [Google Scholar]
- 42. Beard DJ, Murray DW, Gill HS, et al. Reconstruction does not reduce tibial translation in the cruciate‐deficient knee an in vivo study. J Bone Joint Surg Br. 2001;83(8):1098–1103 [DOI] [PubMed] [Google Scholar]
- 43. Decker MJ, Torry MR, Noonan TJ, Sterett WI, Steadman JR. Gait retraining after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2004;85(5):848–856 [DOI] [PubMed] [Google Scholar]
- 44. Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Gait mechanics in chronic ACL deficiency and subsequent repair. Clin Biomech. 2002;17(4):274–285 [DOI] [PubMed] [Google Scholar]
- 45. Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Gait perturbation response in chronic anterior cruciate ligament deficiency and repair. Clin Biomech. 2003;18(2):132–141 [DOI] [PubMed] [Google Scholar]
- 46. Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech. 2004;19(2):136–144 [DOI] [PubMed] [Google Scholar]
- 47. Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three‐dimensional tibiofemoral kinematics of the anterior cruciate ligament‐deficient and reconstructed knee during walking. Am J Sports Med. 2003;31(1):75–79 [DOI] [PubMed] [Google Scholar]
- 48. Gokeler A, Schmalz T, Knopf E, Freiwald J, Blumentritt S. The relationship between isokinetic quadriceps strength and laxity on gait analysis parameters in anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2003;11(6):372–378 [DOI] [PubMed] [Google Scholar]
- 49. Hooper DM, Morrissey MC, Drechsler W, Morrissey D, King J. Open and closed kinetic chain exercises in the early period after anterior cruciate ligament reconstruction. Improvements in level walking, stair ascent, and stair descent. Am J Sports Med. 2001;29(2):167–174 [DOI] [PubMed] [Google Scholar]
- 50. Karanikas K, Arampatzis A, Bruggemann GP. [Graft type‐related versus graft type‐unrelated ACL treatment: influence on adaptation of muscle strength and motor skills]. Z Orthop Unfal.l 2007;145(5):615–621 [DOI] [PubMed] [Google Scholar]
- 51. Karanikas K, Arampatzis A, Bruggemann GP. Motor task and muscle strength followed different adaptation patterns after anterior cruciate ligament reconstruction. Eur J Phys Rehabil Med. 2009;45(1):37–45 [PubMed] [Google Scholar]
- 52. Knoll Z, Kiss RM, Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol. 2004;14(3):287–294 [DOI] [PubMed] [Google Scholar]
- 53. Knoll Z, Kocsis L, Kiss RM. Gait patterns before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(1):7–14 [DOI] [PubMed] [Google Scholar]
- 54. Lewek M, Rudolph K, Axe M, Snyder‐Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17(1):56–63 [DOI] [PubMed] [Google Scholar]
- 55. Roewer BD, Di Stasi SL, Snyder‐Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44(10):1948–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin CS, Chaudhari AM, Dyrby CO, Andriacchi TP. Influence of patellar ligament insertion angle on quadriceps usage during walking in anterior cruciate ligament reconstructed subjects. J Orthop Res. 2009;27(6):730–735 [DOI] [PubMed] [Google Scholar]
- 57. Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2012;46(5):355–359 [DOI] [PubMed] [Google Scholar]
- 58. Webster KE, Wittwer JE, O'Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33(2):247–254 [DOI] [PubMed] [Google Scholar]
- 59. van Eck CF, Schreiber VM, Mejia HA, et al. “Anatomic” anterior cruciate ligament reconstruction: a systematic review of surgical techniques and reporting of surgical data. Arthroscopy. 2010;26(9 Suppl):S2–12 [DOI] [PubMed] [Google Scholar]
- 60. van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single‐ and double‐bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy. 2010;26(2):258–268 [DOI] [PubMed] [Google Scholar]
- 61. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983 [DOI] [PubMed] [Google Scholar]
- 62. Decker LM, Moraiti C, Stergiou N, Georgoulis AD. New insights into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surg Sports Traumatol Arthrosc. 2011 [DOI] [PubMed] [Google Scholar]
- 63. Kapreli E, Athanasopoulos S, Gliatis J, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37(12):2419–2426 [DOI] [PubMed] [Google Scholar]