Abstract
Purpose/Background:
Proper strengthening of the core and upper extremities is important for muscular health, performance, and rehabilitation. Exercise devices have been developed that attempt to disrupt the center of gravity in order to activate the trunk stabilizing muscles. The objective of this study was to analyze the trunk and shoulder girdle muscle activation with double and single oscillating exercise devices (DOD and SOD respectively) in various planes.
Methods:
Twelve male subjects performed three interventions using both devices under randomized conditions: single-handed vertical orientation of DOD and SOD to produce 1) medio-lateral oscillation in the frontal plane 2) dorso-ventral oscillation in the sagittal plane and 3) single-handed horizontal orientation for superior and inferior oscillation in the transverse plane. Electromyographic (EMG) activity during the interventions of the anterior deltoid, triceps brachii, biceps brachii, forearm flexors as well as lower abdominal and back stabilizer muscles was collected, and were normalized to maximal voluntary contractions. A two way repeated measures ANOVA (2x3) was conducted to assess the influence of the devices and movement planes on muscle activation.
Results:
The DOD provided 35.9%, 40.8%, and 52.3% greater anterior deltoid, transverse abdominus (TA)/internal oblique (IO) and lumbo-sacral erector spinae (LSES) activation than did the SOD respectively. Effect size calculations revealed that these differences were of moderate to large magnitude (0.86, 0.48, and 0.61 respectively). There were no significant differences in muscular activation achieved between devices for the triceps brachii, biceps brachii and forearm flexor muscles. Exercise in the transverse plane resulted in 30.5%, 29.5%, and 19.5% greater activation than the sagittal and 21.8%, 17.2%, and 26.3% greater activation than the frontal plane for the anterior deltoid, TA/IO and LSES respectively.
Conclusions:
A DOD demonstrated greater muscular activity for trunk and shoulder muscle activation but does not provide an advantage for limb activation. Overall, oscillating the devices in the transverse plane provided greater muscular activation of the anterior deltoid, TA/IO and LSES than use of the devices during frontal or sagittal plane movements.
Level of evidence:
2c: Outcomes research.
Keywords: Bodyblade®, Core musculature, Flexbar®, Trunk musculature, Shoulder girdle musculature
INTRODUCTION
Power output during limb movement is directly dependent upon the ability of the core musculature to stabilize the spine.1 Low back pain (LBP) is the most common spinal condition2 while joint dislocation and subluxation are common glenohumeral work and athletic injuries that occur in industrialized nations.3 While strengthening of the core muscles has not been directly linked to LBP prevention4, Reeves et al.5 explained that training the core musculature improves the ability of the core stabilizing system to respond to the demands placed upon it, thus providing a buffer of protection against spinal injuries. Increased neuromuscular control and strength of the core or trunk musculature may provide protection when greater forces are needed during performance of athletic skills or certain occupational tasks.6,7,8 High incidences of musculoskeletal pain in the shoulders and arms are reported in workers with highly repetitive jobs (e.g. secretaries) as well as jobs involving forceful contractions (e.g. construction workers).9,10 Increasing the physical capacity or strength of the workers has been suggested as an effective ergonomic intervention for the prevention of shoulder and arm injuries.9,10 Thus, proper strengthening of the core and upper extremities should be considered when attempting to address those individuals who present with shoulder and/or lumbar spine dysfunction.
Exercise devices have been developed that attempt to activate trunk-stabilizing muscles. Their objective is similar to unstable resistance training, which has been shown to increase core muscle activation.11,12,13 Two types of devices have been developed: the Bodyblade®, (Mad Dogg Athletics, Venice CA) a double oscillating device (DOD) and the Thera-band's Flexbar®, (Hygenics Corporation, Akron, Ohio) a single oscillating device (SOD). The original Bodyblade® invented by Bruce Hymanson, in 1991, is a flexible blade ranging from 2.5 to 5 feet (76.2-152.4 cm) in length and weighs 1.5 pounds (0.68 kg), with a handgrip in the center.14 When the Bodyblade® is engaged either in the sagittal, frontal or transverse plane with just a minimal effort, the two ends begin to oscillate at a fixed rate with a natural frequency of 4.5 Hz.14 This oscillation challenges the muscles to keep the joints stable as well as impede or control the ongoing movement.14 The more intensely the individual oscillates the device, the greater the resistance must develop within the muscles in response to the DOD's acceleration.14 In one minute, the ends of the DOD can oscillate 270 times.14 Such a DOD device follows the “principle of inertia”,14 thus, when each end of the DOD moves, inertia acts to ensure continuous movement of the DOD. Rehabilitation professionals use DOD's to address several elements of recovery: power, coordination, endurance, strength, intensity and stabilization both for athletes and the general population.14
In addition to DOD (Bodyblade®), the Flexbar® (SOD) is a resistance device, which weighs about 0.59 kg and is 0.3m in length with a ribbed surface to ensure adequate grasp.15 It is commonly used for upper extremity stabilization and to augment grip strength by performing bending, twisting or oscillating movements.15 The Flexbar® is provided in a variety of resistance levels to match the individual's competency. SOD and DOD are used to induce “vibratory stimuli”, whereby co-activation of the muscles surrounding the shoulder girdle and core produces vibration of these devices with the aim of improving muscle strength and endurance.16
Anecdotal evidence exists for training using DOD and SOD, but little quantitative research has been published. Moreside et al17 evaluated trunk muscle activation patterns, lumbar compressive forces and spine kinematics while using DOD and found that the Bodyblade® has the ability to either augment or decrease spinal stability depending on how Bodyblade® is incorporated into an individual's training. Additionally they reported the frontal plane to be the most effective plane for achieving the activation of the transversus abdominis/internal obliques. Alternatively, Sugimoto et al18 did not find strength gains in young adults (who had no shoulder injury symptoms) when using the DOD to train shoulder internal and external rotation. To date no study has focused on arm and forearm muscle activation (anterior deltoid, triceps brachii, biceps brachii and forearm flexor muscle group) with these devices, nor has any study compared SOD and DOD or evaluated the most effective plane of movement for these devices to elicit trunk and upper extremity musculature activation. Based on the few aforementioned DOD studies, more research is needed to determine the utility of these devices.
The purpose of this study was to quantify and compare trunk and shoulder girdle muscle activation patterns with the movement of DOD and SOD in various planes. Based on the greater mechanical torque of the DOD, it was hypothesized that the DOD would activate the core and shoulder girdle musculature to a greater extent than the SOD. In addition, based on the single published study to date,17 it was hypothesized that using these devices in a frontal plane would lead to greater muscle activation of shoulder and upper limb muscles.
METHODS
Subjects
Twelve male subjects (mean age, 24.1 ± 7.4 years; mean weight, 71.5 ± 15.4 kg; mean height, 172.3 ± 6.5 cm) from Memorial University of Newfoundland University with no history of LBP or previous shoulder or upper extremity injury were recruited for the study. All subjects recruited had previous resistance-training experience (mean = 5.2 ± 6.4 years), and were presently engaged in resistance-training activities involving free weights, elastic bands, resistance machines, and instability devices (e.g. Swiss balls, BOSU™ balls). No subjects had prior experience with DOD or SOD devices. Each subject was required to review and sign a consent form before participation. The Human Research Ethics Authority of Memorial University of Newfoundland approved this study.
Electromyography (EMG) Electrode Preparation and Placement
Based on previously published articles19‐22, bipolar surface EMG electrodes were placed over the anterior deltoid, triceps brachii, biceps brachii, the forearm flexor muscle group, transversus abdominis / internal oblique area (TA/IO) and the lumbosacral erector spinae (LSES) to compare the activation of the muscles. All the electrodes were placed on the dominant side of the body. Before electrode placement, skin surfaces were shaved, abraded using sand paper, and cleaned with alcohol in order to decrease the resistance offered by dead surface skin and tissue oils and as well as to improve the conductivity of the EMG signal. Disposable Ag/Agcl discs electrode (3 cm in diameter) pairs (Kendall Medi-trace 100 series, Chikopee, MA) were placed with an inter-electrode distance of 2 cm. A ground electrode was placed on the acromioclavicular joint (AC) on the dominant side. For the anterior deltoid, the electrodes were placed 4-cm anterior to the AC joint over the belly of anterior deltoid muscle. For triceps brachii and biceps brachii, electrodes were placed at the halfway point over the central belly of triceps brachii and the biceps brachii muscle between the AC joint and lateral epicondyle of the humerus respectively. For the forearm flexor muscle group, electrodes were placed over the proximal one-third of the distance between the lateral epicondyle of the humerus and the radial styloid process. For the TA/IO, electrodes were placed just superior to the inguinal ligament and 1-cm medial to the anterior superior iliac spine. Behm et al19 have used this electrode orientation in previously published studies explaining that the surface EMG electrodes due to possible crosstalk would include activity of both the TA and IO. McGill et al20 reported that surface electrodes adequately represent the EMG amplitude of the deep abdominal muscle within a 15% root mean square (RMS) difference. However, Ng et al21 indicated that electrodes placed medial to the ASIS would receive competing signals from the external obliques and transversus abdominis with the internal obliques. For the LSES, electrodes were placed 2 cm lateral to L5-S1 spinous processes.19 A number of studies have used a similar L5-S1 electrode placement to measure the EMG activity of multifidus.21,22,23,24
MVIC Normalization
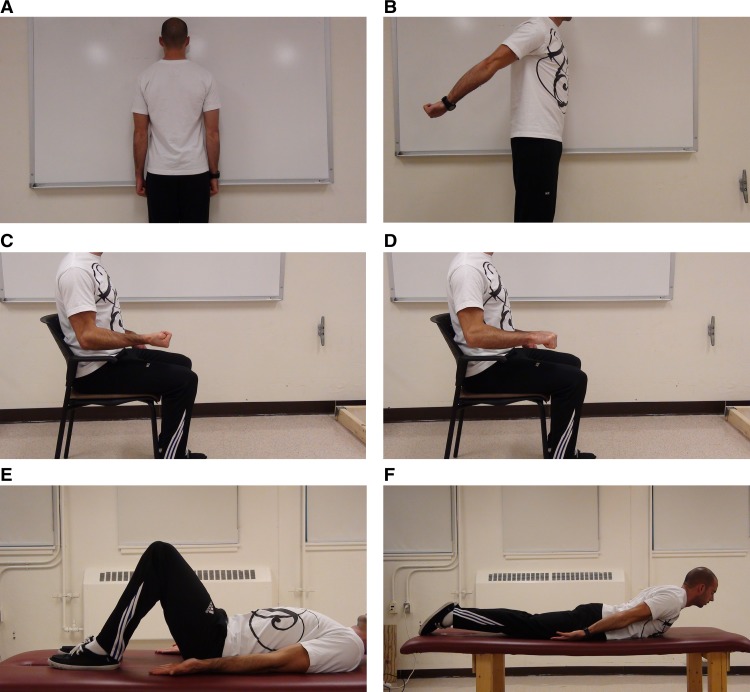
For the normalization of the dominant anterior deltoid, triceps brachii, biceps brachii, and forearm flexor muscle groups, standing was chosen as the initial position and subjects were asked to maintain resisted isometric contractions for a period of 5s. A 2s window of the highest EMG activity from the 5s MVC was used as the reference EMG activity for determining the MVIC used to compare to the activity found during the exercises using the oscillating exercises. Two trials were performed and the highest values were used for normalization. For the anterior deltoid (Figure 1A) subjects were asked to stand facing a wall. Subjects then pushed against the wall with a maximum contraction with their semi-pronated fisted hand and extended elbow. For the triceps brachii (Figure 1B), subjects were asked to extend their arm backwards to their anatomical limit and then maintained the maximum isometric co-contraction at that point. For the biceps brachii (Figure 1C), subjects were asked to flex their elbow, such that upper arm and supinated forearm (fisted hand) were at a right angle. They were then asked to produce a maximum isometric co-contraction. For the forearm flexor muscle group (Figure 1D), subjects were required to position the arm and pronated forearm (fisted hand) at 90°, and perform maximum isometric co-contraction. For the TA/IO Figure 1E), a hollowing maneuver25 was chosen and subjects were asked to lie on their back over the treatment table with their knees flexed and feet flat on the treatment table, arm fully extended along the side of their chest and were required to pull their abdominal muscles towards their spine as hard as they could. For the LSES muscles (Figure 1F), subjects were asked to lie on their stomach on the treatment table, with their arms fully extended by the side of their chest, with legs stabilized by the researcher to avoid lifting of the legs while attempting maximal isometric back extension, which was held parallel to the floor.
Figure 1.
Subjects performing the maximum voluntary contraction testing for (A) Anterior deltoid, (B) Triceps brachii, (C) Biceps brachii (D) Forearm flexor muscle group (E) Transversus abdominis / Internal oblique and (F) Lumbo-sacral erector spinae.
Exercises
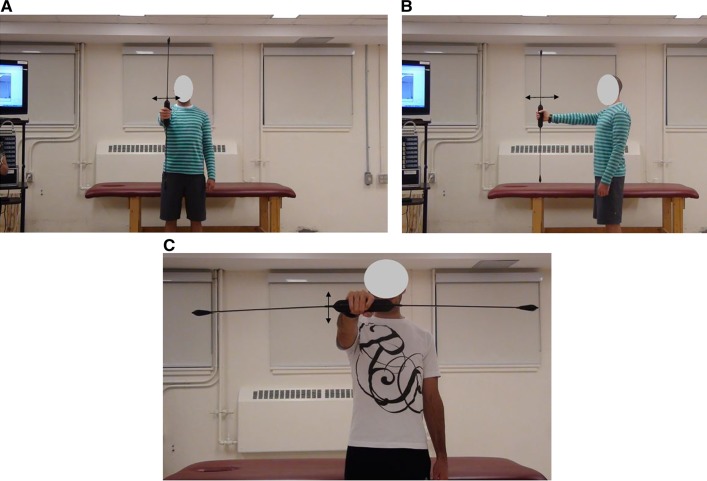
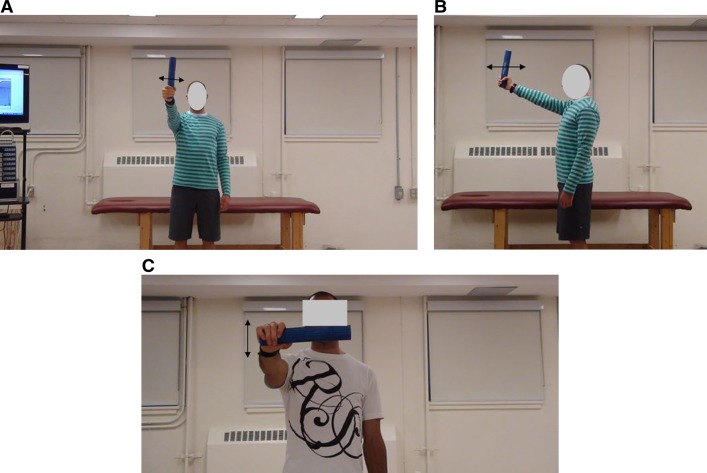
With a randomized conditions design, participants performed the following three movements for the DOD and SOD [Figures 2 and 3]: single-handed vertical orientation of the DOD and SOD to produce 1) medio-lateral oscillation in the frontal plane (Figures 2(A) and 3(A), 2) dorso-ventral oscillation in the sagittal plane [Figure 2(B) and Figure 3(B)], and 3) single-handed horizontal orientation for superior and inferior oscillation in the transverse plane [Figures 2(C) and 3(C)].
Figure 2.
Subjects using double (1A-C) a oscillating device (DOD) in the A) frontal plane (devices medio-lateral oscillation) B) sagittal plane (dorso-ventral oscillation) and C) in the transverse plane (superior and inferior oscillation).
Figure 3.
Subjects using a single (2A-C) oscillating device (SOD) in the A) frontal plane (devices medio-lateral oscillation) B) sagittal plane (dorso-ventral oscillation) and C) in the transverse plane (superior and inferior oscillation).
After a brief demonstration, the subjects practiced with both devices until the researcher was confident that the subject was proficient. This was followed by a variety of movements with the double as well as single oscillating devices, in all three planes of movement. Subjects were asked to separately oscillate a SOD and a DOD for a period of 8-seconds. The initial 2s were a quiet standing period, within the next 5s, subjects were asked to oscillate the devices at their greatest possible amplitude and frequency and during the last 3s were asked to gradually desist. Two trials of each movement were performed. A 3-4 minute rest period was allowed between each trial to minimize the effects of fatigue.25 The order of each trial was randomized. Subjects were encouraged verbally throughout the testing.
The DOD used in this study was a polycarbonate flexible blade (Bodyblade®) device 120 cm in length with a weight 0.68 kg, with a handgrip in the center.14 For the oscillating movements in frontal and transverse planes, the wrist and elbow were held steady (isometrically held) with movement initiated by the shoulder. Moving the DOD in the sagittal plane involved small flexion and extension movements at the elbow and shoulder. The SOD (Flexbar®) was composed of rubber compounds and it weighed 0.59 kg and 0.3 m in length with a ribbed surface. The DOD was grasped at the center with a fully extended arm (single-handed grip) anterior to the dominant shoulder (as shown in Figures 1A-1C). The SOD was held at one end in a single-handed grip similar to the “Statue of Liberty” position. Based on manufacturer (Thera-band®) recommendations, the “Statue of Liberty” position (Figures 3A and 3B) was utilized for movements. SOD with a single-handed grip (primarily isometric wrist contraction) was used with a fully extended arm anterior to the dominant shoulder for the movements in the frontal and transverse planes (Figures 3A and 3B). With sagittal movement the wrist and elbow flexed and extended (Figure 3C), subjects attempted to achieve the greatest amplitude of oscillation.
Data Collection and Processing
Biopac Systems MEC 100 amplifier, (Santa Barbara, CA), with an input impedance of 2 MΩ and common mode rejection ratio of >110 dB minimum (50/60 Hz), was used for data collection. A differential amplifier was used and gain was set at 1000X. The signals were sampled at a rate of 1000 Hz, and then digitized using a 12-bit analog-digital converter (BIOPAC MP 150), which was stored on the computer. Acqknowledge software program (AcqKnowledge III, Biopac System Inc., Holliston, MA) was used for full wave rectification and for filtering (Blackman 61-dB band pass filter between 20 and 500 Hz) the EMG signals and then the mean amplitude of the root mean square (RMS) was computed and analyzed. This value was then normalized to the EMG recorded during a MVIC.
A 2s window from the middle of the 5s oscillation period was chosen from each trial of SOD and DOD. The mean amplitude of the RMS, derived from the average of 2 trials was obtained.
Statistical Analyses
A two way repeated measure ANOVA (2x3) was conducted to assess the influence of the devices and movement planes on muscle activation level. The factors included (1) devices (DOD and SOD) and (2) planes (frontal, transverse and sagittal). The SOD, DOD and the plane of oscillation were used as the independent variables and the percentage of muscle activation as the dependent variable.
A priori statistical power analysis was conducted which determined that approximately twelve subjects would provide an alpha of P < 0.01 with a power of 0.8. Significant differences were detected (P < 0.05); and Dunn's Bonferroni correction (post-hoc test) was incorporated to detect significant interactions. All analyses were conducted using GB Stat: Dynamic Microsystem, Silver Springs Maryland. To infer the magnitude of the outcomes, effect sizes (ES) were calculated.26 The following formula was used to calculate the ES, {Pre-post ES = Posttest mean – Pretest mean/ Pretest Standard Deviation}.27 Cohen27 considered an ES of less than 0.2 as trivial, 0.2-0.41 as small, 0.41-0.70 as moderate and greater than 0.70 as large.
RESULTS
Device x Plane Interactions
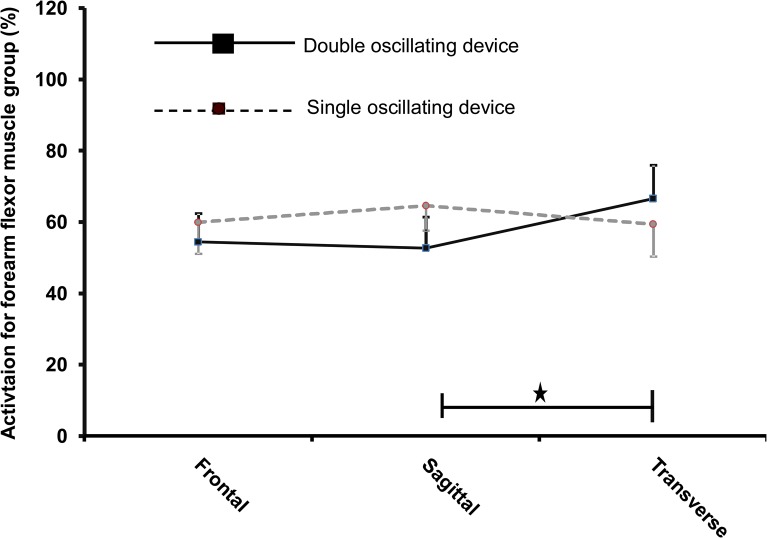
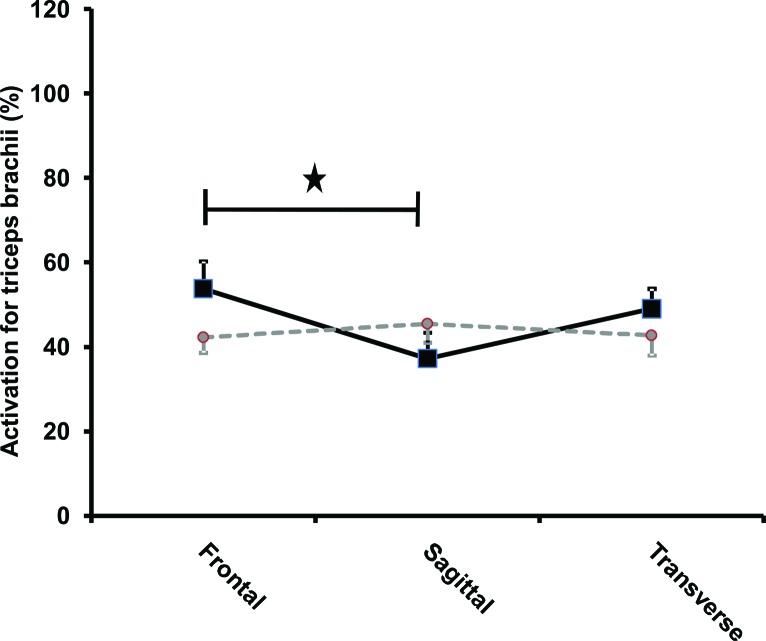
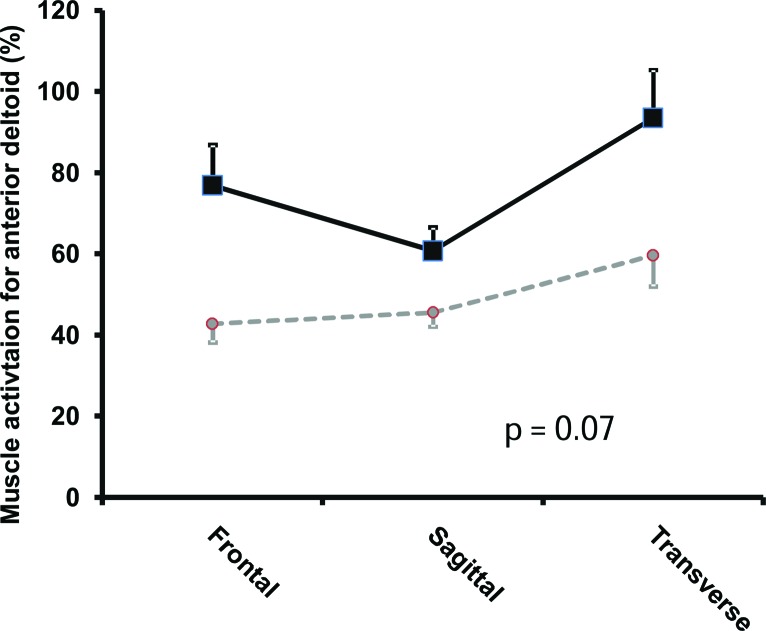
The transverse plane (Figure 4) provided 20.8% (p = 0.04, ES = 0.42) greater forearm flexor muscle group activation with DOD than the sagittal plane. The frontal plane (p = 0.004 ES = 0.73) provided 30.7% significantly greater triceps brachii activation for the DOD than the sagittal plane (Figure 5). There was a trend towards an interaction (p = 0.07) between the planes and the devices for the anterior deltoid (Figure 6).
Figure 4.
Activation of forearm flexor muscle group with respect to planes and devices. A significant (p = 0.0054) interaction was found between the planes and the devices. The transverse plane provided significantly greater forearm flexor muscle group activation with double oscillating device than the sagittal plane (p = 0.005).
Asterisk (*) represents statistical significance of p < 0.05. Squares with full lines represent double oscillating devices (DOD) whereas circles with intermittent lines represent single oscillating devices.
Figure 5.
Activation of triceps brachii with respect to planes and devices. A significant (p = 0.004) interaction was found between the planes and the devices. The frontal plane provided significantly greater triceps brachii activation for the double oscillating device than the sagittal plane (p = 0.004).
Asterisk (*) represents statistical significance of p < 0.05. Squares with full lines represent double oscillating devices (DOD) whereas circles with intermittent lines represent single oscillating devices.
Figure 6.
Graph showing the activation of anterior deltoid with respect to planes and devices. There was a trend (p = 0.07) towards an interaction between the planes and the devices. Squares with full lines represent double oscillating devices (DOD) whereas circles with intermittent lines represent single oscillating devices.
Devices: DOD vs. SOD
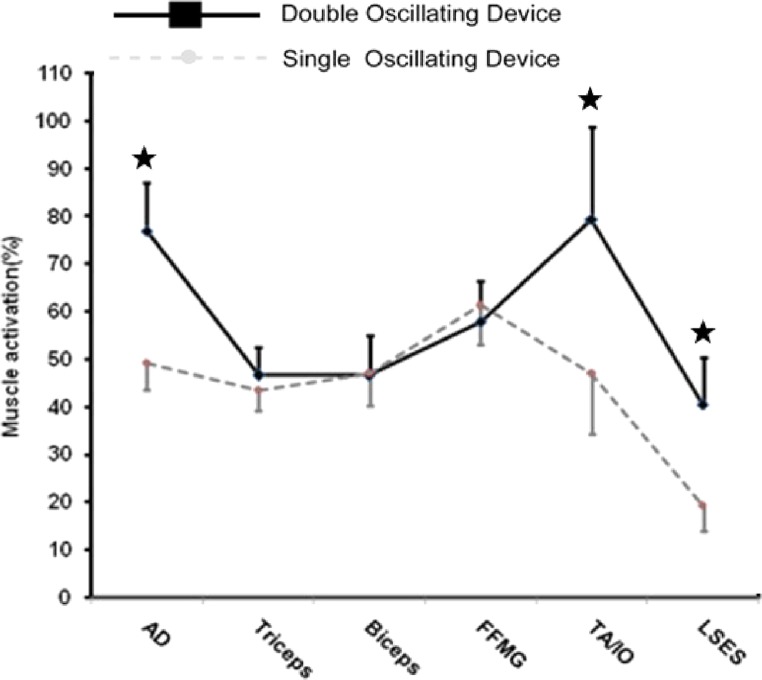
A significant main effect for devices showed overall (mean of all muscles), relative (normalized to MVC) muscle activation for the DOD (58.03% ± 41.53) was 13.4% greater than the SOD (44.6% ± 29.01). The DOD provided 35.9% (p = 0.009, ES = 0.86) greater anterior deltoid activation than did the SOD (Table 1). Although the greater TA/IO (40.8%, p = 0.16, ES = 0.48) and LSES (52.3%, p = 0.06, ES = 0.61) activation with the DOD (Table 1) versus the SOD was not statistically significant, the magnitude of change can be described as moderate. There was no significant effect of devices on activation of triceps brachii, biceps brachii and flexor forearm muscle group (Figure 7).
Table 1.
Effects for device. Acronyms are defined as follows: TA/IO: Transversus Abdominus / Internal Oblique, LSES: Lumbosacral erector spinae. Values represent normalized (% of MVC values) EMG data and are derived from mean EMG activity (mean ± standard deviation).
| Muscle | DOD | SOD | Effect size | p value |
|---|---|---|---|---|
| Anterior deltoid | 76.99% ± 32.04 | 49.29% ±17.98 | 0.86 | 0.000 |
| Triceps brachii | 46.65% ±20.21 | 43.50% ±14.82 | 0.16 | 0.456 |
| Biceps brachii | 46.65% ± 28.76 | 47.13% ±23.73 | 0.02 | 0.939 |
| Forearm flexor muscle group | 57.88% ±30.31 | 61.3% ±28.49 | 0.12 | 0.625 |
| TA/IO | 79.41% ±67.06 | 47.02% ±43.50 | 0.48 | 0.16 |
| LSES | 40.57% ± 34.97 | 19.36% ±17.78 | 0.61 | 0.06 |
Figure 7.
Graph representing the individual amount of muscle activation with respect to devices (both DOD and SOD).
AD=anterior deltoid, FFMG=forearm flexor muscle group, TA/IO=ransversus abdominis/ internal oblique, LSES=lumbo-sacral erector spinae. Asterisks (*) indicate that significantly (p < 0.05) greater activation occurred with the DOD than with the SOD for the respective muscles. Squares with full lines represent double oscillating devices (DOD) whereas circles with intermittent lines represent single oscillating devices.
Planes: Frontal vs. Sagittal vs. Transverse
The transverse plane (Table 2) provided 30.5% (p < 0.05, ES = 0.69) and 21.8% (p = 0.002, ES = 0.5) greater anterior deltoid activation than the sagittal and frontal plane respectively. For the TA/IO, the transverse plane (Table 2) provided 29.5% (p = 0.02, ES = 0.38) and 17.2% (p = 0.006, ES = 0.22) significantly greater activation than did the sagittal and frontal planes respectively. Similarly for the LSES, the transverse plane (Table 2) significantly (p = 0.04) induced 26.3% (p = 0.04, ES = 0.28) and 19.5 % (p = 0.04, ES = 0.21) greater activation than did the frontal and sagittal planes respectively.
Table 2.
Significant main effects for plane of movement. Definition of acronyms are as follow: F = frontal plane, S = sagittal plane, T = transverse plane. Values represent normalized (% of MVC values) EMG data and are derived from mean EMG activity (mean ± standard deviation).
| Muscle | Frontal (F) | Sagittal (S) | Transverse (T) | Effect size |
|---|---|---|---|---|
| Anterior deltoid | 59.80% ±25.25 | 53.11% ± 16.11 | 76.51% ±33.68 | T vs. F = 0.5 T vs. S = 0.69 |
| Triceps brachii | 48.02% ±17.62 | 41.36% ±18.37 | 45.85% ±16.55 | F vs. S= 0.38 Fvs. T = 0.12 |
| Biceps brachii | 55.24% ± 28.77 | 44.45% ± 27.26 | 40.98% ± 22.71 | F vs. S = 0.38 F vs. T = 0.5 |
| Forearm flexor muscle group | 57.23% ± 28.99 | 58.59% ± 27.22 | 62.97% ±31.98 | Tvs. F = 0.12 Tvs. S = 0.14 |
| Transverse abdominus/internal oblique | 61.99% ±64.68 | 52.80% ±43.66 | 74.87% ± 57.49 | T vs. F = 0.22 Tvs. S = 0.38 |
| LSES | 26.06% ± 22.34 | 28.47% ± 24.01 | 35.37% ±32.77 | T vs. F = 0.28 Tvs. S = 0.21 |
There was a trend for the frontal plane (Table 2) to procure 13.8% (p = 0.1, ES = 0.38) and 4.5% (p = 0.1, ES = 0.12) greater triceps brachii activation than the sagittal and the transverse planes respectively, although neither was statistically significant. For the biceps brachii, the frontal plane (Table 2) provided 25.8% (p = .003, ES= 0.5) and 19.5% (p = .01, ES = 0.38) significantly greater activation than the transverse and sagittal planes respectively.
DISCUSSION
The most important findings of this study were (a) the DOD had a significantly greater (large magnitude effect size) anterior deltoid (35.9%) and trends (moderate magnitude effect sizes) for higher TA/IO (40.8%) and LSES (52.28%) activation than the SOD; and (b) the transverse plane [Figure 6] movement resulted in greater activation of the anterior deltoid (moderate magnitude), TA/IO and LSES (small magnitudes).
The first hypothesis can be related to the structural architecture of the Bodyblade®. The mass (0.68 kg) of the DOD is distributed over a long (1.2 m) thin blade whereas the mass (0.59 kg) of the SOD has a more uniform distribution over a shorter cylinder (0.3 m) resulting in greater torque for the DOD versus the SOD. The greater torque of the DOD resulted in moderate to large magnitude greater muscular activation than did the SOD for the anterior deltoid, TA/IO and LSES. This resistive torque challenges the muscles to maintain stability and control the ongoing movement. Furthermore, as the DOD was 13% heavier than the SOD, the increased mass could also have contributed to the increased activation. Similarly, the instability resistance training literature consistently demonstrates high trunk and limb activation with a wide variety of activities from calisthenic type exercises (e.g. push-ups) to full kinetic chain (e.g. squats).11 Lister et al.28 found that DOD is an effective tool for the activation of muscles (as measured by EMG) incorporated during the training and rehabilitation of the shoulder as compared to the conventional interventions such as weight cuffs and Therabands®. Thus whether the instability is derived from oscillating devices or from unstable platforms (e.g. wobble boards, BOSU balls™ rocker boards) or devices (e.g. suspension bands, water filled dumbbells), relatively lower resistive loads or torques compared to the resistance achieved under stable conditions can provide suitable muscle activation for training.
As might be expected, the anterior deltoid as a prime mover of the upper limbs with superior-inferior shoulder movements had overall (combined data from both devices) higher EMG activation with transverse plane movements versus frontal or sagittal plane movements. In contrast to the hypothesis and the results of the Moreside et al17 study, the transverse rather than the frontal plane was the most effective plane for achieving the activation of the TA/IO. When compared to the sagittal and frontal plane, the activation of the TA/IO in the transverse plane with the use of DOD was 29.5% and 17.2% higher respectively. In addition, the transverse plane was more effective for activating the LSES than the frontal (26.3%) plane. However, a previous study reported that the frontal plane (vertical orientation) provided higher activation of the internal oblique.17 This contradiction may be linked to the type (single/ double handed) of grip utilized. A single-handed grip was chosen and utilized in the present study since a double-handed grip could not be used with the SOD. The double-handed grip may provide greater absorption of oscillation by the upper limb musculature. A lower force absorption capacity of a single-handed grip may have resulted in the oscillations of the blades being absorbed to a greater extent by the trunk rather than the limbs. Furthermore, similar to the findings of Behm et al25 the unilateral nature of the SOD may have led to greater torque asymmetries also promoting greater trunk activation.
Lower-load, endurance-type activities have been found to be more suitable for preventing lumbar region injuries as compared to high-load, strength-type activities.29,30,31 It has been reported that a minimum of 25% MVIC of the back muscles is sufficient to provide maximal joint stiffness.32 In addition the efficiency of another lumbar stabilizing muscle such as the multifidus can be improved with training loads of only 30-40% of MVC.33 Since the DOD tended to be more effective than the SOD for activating TA/IO and LSES, the incorporation of DOD activities could be more beneficial in activating the muscles required for a stable core. Moreover DOD exercises for the anterior deltoid and TA/IO can be used for strength training since the activation intensity has been demonstrate to reach approximately 80% of MVIC.34 As the activation intensities for the other muscle tested (triceps brachii, biceps brachii, forearm flexor muscle group and LSES) ranged between 40-60%, they could also promote strength in the untrained or be considered more appropriate for endurance type programs with trained individuals.34 Figure 7 illustrates the extent of activation for each muscle and device in order to determine which device is desirable for training particular muscles. While the DOD is more effective for activation of shoulder and trunk muscles, the portability and lower price range of the SOD may make it a suitable device for providing a training stress (i.e. strength and endurance) for the upper limbs.
Limitations to the study would include the use of only 12 resistance trained male subjects and the limitations of surface EMG recording from deep stabilizing muscles such as the TA/IO and LSES. These results may not transfer specifically to other groups such as those with low back or upper quarter pain or dysfunction.
CONCLUSION
While the DOD was significantly more effective for activating the anterior deltoid, there were non-significant trends with moderate magnitudes differences for higher TA/IO and LSES activation with the DOD compared to the SOD. There were no significant differences between devices in the activation of the triceps brachii, biceps brachii and forearm flexor muscle groups. The transverse plane was the more effective exercise plane overall (combined data from both devices), resulting in greater activation of the anterior deltoid, TA/IO and LSES compared to the frontal and sagittal planes. Hence the DOD used in a transverse plane tends to be more effective for activating the trunk and shoulder muscles while the SOD used in a frontal plane is as effective as a DOD exercise for the arm muscles.
REFERENCES
- 1. Hodges PW, Richardson CA. Relationship between limb movement speed and associated contraction of the trunk muscles. Ergonomics. 1997;40(11):1220–1230. 10.108 0/0014 01397187469. [DOI] [PubMed] [Google Scholar]
- 2. Jabri RS, Helper M, Benzon HT. Overview of Low Back Pain disorders. In: Benzon HT, Raja SN, Molloy RE, et al. Essentials of Pain Medicine and Regional Anesthesia. 2nd ed. Philadelphia, PA: Elsevier‐Churchill Livingstone;2005:311–330 [Google Scholar]
- 3. Hindle P, Davidson EK, Biant LC, et al. Appendicular joint dislocations. Injury. 2013. 10.1016/j.injury.2013.01.043; 10.1016/j.injury.2013.01.043. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Nadler SF, Malanga GA, Bartoli LA, et al. Hip muscle imbalance and low back pain in athletes: Influence of core strengthening. Med Sci Sports Exerc. 2002;34(1):9–16 [DOI] [PubMed] [Google Scholar]
- 5. Reeves NP, Narendra KS, Cholewicki J. Spine stability: The six blind men and the elephant. Clin Biomech (Bristol, Avon). 2007;22(3):266–274. 10.1016/j.clinbiomech. 2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cady LD, Bischoff DP, O'Connell ER, et al. Strength and fitness and subsequent back injuries in firefighters. J Occup Med. 1979;21(4):269–272 [PubMed] [Google Scholar]
- 7. Cairns MC, Foster NE, Wright C. Randomized controlled trial of specific spinal stabilization exercises and conventional physiotherapy for recurrent low back pain. Spine (Phila Pa 1976). 2006;31(19):E670–81. 10.1097/01.brs.0000232787.71938.5d. [DOI] [PubMed] [Google Scholar]
- 8. Caldwell JS, McNair PJ, Williams M. The effects of repetitive motion on lumbar flexion and erector spinae muscle activity in rowers. Clin Biomech (Bristol, Avon). 2003;18(8):704–711 [DOI] [PubMed] [Google Scholar]
- 9. Andersen LL, Jakobsen MD, Pedersen MT, et al. Effect of specific resistance training on forearm pain and work disability in industrial technicians: Cluster randomised controlled trial. BMJ Open. 2012;2(1):e000412‐2011‐000412. Print 2012. 10.1136/bmjopen‐2011‐000412; 10.1136/bmjopen‐2011‐000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundstrup E, Jakobsen MD, Andersen CH, et al. Participatory ergonomic intervention versus strength training on chronic pain and work disability in slaughterhouse workers: Study protocol for a single‐blind, randomized controlled trial. BMC Musculoskelet Disord. 2013;14:67‐2474‐14‐67. 10.1186/1471‐2474‐14‐67; 10.1186/1471‐2474‐14‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behm DG, Drinkwater EJ, Willardson JM, et al. The role of instability rehabilitative resistance training for the core musculature. Strength and Cond J. 2011;33(3): 72–81 [Google Scholar]
- 12. Behm DG, Drinkwater EJ, Willardson JM, et al. Canadian Society for Exercise Physiology. Canadian society for exercise physiology position stand: The use of instability to train the core in athletic and nonathletic conditioning. Appl Physiol Nutr Metab. 2010;35(1):109–112. 10.1139/H09‐128; 10.1139/H09‐128. [DOI] [PubMed] [Google Scholar]
- 13. Behm DG, Drinkwater EJ, Willardson JM, et al. The use of instability to train the core musculature. Appl Physiol Nutr Metab. 2010;35(1):91–108. 10.1139/H09‐127; 10.1139/H09‐127. [DOI] [PubMed] [Google Scholar]
- 14. Bodyblade® Web site Available at: http://www.bodyblade.com Accessed June 22, 2013
- 15. Theraband® Website Available at: www.theraband.com Accessed Accessed June 22, 2013
- 16. Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19(2):183–187 [DOI] [PubMed] [Google Scholar]
- 17. Moreside JM, Vera‐Garcia FJ, McGill SM. Trunk muscle activation patterns, lumbar compressive forces, and spine stability when using the bodyblade. Phys Ther. 2007;87(2):153–163. 10.2522/ptj.20060019. [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto D, Blanpied P. Flexible foil exercise and shoulder internal and external rotation strength. J Athl Train. 2006;41(3):280–285 [PMC free article] [PubMed] [Google Scholar]
- 19. Behm DG, Cappa D, Power GA. Trunk muscle activation during moderate‐ and high‐intensity running. Appl Physiol Nutr Metab. 2009;34(6):1008–1016. 10.1139/H09‐102; 10.1139/H09‐102. [DOI] [PubMed] [Google Scholar]
- 20. McGill S, Juker D, Kropf P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J Biomech. 1996;29(11):1503–1507 [DOI] [PubMed] [Google Scholar]
- 21. Ng JK, Kippers V, Richardson CA. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr Clin Neurophysiol. 1998;38(1):51–58 [PubMed] [Google Scholar]
- 22. Danneels LA, Cagnie BJ, Cools AM, et al. Intra‐operator and inter‐operator reliability of surface electromyography in the clinical evaluation of back muscles. Man Ther. 2001;6(3):145–153. 10.1054/math.2001.0396. [DOI] [PubMed] [Google Scholar]
- 23. Hermann KM, Barnes WS. Effects of eccentric exercise on trunk extensor torque and lumbar paraspinal EMG. Med Sci Sports Exerc. 2001;33(6):971–977 [DOI] [PubMed] [Google Scholar]
- 24. Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976). 1996;21(22):2640–2650 [DOI] [PubMed] [Google Scholar]
- 25. Behm DG, Leonard AM, Young WB, et al. Trunk muscle electromyographic activity with unstable and unilateral exercises. J Strength Cond Res. 2005;19(1):193–201. 2. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Things I have learned (so far). Am. Psych. 1990;45(12):1304–1312 [Google Scholar]
- 27. Cohen J. Statistical Power Analysis for the Biomechanical Sciences. 1st ed. New York: Academic Press; 1969 [Google Scholar]
- 28. Lister JL, Del Rossi G, Ma F, et al. Scapular stabilizer activity during bodyblade, cuff weights, and thera‐band use. J Sport Rehabil. 2007;16(1):50–67 [DOI] [PubMed] [Google Scholar]
- 29. McGill SM. Low back exercises: Evidence for improving exercise regimens. Phys Ther. 1998;78(7):754–765 [DOI] [PubMed] [Google Scholar]
- 30. Gibbons SGT, Comerford MJ. Strength versus stability part I: Concepts and terms. Orthop Division Rev. 2001;2:21–27 [Google Scholar]
- 31. Barr KP, Griggs M, Cadby T. Lumbar stabilization: Core concepts and current literature, part 1. Am J Phys Med Rehabil. 2005;84(6):473–480 [DOI] [PubMed] [Google Scholar]
- 32. Cresswell AG, Thorstensson A. Changes in intra‐abdominal pressure, trunk muscle activation and force during isokinetic lifting and lowering. Eur J Appl Physiol Occup Physiol. 1994;68(4):315–321 [DOI] [PubMed] [Google Scholar]
- 33. Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: Implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon). 1996;11(1):1–15 [DOI] [PubMed] [Google Scholar]
- 34. Kraemer W.J., Fleck S. Resistance training: Exercise Prescription. Physician and Sports Medicine. 1988;16: 69–81 [DOI] [PubMed] [Google Scholar]