Abstract
Background
Community-acquired pneumonia (CAP) causes considerable morbidity and mortality in adults, particularly in the elderly.
Methods
Structured searches of PubMed were conducted to identify up-to-date information on the incidence of CAP in adults in Europe, as well as data on lifestyle and medical risk factors for CAP.
Results
The overall annual incidence of CAP in adults ranged between 1.07 to 1.2 per 1000 person-years and 1.54 to 1.7 per 1000 population and increased with age (14 per 1000 person-years in adults aged ≥65 years). Incidence was also higher in men than in women and in patients with chronic respiratory disease or HIV infection. Lifestyle factors associated with an increased risk of CAP included smoking, alcohol abuse, being underweight, having regular contact with children and poor dental hygiene. The presence of comorbid conditions, including chronic respiratory and cardiovascular diseases, cerebrovascular disease, Parkinson's disease, epilepsy, dementia, dysphagia, HIV or chronic renal or liver disease all increased the risk of CAP by twofold to fourfold.
Conclusion
A range of lifestyle factors and underlying medical conditions are associated with an increased risk of CAP in European adults. Understanding of the types of individual at greatest risk of CAP can help to ensure that interventions to reduce the risk of infection and burden of disease are targeted appropriately.
Keywords: Pneumonia, Respiratory Infection, Clinical Epidemiology
Introduction
Community-acquired pneumonia (CAP) is a cause of considerable morbidity and mortality in adults in developed countries, leading to high rates of hospitalisations, especially in the elderly.1 2 The 2010 Global Burden of Disease Study reported that lower respiratory tract infections, including pneumonia, are the fourth most common cause of death globally, exceeded only by ischaemic heart disease, strokes and chronic obstructive pulmonary disease (COPD), and they are the second most frequent reason for years of life lost.3 Within Europe, CAP is the leading cause of death due to infection,2 with approximately 90% of deaths due to pneumonia occurring in people aged >65 years.4 Pneumonia places a considerable burden on healthcare resources and society, with associated annual costs in Europe estimated at approximately €10 billion, mainly due to hospitalisation and lost working days.5
Several risk factors for CAP are recognised, including age >65 years,1 6 7 smoking,6 alcoholism,7 immunosuppressive conditions,7 and conditions such as COPD,8 cardiovascular disease, cerebrovascular disease, chronic liver or renal disease, diabetes mellitus and dementia.9 Although many European studies have reported on the incidence of CAP and associated risk factors, no comprehensive overviews of these data are currently available. This literature review was conducted to generate up-to-date information on the incidence of CAP in adults in Europe, and of the risk factors for contracting CAP. A secondary objective was to collect data on the rates of comorbidities in patients with CAP.
Methods
The PubMed database was searched using the following search string: pneumonia AND English AND 2005/01/01–2012/07/31 AND risk NOT clinical trial, phase i OR clinical trial, phase ii OR clinical trial, phase iii OR controlled clinical trial OR randomised controlled trial OR case reports OR practice guideline OR editorial OR review OR cost OR cost effectiveness OR efficacy OR immunogenicity OR economic OR nosocomial. Additional searches used the same search string, but replaced ‘risk’ with either ‘comorbidity’ or ‘co-morbidity’.
Papers were included if they reported observational studies performed in Western European countries (Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, UK) and presented data from individuals aged >15 years on any of the following: incidence of CAP in at-risk individuals, defined as those with underlying risk factors for contracting CAP (table 1); risk factors for CAP; comorbidities in patients with CAP; pharmacotherapeutic agents associated with an increase or decrease in the risk of CAP; pathogens identified in patients with CAP. Studies that focused on nosocomial or healthcare acquired pneumonia were excluded.
Table 1.
Risk categories for community-acquired pneumonia included in the review
| Immunocompetent at risk | Immunocompromised at risk |
|---|---|
|
|
CNS, central nervous system; IPD invasive pneumococcal disease.
Included papers were reviewed in full and data on the study setting and methodology, characteristics of the study population, incidence of CAP, risk factors for CAP (ORs or relative risks (RRs), and 95% CIs) reported in case-control studies, and observational data on rates of comorbidities, associated pharmacotherapies and pathogens were collected. If more than one paper reported different aspects of the same study, all relevant papers were included. Where the same data were reported in more than one paper, the earliest published paper was selected.
Analysis of the included papers was descriptive and no meta-analyses of data were performed. Unless otherwise stated, all data are reported as OR (95% CI) or RR (95% CI).
Results
Included studies
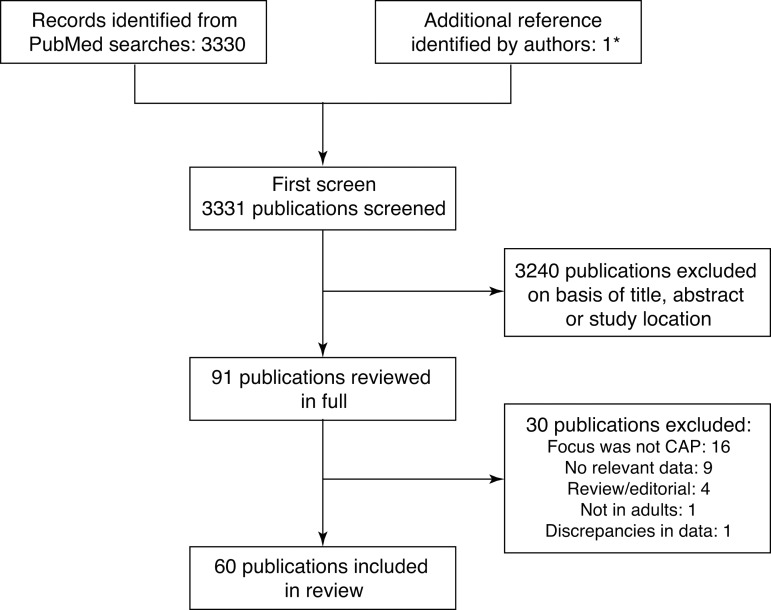
Of the 3330 references identified, 3240 were excluded on the basis of the title or abstract. The authors identified one additional reference10 that did not include the terms ‘risk’ or ‘co-morbidity’/'comorbidity’ in the title or abstract and was therefore not identified in the PubMed searches. However, it satisfied the other inclusion criteria. Final screening of the full papers identified 61 references meeting the inclusion and exclusion criteria, of which one paper11 was later excluded due to data discrepancies that we were unable to resolve by correspondence with the author (figure 1).
Figure 1.
Summary of the study selection procedure. CAP, community acquired pneumonia. *One study did not include the terms ‘risk’ or ‘co-morbidity’/'comorbidity’ in either the title or abstract and so was not identified in the PubMed searches; however, ‘risk factors’ was included in the list of MeSH terms for the article.
Of the 60 publications, a majority (34) focused on hospitalised patients. Included studies were from Denmark (n=7), France (n=5), Germany (n=5), Greece (n=1), Italy (n=4), The Netherlands (n=3), Spain (n=23) and the UK (n=12). Study designs and populations are summarised in online supplementary table S1. Most studies included adults of all ages. However, five studies considered only patients aged ≥65 years,12–16 two included patients aged 50–65 years,17 18 two included patients aged ≥45 years19 20 and single studies included patients aged ≥30 years,21 ≥40 years22 or 16–40 years.23 Six studies included only patients infected with HIV.24–29
Most studies included patients with pneumonia of any aetiology, but six were performed in patients with pneumonia due to a specified bacterial agent: four studies in patients with Legionella pneumophila infection,30–33 and one each in patients with Haemophilus influenzae34 or Gram-negative bacteria35 infections.
Incidence of CAP
The incidence of CAP was reported in 16 studies, from Denmark (n=2),17 18 France (n=3),24 26 29 Germany (n=1),36 Italy (n=2),27 37 Spain (n=5)16 25 38–40 and the UK (n=3).19 41 42 Data are summarised in table 2, with more details available in online supplementary table S2.
Table 2.
Incidence of community-acquired pneumonia (CAP) in adults in Europe
| Study | Country; region | Study period | CAP incidence (95% CI) |
|---|---|---|---|
| Overall population | |||
| Almirall et al38 | Spain; east coast | 1 November 1999–30 November 2000 | Per 1000 population >14 years: 1.54 |
| Gutierrez et al39 | Spain; Alicante | 15 October 1999–14 October 2001 | Per 1000 person-years: Overall, 1.230 |
| Men, 1.556 | |||
| Women, 0.911 | |||
| Rodriguez et al42 | UK; national | 1 January 2000–31 December 2005 | Primary care patients, per 1000 person-years: Overall, 1.07 (1.04 to 1.09) |
| Women, 0.93 (0.89 to 0.96) | |||
| Men, 1.22 (1.18 to 1.26) | |||
| Viegi et al37* | Italy; national | 15 February 1999–14 February 2000 | Annual incidence per 1000 population: Overall, 1.703 |
| Males, 1.692 | |||
| Females, 1.713 | |||
| Vila-Corcoles et al16 | Spain; Tarragona | 1 January 2002–30 April 2005 | Age ≥65 years, per 1000 person-years: Overall, 14.0 (12.7 to 15.3) |
| Men, 19.2 (17.1 to 21.6) | |||
| Women, 10.0 (8.6 to 11.5) | |||
| Hospitalisation for CAP | |||
| Bewick et al41 | UK; Nottingham | September 2008–September 2010 | Per 1000 population ≥16 years: Overall, 1.097 |
| Ewig et al36 | Germany; national | 2005 and 2006 | Per 1000 population/year ≥18 years: 2005, 2.75 2006, 2.96 |
| Mean incidence: Men, 3.21 Women, 2.52 | |||
| Kornum et al17 | Denmark; Copenhagen and Aarhus | December 1993–April 2008 | Per 1000 person-years, >50 years: Men, 4.2 Women, 3.4 |
| Kornum et al18 | Denmark; Copenhagen and Aarhus | December 1993–April 2008 | Per 1000 person-years, >50 years: Men, 4.25 Women, 3.28 |
| Patients with COPD | |||
| Müllerova et al19 | UK; England and Wales | 1 January 1996–31 December 2005 | Per 1000 patient-years: Overall, 22.4 (21.7 to 23.2) |
| Women, 21.4 (20.4 to 22.5) | |||
| Men, 23.1 (22.1 to 24.2) | |||
| Immunocompromised individuals | |||
| Perez-Sola et al40† | Spain; national | February 2000–January 2006 | Patients with rheumatic diseases treated with TNF antagonists, per 1000 patient-years: 5.97 (4.87 to 7.25) |
| HIV-infected individuals | |||
| Bénard et al24 | France; Aquitaine | 2000–2007 | Per 1000 patient-years: Overall: 12.0 (9.9 to 14.0) |
| Curran et al25 | Spain; Barcelona | January 2000–December 2005 | Cases/1000 patients/year: |
| 2000, 30.90 | |||
| 2001, 31.80 | |||
| 2002, 25.70 | |||
| 2003, 21.90 | |||
| 2004, 20.50 | |||
| 2005, 24.00 | |||
| Le Moing et al26 | France; national | May 1997–December 2001 | Hospitalisation for first episode of bacterial pneumonia in protease inhibitor-treated patients: 8/1000 patient-years (3–13) |
| Madeddu et al27‡ | Italy; northern Sardinia | January 1999–December 2004 | Per 1000 inpatients/year: 1999, 177 |
| 2004, 280 | |||
| Saindou et al29 | France; Lyon | 1993–2004 | Pneumococcal pneumonia, per 1000 patient-years: Cohort followed 1993–1 July 1996 (pre-HAART), 10.6 (5.4 to 15.7) |
| Cohort followed before 1 July 1996–2004 (pre-HAART and HAART era), 1.5 (0.9 to 2.1) | |||
| Cohort followed 1 July 1996–2004 (HAART era), 2.5 (1.4 to 3.6) | |||
Incidence rates standardised per 1000 population or per 1000 person-years; original study data are available in online supplementary table S2.
*This study included data for 10 children aged <14 years.
†In this study, ‘pneumonia’ included fungal and viral aetiologies.
‡A majority of patients (84%) in this study were also intravenous drug users.
COPD, chronic obstructive pulmonary disease; HAART, highly active antiretroviral therapy; TNF, tumour necrosis factor.
Differences in study populations and measures used for incidence rates make it difficult to make direct comparisons across studies. Nevertheless, several trends were apparent. The overall annual incidence of CAP in adults ranged between 1.07 to 1.2 per 1000 person-years and 1.54 to 1.7 per 1000 population37 38 42 43 (table 2). Rates of hospitalisation for CAP were typically higher than overall incidence rates; for example, a German study36 reported rates of 2.75 and 2.96 per 1000 population/year aged ≥18 years in 2005 and 2006, respectively (table 2). Overall CAP incidence and hospitalisation for CAP were higher in men than in women. The overall incidence per 1000 person-years in a UK study was 1.22 (1.18 to 1.26) in men compared with 0.93 (0.89 to 0.96) in women,42 whereas a study in Denmark in men and women aged >50 years reported rates of hospitalisation for pneumonia per 1000 person-years of 4.2 in men and 3.4 in women.17
The incidence of CAP increased with age and with the presence of comorbidities (see online supplementary table S2). Among individuals aged ≥65 years in Spain, the incidence per 1000 person-years was 14.0 (12.7 to 15.3).16 A study of patients with COPD reported the highest overall incidence of 22.4 (21.7 to 23.2) per 1000 person-years, with rates of 23.1 (22.1 to 24.2) and 21.4 (20.4 to 22.5) in men and women, respectively.19
High incidence rates were also reported in immunocompromised patients (table 2). Among patients with rheumatic diseases in Spain treated with tumour necrosis factor antagonists, the incidence per 1000 patient-years was 5.97 (4.87 to 7.25).40 The incidence in patients with HIV in France, was 12.0 (9.9 to 14.0) per 1000 patient-years.24 However, highly active antiretroviral therapy (HAART) appears to reduce the risk of CAP, with another French study reporting a reduction from 10.6 (5.4 to 15.7) per 1000 patient-years in the pre-HAART era to 2.5 (1.4 to 3.6) in the post-HAART era.29
Lifestyle factors and risk of CAP
The potential association between lifestyle factors and the risk of CAP was investigated in 12 case-control studies, performed in France (n=1),30 Germany (n=1),44 The Netherlands (n=1),23 45 Spain (n=2)12 38 and the UK (n=7).19–22 42 46 47 Study details are summarised in online supplementary table S3.
There was consistent evidence that smoking was associated with an increased risk of CAP.19–23 38 42 46 47 Compared with non-smokers (OR 1.00), the risk of CAP was increased in current smokers (crude ORs: 1.37 (1.14 to 1.64) to 1.81 (1.53 to 2.15); adjusted ORs: 0.99 (0.86 to 1.14) to 2.00 (1.20 to 3.36)) and former smokers (crude ORs: 1.34 (1.11 to 1.62) to 1.40 (1.17 to 1.68); adjusted OR: 1.04 (0.90 to 1.2)).
Compared with individuals who consumed no alcohol (OR 1.00), consumption of ≤40 g alcohol daily appeared to protect against CAP (21–40 g/day, crude ORs: 0.53 (0.22 to 1.25) and 0.88 (0.63 to 1.22)).23 38 However, the risk increased in individuals with higher consumption (>41 g/day, crude OR: 1.59 (0.59 to 4.25)23; >80 g/day, crude OR: 2.34 (1.13 to 4.85)38) or with a history of alcohol abuse/alcoholism (crude ORs: 1.85 (1.19 to 2.88)21 and 1.62 (0.91 to 2.91)47).
Being underweight was generally associated with an increased risk of CAP (crude ORs: 1.04 (0.57 to 1.89) to 2.20 (1.57 to 3.09)23 38 44 47) compared with normal bodyweight (OR 1.00). A reduced risk was seen in individuals classified as overweight (crude ORs: 0.6 (0.5 to 0.7) to 0.89 (0.72 to 1.09)21 23 38 44 47; adjusted ORs: 0.6 (0.5 to 0.7)44 and 0.78 (0.67 to 0.90)19), whereas those classified as obese had either a lower risk (crude ORs: 0.66 (0.58 to 0.75) to 0.81 (0.66 to 0.99)21 38 44 47; adjusted ORs: 0.7 (0.5 to 0.9)44 and 0.71 (0.60 to 0.85)19) or the same risk (crude OR 1.04 (0.57 to 1.89)23) as those of normal weight.
Household arrangements were also associated with the risk of CAP. Living in a household of over 10 people was associated with a crude OR of 2.20 (1.21 to 4.00).38 Regular contacts with children also increased the risk of CAP (crude OR: 1.48 (1.26 to 1.75)38). Two studies found that having children in the household increased the adjusted OR from 1.00 for ‘no children’ to 3.2 (1.5 to 7.0)44 or 3.41 (1.57 to 7.41)23 for three or more children. There was no clear evidence regarding the influence of contact with pets; one study demonstrated an increased risk of CAP (crude OR 1.37 (1.18 to 1.60)38), whereas a study in young adults (aged 16–40 years) found a decreased risk (crude OR 0.85 (0.58 to 1.24)23).
Higher levels of education were associated with a lower risk of CAP.23 38 44 Compared with individuals with a low level of education (OR 1.00), risk declined in those with an intermediate (secondary; crude ORs: 0.69 (0.41 to 1.19) to 0.86 (0.72 to 1.01)) or high level (university; crude ORs: 0.67 (0.41 to 1.10) to 0.78 (0.64 to 0.96)).23 38 In another study, individuals with ≥12 years of education had a lower risk of CAP (adjusted OR 0.8 (0.6 to 1.1)) compared with those who had ≤9 years of education (OR 1.00).44
Two studies found that visiting the dentist was associated with a decreased risk of CAP (in the past month, crude OR 0.71 (0.55 to 0.92)38; in the past year, OR 0.59 (0.34 to 1.04)23). In contrast, one study found that frequent visits to the general practitioner in the previous year were associated with a substantial increase in the risk of CAP (1–4 visits, OR 1.00; ≥30 visits, crude OR 3.73 (3.14 to 4.42)).21
Comorbid conditions and risk of CAP
The association between comorbidities and the risk of CAP was investigated in 14 case-control studies (Denmark (n=1),48 Germany (n=1),44 The Netherlands (n=1),23 Spain (n=2)12 38 and the UK (n=9)14 19–22 42 46 47 49 (see online supplementary table S4).
A history of respiratory disease was associated with an increased risk of CAP. A history of pneumonia increased the risk of a subsequent episode (crude ORs: 2.39 to 6.25 (1.83 to 21.40)22 38 44). Patients with chronic respiratory diseases, including COPD, bronchitis or asthma, had a twofold to fourfold increase in the risk of CAP (crude ORs: 2.17 (1.99 to 2.37) to 3.92 (3.67 to 4.18)12 14 21–23 38 44 46 47). Additional data also support this association. One study reported an adjusted OR of 2.47 (2.37 to 2.58) for chronic respiratory disease,20 and another study reported adjusted RRs of 2.82 (2.45 to 3.24) for COPD and 1.58 (1.44 to 1.74) for asthma.42 Patients with at least one respiratory tract infection in the past year were also at increased risk of CAP (crude ORs: 1.57 (1.35 to 1.84)38 to 4.5 (3.7 to 5.4)44). In young adults, the risk of CAP increased in line with the number of infections over the previous 6 years (1–2 infections, adjusted OR 1.49 (0.87 to 2.56); >3 infections, adjusted OR 4.84 (1.24 to 18.9)).23
Chronic cardiovascular disease increased the risk of CAP up to threefold (crude ORs from 1.4 (1.2 to 1.5) to 3.2 (2.6 to 4.1)12 21 22 38 44 46 47 49). Additional studies supported an association between chronic heart disease (adjusted ORs: 1.63 (1.54 to 1.72)46 and 1.66 (1.59 to 1.73)20) or heart failure (adjusted ORs: 2.19 (0.69 to 6.95)12 and 1.37 (1.20 to 1.57)19; adjusted RR: 2.63 (2.21 to 3.14)42) and the risk of CAP.
Cerebrovascular disease/stroke and dementia approximately doubled the risk of CAP (for cerebrovascular disease/stroke, crude ORs: 1.86 (1.74 to 1.99) to 2.37 (2.19 to 2.57),14 21 46 49 adjusted ORs: 1.08 (0.93 to 1.26)19 and 1.68 (1.58 to 1.77),20 adjusted RR: 1.42 (1.25 to 1.61)42 for dementia, crude ORs: 2.12 (0.91 to 4.94) to 2.41 (2.11 to 2.75),14 38 46 adjusted ORs: 2.64 (1.86 to 3.75)19 and 2.68 (2.42 to 2.97)20). Other neurological or psychiatric conditions were also associated with an increased risk of CAP in some studies (epilepsy, crude ORs: 2.81 (1.83 to 4.30) and 2.83 (1.11 to 7.21)21 38; Parkinson's disease, crude ORs: 1.82 (1.52 to 2.19) and 1.87 (1.60 to 2.19)14 46; multiple sclerosis, crude OR 3.20 (2.40 to 4.26)46). Crude ORs for CAP in patients with depression or bipolar disorder ranged from 1.75 (1.65 to 1.86) to 2.54 (1.03 to 6.26).14 21 23 However, the association with depression may have been confounded by other factors, as other studies reported an adjusted OR of 1.13 (0.99 to 1.28)19 or an adjusted RR of 1.30 (1.19 to 1.40).42
Two studies in elderly patients found a strong association between dysphagia and risk of CAP. A large database study in patients aged ≥65 years reported a crude OR of 2.10 (1.85 to 2.38),14 whereas a small study in patients aged ≥70 years reported a crude OR of 16.3 (4.57 to 58.2) and an adjusted OR of 11.9 (3.03 to 46.9).12
Data from several studies suggested that diabetes mellitus was associated with a moderate increase in the risk of CAP (crude ORs: 1.43 (1.11 to 1.92) to 1.54 (1.44 to 1.65),21 38 46 adjusted ORs: 1.07 (0.89 to 1.28)19 and 1.33 (1.26 to 1.41),20 adjusted RRs: 1.26 (1.21 to 1.31)48 and 1.28 (1.13 to 1.44)42).
Cancer was also associated with a moderate increase in the risk of CAP (crude ORs: 1.42 (1.04 to 1.92)38 and 1.70 (1.58 to 1.82),46 adjusted ORs: 1.42 (1.3 to 1.56)46 and 1.36 (1.24 to 1.49),20 adjusted RR: 1.37 (1.22 to 1.54)42). One study reported a fivefold higher risk in patients with lung cancer (crude OR: 4.73 (3.58 to 6.25)).46
Chronic liver or renal disease increased the risk of CAP approximately twofold (chronic liver disease, crude ORs: 1.67 (0.99 to 2.82) to 2.24 (1.74 to 2.89),38 44 46 adjusted ORs: 1.87 (1.43 to 2.44)46 and 1.85 (1.48 to 2.31)20; chronic renal disease, crude ORs: 1.7 (1.1 to 2.8)44 and 2.15 (1.81 to 2.56),46 adjusted ORs: 1.72 (1.43 to 2.07)46 and 1.78 (1.53 to 2.07)20).
Associations between conditions affecting immune function and the risk of CAP were reported. There was a moderate increase in risk in patients with rheumatoid arthritis (crude ORs: 1.46 (1.14 to 1.88)21 and 2.02 (1.79 to 2.29),46 adjusted ORs: 1.84 (1.62 to 2.10)46 and 1.83 (1.64 to 2.03),20 adjusted RR: 1.37 (1.12 to 1.69)42). Additionally, there was over a twofold increase in risk in patients with asplenia (adjusted OR: 2.58 (1.80 to 3.71)46) or with HIV or AIDS (adjusted ORs: 2.48 (1.34 to 4.58)46 and 5.90 (2.55 to 13.64)20).
In addition to the above medical conditions, a moderate increase in risk of CAP was reported in patients with anaemia (adjusted RR: 1.43 (1.25 to 1.62)).42
Hospitalisation in the previous 5 years was associated with an increased risk of CAP (crude ORs: 1.6 (1.4 to 1.9)44 and 1.68 (1.44 to 1.96)38). An adjusted RR of 1.77 (1.59 to 1.97) was calculated in patients with more than one hospitalisation in the previous year.42 The risk of CAP was increased in patients who had undergone either bronchoscopy (crude OR: 2.09 (1.07 to 4.06)) or passage of a nasogastric tube (crude OR: 3.21 (1.17 to 8.77)) during the previous year.38
Comorbid conditions in patients with CAP
The frequency of comorbidities in patients diagnosed with CAP was presented in 39 studies (7 case-control studies of observational data,15 19 45 50–53 and 31 observational, cohort studies10 13 16 27 28 31–37 41 43 54–70). Study details are summarised in online supplementary table S5.
The most common comorbidities were chronic respiratory diseases (up to 68% of patients), chronic heart disease (up to 47%) or heart failure (up to 46%), diabetes mellitus, cerebrovascular diseases and dementia (all up to 33%; table 3). Chronic liver and chronic renal diseases were observed in up to 20% and 27% of patients, respectively. The frequency of comorbidities was generally higher in patients aged ≥65 years compared with those aged <65 years, and in patients with COPD, chronic renal failure or cirrhosis compared with those without such conditions (see online supplementary table S5).
Table 3.
Frequency of comorbid conditions in adults with community-acquired pneumonia
| Comorbid condition | Number of cohorts with data* | Patients with condition (%) |
|---|---|---|
| Previous pneumonia | 10 | 3.2–33.8 |
| Chronic respiratory disease | 25 | 9.7–68 |
| COPD | 21 | 9.4–62 |
| Asthma | 9 | 3–50.0 |
| Chronic heart disease | 23 | 10–47.2 |
| Heart failure | 27 | 1.0–46 |
| Diabetes mellitus | 48 | 4.9–33.0 |
| Cerebrovascular disease/stroke | 26 | 3.2–33 |
| Dementia | 12 | 1.1–33.6 |
| Cancer | 33 | 4.3–18.0 |
| Chronic liver disease | 36 | 0.3–20 |
| Chronic renal disease | 39 | 0.5–26.7 |
*For studies that only reported data separately for each cohort, all cohorts were included; for studies that reported data for the overall study population, the summary data were used.
COPD, chronic obstructive pulmonary disease.
Discussion
This review represents a comprehensive compilation of data about the incidence of and risk factors for CAP in adults in Western Europe.
Notwithstanding the heterogeneity of the populations studied and measures of incidence rates used, overall the annual incidence was 1.07–1.7 per 1000 population. Studies consistently showed that the incidence was higher in men than in women, and that it increased with age; in patients aged ≥65 years, an incidence rate of 14 cases per 1000 person-years was reported.16 These findings are consistent with those of a recent review of European incidence rates published between 1990 and 2007.1 Also in line with previous studies of CAP epidemiology, incidence rates were higher in patients with comorbidities such as COPD,8 and in patients with HIV compared with those without HIV.71 Possible explanations for the higher rates of hospitalisation for CAP compared with overall incidence rates include the inclusion of data from different countries (Italy,37 Spain38 43 and the UK42 for overall incidence rates; Denmark,17 18 Germany36 and the UK41 for hospitalisation) reflecting national differences in medical practice, and that Danish studies were performed in patients aged >50 years,17 18 and so represent a population at increased risk of CAP.1 2
Importantly, this review included data obtained from observational and case-control studies. While observational studies provide valuable data on the rates of comorbidities observed in patients with CAP, they do not permit their identification as risk factors for infection. However, case-control studies of patients allow us to establish which comorbidities are indeed risk factors for CAP.
Pooled data from observational studies demonstrated the overall burden of CAP in patients with other medical conditions.10 13 16 27 28 31–37 41 43 54–70 Chronic respiratory diseases, cardiovascular diseases, cerebrovascular diseases, dementia and diabetes mellitus were the most frequently observed comorbidities. Up to two-thirds of patients had a chronic respiratory disease and almost half had a chronic cardiovascular disease, highlighting the need for appropriate management of these patients to reduce their risk of CAP.
Lifestyle factors such as smoking, high alcohol intake, being underweight, living in a large household or having regular contact with children were associated with an increased risk of CAP.12 19–23 30 38 42 44 46 47 Smoking is an established risk factor for CAP,6 72 probably due to its adverse effects on the respiratory epithelium and the clearance of bacteria from the respiratory tract.73 Alcoholism has been associated with defects in innate and adaptive immunity,74 and is a recognised CAP risk factor.7 Smoking and excessive alcohol consumption are major health risks globally and are targets for interventions to reduce the global burden of disease.75 Ensuring that patients make appropriate lifestyle changes would help to reduce the overall burden of CAP. Being underweight may predispose patients to CAP due to the consequences of undernutrition or underlying conditions on immune function.6 44 72 76 Assessment of the nutritional status of vulnerable patients might help to identify those at increased risk of CAP. Regular contact with children has also been identified previously as a risk factor for CAP, possibly due to the high carriage of Streptococcus pneumoniae by children.44 77 Appropriate measures for infection control may be advisable in vulnerable patients who are in regular contact with children.
Some lifestyle factors may provide protection against CAP. Young adults who consumed <40 g of alcohol per day had a lower risk of CAP than those who drank no alcohol,23 potentially because individuals who consumed no alcohol had other comorbidities that increased the risk of CAP. However, light-to-moderate alcohol intake has been reported to reduce the risk of atherosclerosis and cardiovascular disease,78 79 due to the antioxidant activities of alcohol,78 and this may also protect against CAP. Adherence to good dental hygiene was also associated with a reduced risk of CAP. Poor oral care has previously been identified as a risk factor for nursing-home acquired pneumonia, possibly due to the colonisation of the oral cavity by respiratory pathogens,80 and this risk may also be relevant for CAP. Finally, a higher level of education appeared to reduce the risk of CAP compared with a low level of education, as reported previously for invasive pneumococcal disease.81 A similar protective association of higher educational levels has also been described in relation to cardiovascular risk factors.82 Measures to reduce social and health inequalities could have the benefit of reducing costs associated with diseases like CAP.
The review also provides robust evidence that several comorbidities are associated with an increased risk of CAP, including a history of respiratory disease (including pneumonia) and cardiovascular disease. Patients with COPD are recognised as having a high risk of CAP8 and are targets for vaccination against influenza and pneumococcal disease,83 84 as are patients with chronic cardiovascular diseases.84–86
Patients with cerebrovascular disease or stroke, and neurological disorders (dementia, epilepsy, Parkinson's disease and multiple sclerosis) had approximately twice the risk of CAP compared with individuals without these conditions; dysphagia was also associated with a substantial increase in risk. The use of sedative medications and problems with swallowing might contribute to the risk of CAP in patients with dementia,19 43 probably due to aspiration and its associated risk of pneumonia.87 This could apply to patients with other neurological disorders.
Other comorbid conditions associated with an increased risk of CAP in the present study, including diabetes mellitus, cancer, chronic liver or renal disease, and impaired immune function, have previously been identified as risk factors for CAP.9
The main strength of this review is that many of the included publications were of case-control studies performed in large numbers of patients from registries or primary care databases, rather than small, single-centre studies, providing reassurance that the included studies provide a good representation of CAP in European populations.
This review also has some limitations. Patient registries and primary care databases are dependent on the quality of the information included in the records, and rely on the accuracy of the individuals responsible for entering diagnostic codes and demographic data. However, the inclusion of several thousand patients in such studies should help to minimise any potential introduced bias. Most of the included studies were based on patient populations in either Spain (23 studies10 12 13 16 25 32 33 35 38–40 43 50 54–59 63 65 68 69) or the UK (12 studies14 19–22 41 42 46 47 49 53 66), and this could limit the validity of the review for extrapolating the data to other European populations. Only those studies that were indexed in the PubMed database were included, and data from, for example, national surveillance databases were not included. Nevertheless, we believe it provides a good representation of the incidence and risk factors for CAP in European countries.
Lifestyle interventions, such as stopping smoking, reducing alcohol consumption, having regular dental checks and maintaining good nutritional status could help to reduce the burden of CAP. Patients with conditions such as chronic respiratory, cardiovascular and neurological diseases should be managed in accordance with current clinical guidelines to optimise their overall health status, and elderly patients should try to minimise contact with children who have acute viral respiratory infections. Finally, adults at risk of CAP should be vaccinated against influenza and pneumococcal pneumonia to reduce the risk of lower respiratory tract infections, in accordance with current guidelines (table 4).88 89
Table 4.
Bundles for lifestyle interventions to reduce the risk of CAP in adults
| Risk factor | Evidence | Recommendation |
|---|---|---|
| Smoking | Risk of CAP increased in current and former smokers (9 studies)19–23 38 42 46 47 | Smoking cessation |
| Alcohol consumption | Risk of CAP increased with high consumption or history of alcohol abuse (4 studies)21 23 38 47 | Reduce alcohol consumption |
| Nutritional status | Being underweight was generally associated with an increased risk of CAP (4 studies)23 38 44 47 | Dietary advice to ensure good nutritional status |
| Contact with children | Regular contact with children increased the risk of CAP (3 studies)23 38 44 | Avoid contacts with children with lower respiratory tract infections |
| Dental hygiene | Risk of CAP decreased in individuals with a recent (within past year) dental visit (2 studies)23 38 | Ensure regular dental visits |
| Vaccination against influenza and Streptococcus pneumoniae | Current guidelines88 89 | Ensure compliance with guidelines |
CAP, community-acquired pneumonia.
All but six of the studies included patients with pneumonia of any aetiology. S pneumoniae is the most frequently isolated pathogen from patients with CAP in Europe,1 and has been estimated to be the cause of 30–50% cases of CAP requiring hospitalisation in adults in developed countries.90 A 23-valent pneumococcal polysaccharide vaccine is recommended in some countries for the routine vaccination of adults aged ≥65 years, and for patients at increased risk of CAP.85 86 However, there is little evidence that it is effective in elderly people or adults with chronic diseases.91 92 A 13-valent pneumococcal conjugate vaccine (PCV-13) is available for the prevention of pneumonia and invasive pneumococcal disease caused by PCV-13 serotypes in adults aged ≥18 years.93 Efficacy of PCV-13 for the prevention of a first episode of vaccine serotype-specific pneumococcal CAP in community-dwelling adults aged ≥65 years is being investigated in the ongoing Community Acquired Pneumonia Immunisation Trial in Adults.94
In conclusion, this review of risk factors for CAP in European adults has highlighted the range of lifestyle factors and underlying medical conditions that are associated with an increased risk of infection. Lifestyle factors included smoking, alcohol abuse, being underweight and regular contact with children, whereas patients with chronic respiratory or cardiovascular diseases, cerebrovascular disease, epilepsy, dementia, dysphagia, HIV, or chronic renal or liver disease were all at increased risk of CAP. Greater understanding of the types of individuals at risk of CAP can help to ensure that interventions to reduce the risk of infection and burden of disease are targeted appropriately.
Supplementary Material
Acknowledgments
The authors take full responsibility for the content of this article and thank Neostar Communications Limited, Oxford, UK (supported by Pfizer, France), for their assistance in preparing the manuscript, including preparing the first draft in close collaboration with the authors and the collation of author comments.
Footnotes
Contributors: AT, WEP, GV and FB approved the literature search, commented on drafts of the manuscript and approved the final draft. AT is guarantor. Nathalie Dartois (Pfizer Ltd, Paris, France) discussed the manuscript concept with AT and reviewed drafts of the manuscript. Neostar Communications collaborated closely with the authors throughout the development of the manuscript and were responsible for performing the literature search, preparing the first draft of the article and providing author comments.
Funding: Pfizer supported Neostar Communications for preparation of the manuscript in close collaboration with the authors.
Competing interests: AT has received consulting fees/honorarium from Astra-Zeneca, Bayer, Curetis, GlaxoSmithKline, Pfizer and Polyphor. WEP has received consulting fees/honorarium from Pfizer; fees for board membership from Astellas, AstraZeneca, Bayer, GlaxoSmithKline Biologicals, Merck-Shering and Pfizer; and his institution has received research grants for investigator-initiated research from AstraZeneca, Bayer, Pfizer and Sanofi-Aventis. GV's institution has received consulting fees/honorarium from Pfizer. FB has received financial support for travel to meetings from Pfizer; consultancy fees from AstraZeneca, Pfizer and Zambon; fees for board membership from AstraZeneca, Chiesi, GlaxoSmithKline, Novartis and Pfizer; lecture fees/speaker bureaus fees from AstraZeneca, Chiesi, Novartis, Pfizer and Zambon; and his institution has received grants from Chiesi, Novartis, Pfizer and Zambon.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012;67:71–9 [DOI] [PubMed] [Google Scholar]
- 2.Blasi F, Mantero M, Santus P, et al. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect 2012;18:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Commission Health statistics. Atlas on mortality in the European Union. Luxembourg: Office for Official Publications of the European Communities, 2008 [Google Scholar]
- 5.Pneumonia. In: European lung white book 2nd edn. Sheffield, UK: European Respiratory Society/European Lung Foundation, 2003:55–65 [Google Scholar]
- 6.Baik I, Curhan GC, Rimm EB, et al. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 2000;160:3082–8 [DOI] [PubMed] [Google Scholar]
- 7.Koivalu I, Sten M, Makela PH. Risk factors for pneumonia in the elderly. Am J Med 1994;96:313–20 [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med 2009;103:224–9 [DOI] [PubMed] [Google Scholar]
- 9.Polverino E, Torres Marti A. Community-acquired pneumonia. Minerva Anestesiol 2011;77:196–211 [PubMed] [Google Scholar]
- 10.de Roux A, Cavalcanti M, Marcos MA, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest 2006;129:1219–25 [DOI] [PubMed] [Google Scholar]
- 11.Jover F, Cuadrado JM, Andreu L, et al. A comparative study of bacteremic and non-bacteremic pneumococcal pneumonia. Eur J Intern Med 2008;19:15–21 [DOI] [PubMed] [Google Scholar]
- 12.Almirall J, Rofes L, Serra-Prat M, et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J 2013;41:923–8 [DOI] [PubMed] [Google Scholar]
- 13.Cabre M, Serra-Prat M, Palomera E, et al. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2010;39:39–45 [DOI] [PubMed] [Google Scholar]
- 14.Hennessy S, Bilker WB, Leonard CE, et al. Observed association between antidepressant use and pneumonia risk was confounded by comorbidity measures. J Clin Epidemiol 2007;60:911–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trifiro G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med 2010;152:418–40 [DOI] [PubMed] [Google Scholar]
- 16.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, et al. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med 2009;103:309–16 [DOI] [PubMed] [Google Scholar]
- 17.Kornum JB, Norgaard M, Dethlefsen C, et al. Obesity and risk of subsequent hospitalisation with pneumonia. Eur Respir J 2010;36:1330–6 [DOI] [PubMed] [Google Scholar]
- 18.Kornum JB, Due KM, Norgaard M, et al. Alcohol drinking and risk of subsequent hospitalisation with pneumonia. Eur Respir J 2012;39:149–55 [DOI] [PubMed] [Google Scholar]
- 19.Mullerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respir Med 2012;106:1124–33 [DOI] [PubMed] [Google Scholar]
- 20.Vinogradova Y, Coupland C, Hippisley-Cox J. Risk of pneumonia in patients taking statins: population-based nested case-control study. Br J Gen Pract 2011;61:e742–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlienger RG, Fedson DS, Jick SS, et al. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy 2007;27:325–32 [DOI] [PubMed] [Google Scholar]
- 22.Myles PR, Hubbard RB, McKeever TM, et al. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009;18:269–75 [DOI] [PubMed] [Google Scholar]
- 23.Teepe J, Grigoryan L, Verheij TJ. Determinants of community-acquired pneumonia in children and young adults in primary care. Eur Respir J 2010;35:1113–17 [DOI] [PubMed] [Google Scholar]
- 24.Benard A, Mercie P, Alioum A, et al. Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS ONE 2010;5:e8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran A, Falco V, Crespo M, et al. Bacterial pneumonia in HIV-infected patients: use of the pneumonia severity index and impact of current management on incidence, aetiology and outcome. HIV Med 2008;9:609–15 [DOI] [PubMed] [Google Scholar]
- 26.Le Moing V, Rabaud C, Journot V, et al. Incidence and risk factors of bacterial pneumonia requiring hospitalization in HIV-infected patients started on a protease inhibitor-containing regimen. HIV Med 2006;7:261–7 [DOI] [PubMed] [Google Scholar]
- 27.Madeddu G, Porqueddu EM, Cambosu F, et al. Bacterial community acquired pneumonia in HIV-infected inpatients in the highly active antiretroviral therapy era. Infection 2008;36:231–6 [DOI] [PubMed] [Google Scholar]
- 28.Manno D, Puoti M, Signorini L, et al. Risk factors and clinical characteristics associated with hospitalization for community-acquired bacterial pneumonia in HIV-positive patients according to the presence of liver cirrhosis. Infection 2009;37:334–9 [DOI] [PubMed] [Google Scholar]
- 29.Saindou M, Chidiac C, Miailhes P, et al. Pneumococcal pneumonia in HIV-infected patients by antiretroviral therapy periods. HIV Med 2008;9:203–7 [DOI] [PubMed] [Google Scholar]
- 30.Che D, Campese C, Santa-Olalla P, et al. Sporadic community-acquired Legionnaires’ disease in France: a 2-year national matched case-control study. Epidemiol Infect 2008;136:1684–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chidiac C, Che D, Pires-Cronenberger S, et al. Factors associated with hospital mortality in community-acquired legionellosis in France. Eur Respir J 2012;39:963–70 [DOI] [PubMed] [Google Scholar]
- 32.Sopena N, Force L, Pedro-Botet ML, et al. Sporadic and epidemic community legionellosis: two faces of the same illness. Eur Respir J 2007;29:138–42 [DOI] [PubMed] [Google Scholar]
- 33.Sopena N, Pedro-Botet L, Mateu L, et al. Community-acquired legionella pneumonia in elderly patients: characteristics and outcome. J Am Geriatr Soc 2007;55: 114–19 [DOI] [PubMed] [Google Scholar]
- 34.Kofteridis D, Samonis G, Mantadakis E, et al. Lower respiratory tract infections caused by Haemophilus influenzae: clinical features and predictors of outcome. Med Sci Monit 2009;15:CR135–39 [PubMed] [Google Scholar]
- 35.Ruiz LA, Gomez A, Jaca C, et al. Bacteraemic community-acquired pneumonia due to Gram-negative bacteria: incidence, clinical presentation and factors associated with severity during hospital stay. Infection 2010;38:453–8 [DOI] [PubMed] [Google Scholar]
- 36.Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009;64:1062–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viegi G, Pistelli R, Cazzola M, et al. Epidemiological survey on incidence and treatment of community acquired pneumonia in Italy. Respir Med 2006;100:46–55 [DOI] [PubMed] [Google Scholar]
- 38.Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J 2008;31:1274–84 [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez F, Masia M, Mirete C, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect 2006;53:166–74 [DOI] [PubMed] [Google Scholar]
- 40.Perez-Sola MJ, Torre-Cisneros J, Perez-Zafrilla B, et al. Infections in patients treated with tumor necrosis factor antagonists: incidence, etiology and mortality in the BIOBADASER registry. Med Clin (Barc) 2011;137:533–40 [DOI] [PubMed] [Google Scholar]
- 41.Bewick T, Sheppard C, Greenwood S, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012;67:540–5 [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez LA, Ruigomez A, Wallander MA, et al. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009;20:800–6 [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez F, Masia M, Rodriguez JC, et al. Epidemiology of community-acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of Spain. Clin Microbiol Infect 2005;11:788–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnoor M, Klante T, Beckmann M, et al. Risk factors for community-acquired pneumonia in German adults: the impact of children in the household. Epidemiol Infect 2007;135:1389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Garde EM, Souverein PC, van den Bosch JM, et al. Angiotensin-converting enzyme inhibitor use and pneumonia risk in a general population. Eur Respir J 2006;27:1217–22 [DOI] [PubMed] [Google Scholar]
- 46.Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract 2009;59:e329–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Garde EM, Hak E, Souverein PC, et al. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax 2006;61:957–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kornum JB, Thomsen RW, Riis A, et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 2008;31:1541–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Garde EM, Souverein PC, Hak E, et al. Angiotensin-converting enzyme inhibitor use and protection against pneumonia in patients with diabetes. J Hypertens 2007;25:235–9 [DOI] [PubMed] [Google Scholar]
- 50.Almirall J, Bolibar I, Serra-Prat M, et al. Inhaled drugs as risk factors for community-acquired pneumonia. Eur Respir J 2010;36:1080–7 [DOI] [PubMed] [Google Scholar]
- 51.Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007;167:950–5 [DOI] [PubMed] [Google Scholar]
- 52.Nielsen AG, Nielsen RB, Riis AH, et al. The impact of statin use on pneumonia risk and outcome: a combined population-based case-control and cohort study. Crit Care 2012;16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008;149:391–8 [DOI] [PubMed] [Google Scholar]
- 54.Carratala J, Mykietiuk A, Fernandez-Sabe N, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 2007;167:1393–9 [DOI] [PubMed] [Google Scholar]
- 55.Cilloniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care 2011;15:R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cilloniz C, Ewig S, Polverino E, et al. Pulmonary complications of pneumococcal community-acquired pneumonia: incidence, predictors, and outcomes. Clin Microbiol Infect 2012;18:1134–42 [DOI] [PubMed] [Google Scholar]
- 57.Falguera M, Carratala J, Ruiz-Gonzalez A, et al. Risk factors and outcome of community-acquired pneumonia due to Gram-negative bacilli. Respirology 2009;14:105–11 [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Vidal C, Carratala J, Fernandez-Sabe N, et al. Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin Microbiol Infect 2009;15:1033–8 [DOI] [PubMed] [Google Scholar]
- 59.Giannella M, Pinilla B, Capdevila JA, et al. Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clin Microbiol Infect 2012;18:786–94 [DOI] [PubMed] [Google Scholar]
- 60.Holm A, Nexoe J, Bistrup LA, et al. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br J Gen Pract 2007;57:547–54 [PMC free article] [PubMed] [Google Scholar]
- 61.Klapdor B, Ewig S, Pletz MW, et al. Community-acquired pneumonia in younger patients is an entity on its own. Eur Respir J 2012;39:1156–61 [DOI] [PubMed] [Google Scholar]
- 62.Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008;32:139–46 [DOI] [PubMed] [Google Scholar]
- 63.Liapikou A, Polverino E, Ewig S, et al. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J 2012;39:855–61 [DOI] [PubMed] [Google Scholar]
- 64.Migliorati PL, Boccoli E, Bracci LS, et al. A survey on hospitalised community-acquired pneumonia in Italy. Monaldi Arch Chest Dis 2006;65:82–8 [DOI] [PubMed] [Google Scholar]
- 65.Molinos L, Clemente MG, Miranda B, et al. Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect 2009;58:417–24 [DOI] [PubMed] [Google Scholar]
- 66.Myint PK, Lowe D, Stone RA, et al. U.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbations. Respiration 2011;82:320–7 [DOI] [PubMed] [Google Scholar]
- 67.Thomsen RW, Riis A, Kornum JB, et al. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med 2008;168:2081–7 [DOI] [PubMed] [Google Scholar]
- 68.Viasus D, Garcia-Vidal C, Cruzado JM, et al. Epidemiology, clinical features and outcomes of pneumonia in patients with chronic kidney disease. Nephrol Dial Transplant 2011;26:2899–906 [DOI] [PubMed] [Google Scholar]
- 69.Viasus D, Garcia-Vidal C, Castellote J, et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011;90:110–18 [DOI] [PubMed] [Google Scholar]
- 70.von Baum H, Welte T, Marre R, et al. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J 2010;35:598–605 [DOI] [PubMed] [Google Scholar]
- 71.Sogaard OS, Lohse N, Gerstoft J, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995–2007: a Danish population-based, nationwide cohort study. Clin Infect Dis 2008;47:1345–53 [DOI] [PubMed] [Google Scholar]
- 72.Almirall J, Gonzalez CA, Balanzó X, et al. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 1999;116:375–9 [DOI] [PubMed] [Google Scholar]
- 73.Dye JA, Adler KB. Effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax 1994;49:825–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol 2002;2:205–9 [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization The World health Report 2002. Reducing risks, promoting healthy life. Geneva, Switzerland: World Health Organization, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Hedlund J, Hansson LO, Ortqvist A. Short- and long-term prognosis for middle-aged and elderly patients hospitalised with community-acquired pneumonia: impact of nutritional and inflammatory factors. Scand J Infect Dis 1995;27:32–7 [DOI] [PubMed] [Google Scholar]
- 77.Hendley JO, Sande MA, Stewart PM, et al. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis 1975;132:55–61 [DOI] [PubMed] [Google Scholar]
- 78.Arranz S, Chiva-Blanch G, Valderas-Martínez P, et al. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012;4:759–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quagliarello V, Ginter S, Han L, et al. Modifiable risk factors for nursing home-acquired pneumonia. Clin Infect Dis 2005;40:1–6 [DOI] [PubMed] [Google Scholar]
- 81.Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med 2000;342:681–9 [DOI] [PubMed] [Google Scholar]
- 82.Winkleby MA, Jatulis DE, Frank E, et al. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992;82:816–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaillat J. Should patients with chronic obstructive pulmonary disease be vaccinated against pneumococcal diseases? Expert Rev Respir Med 2009;3:585–96 [DOI] [PubMed] [Google Scholar]
- 84.World Health Organization Vaccines against influenza. WHO position paper—November 2012. Wkly Epidemiol Rec 2012;87:461–76 [PubMed] [Google Scholar]
- 85.World Health Organization 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008;83:373–84 [PubMed] [Google Scholar]
- 86.Advisory Committee on Immunization Practices Recommended adult immunization schedule: United States, 2009. Ann Intern Med 2009;150:40–4 [DOI] [PubMed] [Google Scholar]
- 87.Loeb M, McGeer A, McArthur M, et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. JAMA 1999;159:2058–64 [DOI] [PubMed] [Google Scholar]
- 88.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138–80 [DOI] [PubMed] [Google Scholar]
- 90.World Health Organization Pneumococcal vaccines. WHO position paper—2012. Wkly Epidemiol Rec 2012;87:129–44 [PubMed] [Google Scholar]
- 91.Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009;180:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008;1:CD000422.10.1002/14651858.CD000422.pub2 [DOI] [PubMed] [Google Scholar]
- 93.European Medicines Agency Prevenar 13. http://www.medicines.org.uk/emc/medicine/22689/SPC/Prevenar+13+suspension+for+injection/ (accessed 29 Jul 2013).
- 94.Hak E, Grobbee DE, Sanders EA, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008;66:378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.