Abstract
We have previously shown that in renal cortex, COX-2 expression is localized to macula densa and surrounding cortical thick ascending limb of Henle (cTALH). Dietary salt restriction increases local expression of COX-2, which mediates renin production and secretion. Given that decreased luminal chloride [Cl–] at the level of the macula densa increases renin production and secretion, we investigated the role of extracellular ion concentration on COX-2 expression. Quiescent rabbit cTALH cells were incubated in a physiological salt solution containing high or low levels of NaCl. Immunoreactive COX-2 expression increased significantly in the low NaCl solution. COX-2 expression also increased after administration of the Na+/K+/2Cl– cotransport inhibitor, bumetanide. Selective substitution of chloride led to increased COX-2 expression, whereas selective substitution of sodium had no effect. The p38 MAP kinase inhibitor PD169316 decreased low NaCl-induced COX-2 expression. Low-salt or low-chloride medium induced cultured cTALH to accumulate ≥ 3-fold higher levels of pp38, the activated (phosphorylated) form of p38; low-salt medium also increased pJNK and pERK levels. Feeding rats a low-salt diet for 14 days induced a significant increase in renal cortical pp38 expression, predominantly in the macula densa and cTALH. These results suggest that reduced extracellular chloride leads to increased COX-2 expression, which may be mediated by activation of a p38-dependent signaling pathway.
Introduction
In the mammalian kidney, the macula densa is involved in regulation of tubuloglomerular feedback and renin release by sensing alterations in luminal chloride (1–4). To our knowledge, Kotchen et al. were the first to consider the possibility that intraluminal chloride concentrations may be the proximate mediator of macula densa regulation of renin secretion (5), and subsequent studies strongly suggested a preeminent role for extracellular chloride in macula densa regulation of renin secretion (6–8). The use of the isolated perfused juxtaglomerular (JGA) preparation provided definitive confirmation of the role of alterations of luminal chloride in regulation of renin secretion (9). Ion substitution experiments of tubular perfusate demonstrated that substitution of other cations for sodium did not affect renin secretion, whereas substitution of other anions for chloride led to increased renin secretion (10). Increasing luminal NaCl from 25 to 80 mmol/L decreased renin secretion sixfold, whereas further increases had no effect on renin secretion, indicating the t1/2 for Cl– to be approximately 30 mmol (8).
Macula densa sensing of luminal chloride concentration is dependent on net apical transport, mediated by the luminal Na+/K+/2Cl– cotransport (11). The Na+/K+/2Cl– cotransporter possesses a high affinity for Na+ and K+, such that minimal alterations in transport occur with physiological changes of Na+ or K+ concentrations; however, the affinity for chloride is lower and falls within the range of loop chloride values, thereby resulting in an uptake mechanism that is very sensitive to any change in luminal chloride (12). The role of the Na+/K+/2Cl– cotransport in this macula densa sensing is further supported by the observation that loop diuretics, which inhibit Na+/K+/2Cl– cotransport, increase renin activity, even in the absence of volume depletion (8, 13, 14).
Studies in experimental animals and in humans have indicated that prostaglandins are important mediators of this macula densa–regulated renin release (15–18), and studies using the isolated perfused JGA preparation demonstrated that nonselective nonsteroidal antiinflammatory drugs (NSAIDs) prevented the increases in renin release mediated by macula densa sensing of decreases in luminal NaCl (19). NSAIDs inhibit the enzymatic activity of cyclooxygenases, which prevent conversion of arachidonic acid to prostaglandin G2 and thence to prostaglandin H2 (20). There are two separate gene products with cyclooxygenase activity, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). The gene for the “constitutive” cyclooxygenase, COX-1, encodes a 2.9-kb transcript, and the gene for COX-2, the “inducible” cyclooxygenase, encodes a 4.5-kb transcript and increases in response to inflammatory or mitogenic stimuli. In the kidney, COX-1 has been localized to mesangial cells, arteriolar endothelial cells, parietal epithelial cells of Bowman’s capsule, and cortical and medullary collecting ducts, but not to macula densa or cortical thick ascending limb of Henle (cTALH). In contrast, in all mammalian species examined to date (rat, mouse, dog, rabbit, human), there is localized and regulable COX-2 expression in macula densa cells and surrounding cTALH cells (21–25).
Conditions in which the macula densa has been shown to mediate renin expression and secretion, such as dietary salt deficiency, renovascular hypertension, or treatment with angiotensinogen-converting enzyme (ACE) inhibitors or with loop diuretics, all increase macula densa/cTALH COX-2 expression (21, 26–29), and COX-2–selective inhibitors blunt increases in renin in response to salt-deficient diets (30), ACE inhibition (26), and experimental renovascular hypertension (27). Direct evidence for a role of COX-2 in macula densa–mediated renin release has recently been provided by Traynor et al., who determined that in an isolated JGA preparation, increased renin release in response to lowering the perfusate NaCl concentration was blocked by a COX-2–selective inhibitor but not by a COX-1–selective inhibitor (31). Although these studies indicated that COX-2–derived prostaglandins are involved in macula densa–mediated renin release, the signals regulating COX-2 expression have not yet been determined. The present studies therefore examined whether extracellular ionic composition might influence COX-2 expression.
Methods
Materials.
Goat polyclonal anti-murine COX-2 antibody, mouse monoclonal anti-pp38, anti-pJNK, anti-pERK antibody, goat polyclonal anti-p38 antibody, and ATF-2 were from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Goat anti-human uromucoid (Tamm Horsfall) antibody was from ICN Biochemicals Inc. (Costa Mesa, California, USA). Anti-goat IgG (H+L) was from Vector Laboratories (Burlingame, California, USA), and biotin-labeled mouse anti-rabbit IgG (H+L) antibodies were from Pierce Chemical Co. (Rockford, Illinois, USA). PD98059, PD169316, H7, H8, and 2′, 5′-dideoxyadenosine were from Calbiochem-Novabiochem Corp. (La Jolla, California, USA). 32P-CTP (3,000 Ci/mmol), Enhanced Chemiluminescence Kit (ECL), and ECL Hyperfilm were from Amersham Life Sciences Inc. (Arlington Heights, Illinois, USA). BCA protein Assay Reagent kit and Immunopure ABC peroxidase staining kit were from Pierce Chemical Co. Other reagents were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA).
Animals.
Male Sprague-Dawley rats (Harlan Bioproducts for Science Inc., Indianapolis, Indiana, USA), initially weighing 250 g, either were maintained on normal rat chow or were given a single intraperitoneal dose of furosemide (1 mg/kg) and then placed on rat chow deficient in sodium (0.02–0.03% Na+) (ICN Biochemicals Inc.) for 2 weeks.
Primary culture of rabbit cTALH cells.
cTALH cells were isolated from homogenates of rabbit renal cortex by immunodissection with anti-Tamm Horsfall antibody, as described previously (26, 32–34). Briefly, the renal cortex was dissected, minced, and digested with 0.1% collagenase. After blocking with 10% BSA, the sieved homogenates were incubated with goat anti-human Tamm Horsfall antiserum (50 mg/mL) for 30 minutes on ice, followed by washing and addition to plastic Petri dishes coated with anti-goat IgG (8 mg/mL). Attached cells resistant to washing were dislodged and grown to confluence in DME/F12 with 10% FCS. Quiescent cTALH cells (grown overnight in the absence of FCS) were then exposed to the various solutions or agents for the indicated time before protein isolation.
p38 kinase activity assay.
The assay procedure was modified from methods published previously (35). Confluent quiescent cTALH cells were exposed to the indicated condition for 3 hours, then washed with ice-cold PBS and lysed with lysis buffer (20 mM Tris [pH 7.4], 137 mM NaCl, 2 mM EDTA, 1% Triton X-100, 25 mM glycerophosphate, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 10 glycerol, 1 mM PMSF, and 1 μg leupeptin). After brief centrifugation, an aliquot of the cell suspension was removed for protein measurement; subsequently, 150 μg of protein was incubated with anti-p38 and precipitated with protein agarose AG. The precipitates were washed five times with kinase buffer (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl, 2 mM DTT, 0.1 mM sodium vanadate, and 25 μM ATP), and dissolved with kinase buffer, followed by kinase reaction with ATF-2 (Santa Cruz Biotechnology Inc.) and 32P-ATP at 30° for 20 minutes. The kinase reaction was stopped by centrifugation at 12,000 g for 2 minutes and an equal volume sample buffer was added to the supernatant, boiled for 5 minutes, and separated by 10% SDS-PAGE. After drying the gel, it was exposed to Kodak X-Omat AR film (Eastman Kodak Co., Rochester, New York, USA) with an intensifying screen at –70° C.
Immunoblotting.
Cells or renal cortex was homogenized with RIPA buffer and centrifuged, an aliquot taken for protein measurement, and the rest heated to 100°C for 5 minutes with sample buffer. Renal cortical microsomes were isolated as described previously (21). Equal volumes of protein were separated on 8% (for COX-2) or 12% SDS gels under reducing conditions and transferred to Immobilon-P transfer membranes (Millipore, Bedford, Massachusetts, USA). The blots were blocked overnight with 100 mM Tris-HCl (pH 7.4) containing 5% nonfat dry milk, 3% albumin, and 0.5% Tween-20, followed by incubation for 16 hours with the primary antibody. The secondary biotinylated antibody was detected using avidin and biotinylated horseradish peroxidase (Pierce Chemical Co.) and exposed on film using ECL.
Immunohistochemistry.
Under deep anesthesia with pentobarbital (70 mg/kg intraperitoneally), rats were exsanguinated with 50 mL/100 g heparinized saline (0.9% NaCl, 2 U/mL heparin, 0.02% sodium nitrite) through a transcardial aortic cannula and fixed with glutaraldehyde-periodate acid saline (GPAS), as described previously (26). GPAS contains final concentrations of 2.5% glutaraldehyde, 0.01 M sodium metaperiodate, 0.04 M sodium phosphate, 1% acetic acid, and 0.1 M NaCl and provides excellent preservation of tissue structure and pp38 antigenicity. The fixed kidneys were dehydrated through a graded series of ethanols, embedded in paraffin, sectioned at 4-μm thickness, and mounted on glass slides. The first antibody (rabbit polyclonal anti-pp38 [New England Biolabs Inc., Beverly, Massachusetts, USA] or polyclonal rabbit anti-murine COX-2 antiserum [Cayman Chemical, Ann Arbor, Michigan, USA]) was localized using Vectastain ABC-Elite (Vector Laboratories) with diaminobenzidine as the chromogen, followed by a light counterstain with toluidine blue.
Statistical analysis.
All values are presented as mean ± SEM. ANOVA and Bonferroni t tests were used for statistical analysis, and differences were considered significant when P < 0.05.
Results
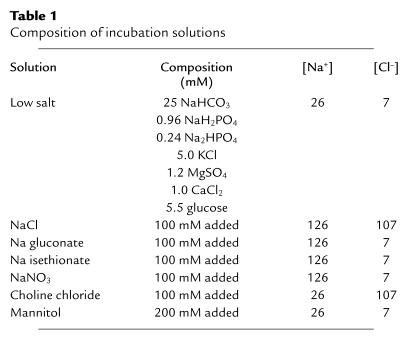
To determine the role of alterations in extracellular ions on expression of COX-2, quiescent cultured cTALH cells were incubated in solutions of varying ionic composition, indicated in Table 1. Immunoreactive COX-2 was expressed as the fold increase compared with quiescent control cells maintained in DME/F12.
Table 1.
Composition of incubation solutions
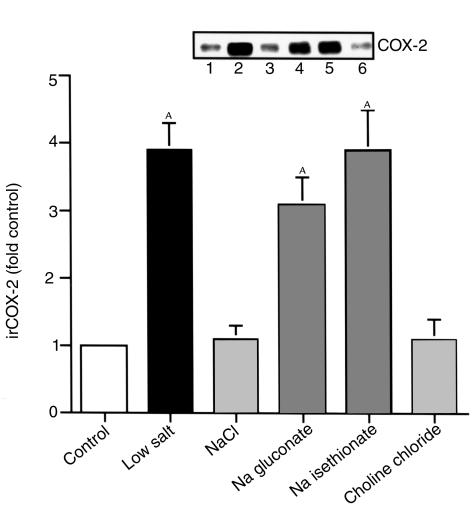
When cTALH were incubated in low-salt medium for 6 hours, immunoreactive COX-2 expression increased significantly (3.9 ± 0.4–fold control; n = 13; P < 0.01) (Figure 1). When the cells were incubated in the same solution in which 100 mM NaCl had been added (NaCl solution), COX-2 expression was not different than in cells incubated in DME/F12 (1.1 ± 0.2–fold control; n = 5). Similarly, when 100 mM choline chloride was added (final [Cl–] = 107 mM), COX-2 expression was not increased above base line (1.1 ± 0.3–fold control; n = 5). In contrast, when additional sodium was added with either Na gluconate or Na isethionate, COX-2 expression was significantly increased (3.1 ± 0.4– and 3.9 ± 0.6–fold control, respectively; n = 5; P < 0.01 compared with control) (Figure 1). Similar increases were seen with NaNO3 substitution (2.5 ± 0.15–fold control; n = 3; P < 0.02 compared with control). A time course of COX-2 expression after exposure of cTALH cells to low-salt solution indicated detectable increases in expression within 1 hour, an apparent peaking within 6 hours (3.9 ± 0.4–fold control), and increased expression for up to 16 hours (3.4 ± 0.5–fold control; P < 0.01) (Figure 2).
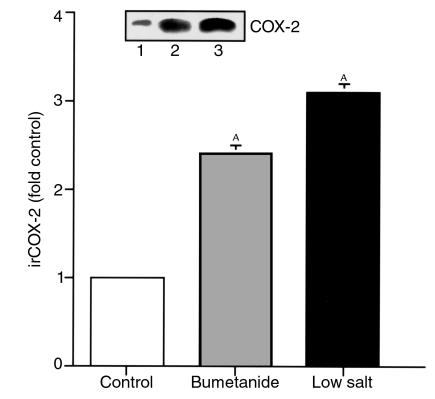
Figure 1.
Decreased extracellular [Cl–] increases immunoreactive COX-2 (irCOX-2) expression in cultured cTAL cells. Quiescent primary cultured rabbit cTAL cells were incubated with various media (described in Table 1) for 6 hours: control, low salt, NaCl, Na gluconate, Na isethionate, and choline chloride. AP < 0.01 compared with control. Inset: representative experiment.
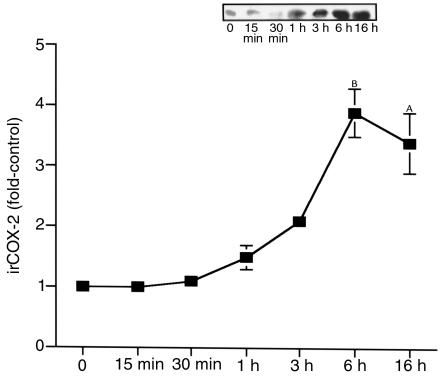
Figure 2.
Time course of COX-2 expression in cTAL induced by low-salt medium. Primary cultured cTAL cells were incubated in low-salt medium for the indicated time. (n = 4–13). AP < 0.05, BP < 0.01 compared with base line. Inset: representative experiment.
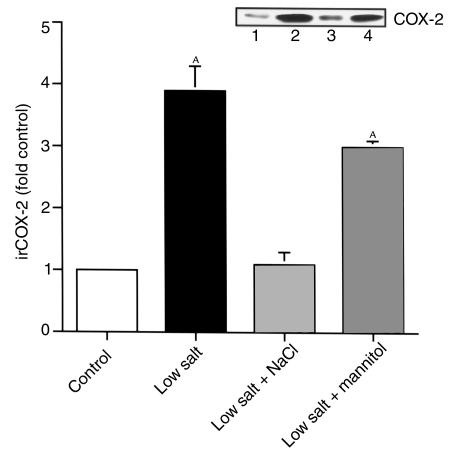
To investigate whether decreases in extracellular osmolarity were involved in the increased COX-2 expression in response to low extracellular NaCl, 200 mM mannitol was added to low-salt medium. Under these conditions, COX-2 expression was still significantly increased (3.0 ± 0.1–fold control; n = 3, P < 0.05) (Figure 3). cTALH cells exposed to bumetanide (50 μM) in the NaCl solution increased immunoreactive COX-2 expression significantly (2.4 ± 0.1–fold control; n = 4; P < 0.01 compared with control) (Figure 4).
Figure 3.
Effects of mannitol addition to low-salt medium. Lane 1, control; lane 2, low salt; lane 3, Low salt + NaCl; lane 4, low salt + 200 mM mannitol. AP < 0.01 compared with control. Inset: representative experiment.
Figure 4.
Bumetanide increases COX-2 expression in cultured cTAL. Lane 1, control; lane 2, bumetanide (50 μM) in NaCl solution; lane 3, low salt. AP < 0.01 compared with control. Inset: representative experiment.
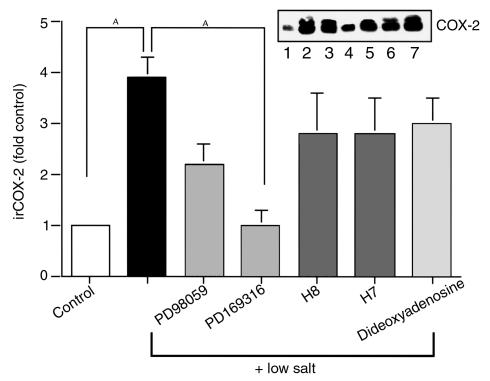
To identify possible signaling pathways involved in the induction of COX-2 expression by altered extracellular ionic content, cTALH cells were incubated in low-salt solution in the presence of kinase inhibitors. As indicated in Figure 5, incubation with the protein kinase C inhibitor, H7 (1 μM); the protein kinase A inhibitor, H8 (1 μM); or the adenylate cyclase inhibitor, dideoxyadenosine (100 μM), failed to attenuate significantly the elevated immunoreactive COX-2 expression (2.8 ± 0.7–, 2.8 ± 0.8–, and 3.0 ± 0.5–fold control, respectively; n = 5; P < 0.05 compared with control). In contrast, the p38 mitogen-activated protein kinase–specific (MAPK-specific) inhibitor, PD169316 (1 μM), significantly blocked COX-2 upregulation induced by low-salt medium (1.0 ± 0.3–fold control; n = 5; P < 0.05 compared with low salt only). The MEK1 inhibitor, PD98059 (1 μM), produced numerical but not statistically significant decreases in COX-2 expression (2.2 ± 0.4–fold control; n = 5; P = NS compared with low salt only).
Figure 5.
The p38 specific inhibitor, PD169316, prevents increases in the COX-2 expression in cTAL cells exposed to low-salt medium. Quiescent cTAL cells were maintained in DME/F12 medium (lane 1), incubated for 6 hours in low-salt medium alone (lane 2), or in low-salt medium in the presence of MEK1 inhibitor, PD98059 (1 μM) (lane 3); p38 MAP kinase inhibitor, PD169316 (1 μM) (lane 4); PKA kinase inhibitor, H8 (1 μM) (lane 5); PKC kinase inhibitor, H7 (1 μM) (lane 6); or adenylate cyclase inhibitor, dideoxyadenosine (100 μM) (lane 7) (n = 5). AP < 0.01 compared with control. Inset: representative experiment.
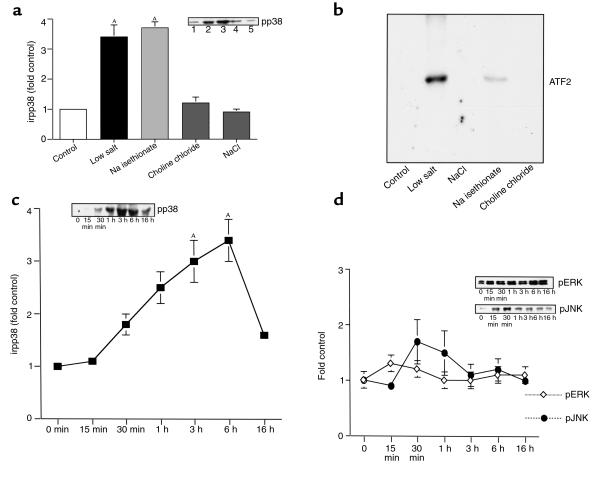
Immunostaining with an antibody that recognizes specifically the phosphorylated form of p38 indicated that pp38 expression increased significantly in cultured cTALH incubated for 6 hours in low-salt medium (3.4 ± 0.4–fold control; n = 8; P < 0.01 compared with control) (Figure 6a). There were no increases in COX-2 expression in cells incubated in NaCl or choline chloride solutions (0.9 ± 0.1– and 1.2 ± 0.2–fold control; n = 5; P = NS compared with control), but selective removal of extracellular chloride (Na iºsethionate) induced the same increase in pp38 expression as low salt (3.3 ± 0.2–fold control; n = 5; P < 0.01 compared with control). Total immunoreactive p38 expression was unchanged (data not shown). p38 activity in cultured cTALH was also measured using ATF-2 as a substrate. p38 activity was low in cTALH in DME/F12 or NaCl or choline chloride solutions but was increased when incubated in low-salt or Na isethionate solutions (Figure 6b).
Figure 6.
(a) Decreased extracellular [Cl–] increases pp38 expression in cultured cTAL cells. Cultured cTAL cells were incubated in the indicated solution for 6 hours. (lane 1) control; (lane 2) low salt; (lane 3) Na isethionate; (lane 4) choline chloride; (lane 5) NaCl. AP < 0.01 compared with control; n = 5. Inset: representative experiment. (b) Decreased extracellular [Cl-] increases p38 kinase activity in cTAL cells. cTAL cells were incubated in different media for 6 hours, and p38 kinase activity was measured as described in Methods, using ATF2 as substrate. (lane 1) control; (lane 2) low salt; (lane 3) NaCl; (lane 4) Na isethionate; and (lane 5) choline chloride. (c) Time course of pp38 expression in cTAL incubated in low-salt medium. Cells were incubated in low-salt medium 0–16 hours (n = 3). AP < 0.01 compared with base line. (d) Time course of pJNK and pERK expression in cTAL incubated in low-salt medium.
pp38 expression increased in response to low salt by 30 minutes, with a peak at 3–6 hours (3.0 ± 0.3–fold and 3.4 ± 0.4–fold, respectively; n = 3; P < 0.01 compared with control) (Figure 6c). Determination of the time course of expression of the activated forms of other MAPKs, activated jun kinase (pJNK) and activated ERK (pERK) indicated relatively small increases in expression above base line, with early and transient peak increases (pJNK: 1.7 ± 0.4–fold control at 30 minutes; pERK: 1.3 ± 0.1–fold control at 15 minutes; n = 3) (Figure 6d).
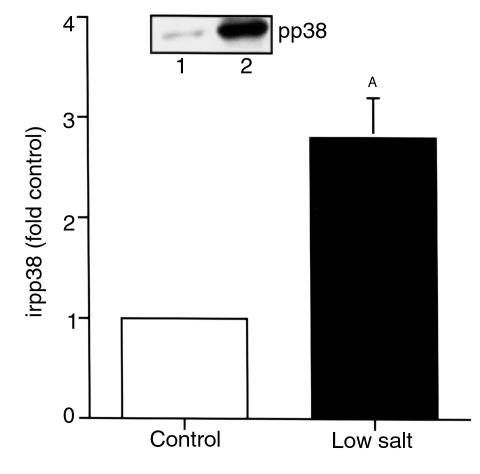
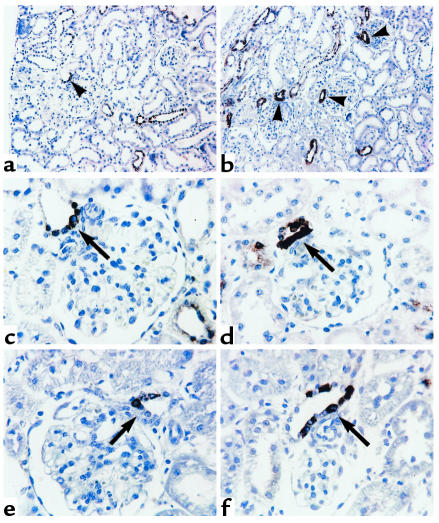
To determine whether alterations in p38 activity are observed in vivo in conditions in which macula densa/cTALH COX-2 expression is increased, rats were placed on a salt-deficient diet for 14 days. In renal cortex, a low-salt diet induced a significant increase in immunoreactive pp38 expression (3.9 ± 0.6; n = 3; P < 0.05 compared with control) (Figure 7). In control rat cortex, immunoreactive pp38 expression was predominantly localized to the macula densa and cTALH (Figure 8, a and c). The localization of pp38 expression was similar in the animals on a low-salt diet, but the intensity of expression was increased (Figure 8, b and d), consistent with the increases detected by immunoblotting and in a similar distribution as renal cortical COX-2 expression (Figure 8, e and f).
Figure 7.
Expression of pp38 in rat renal cortex in response to dietary salt restriction. Male Sprague-Dawley rats (250 g) were maintained on normal chow or a salt-deficient diet for 14 days. Renal cortical pp38 increased significantly in low-salt rats. AP < 0.05 compared with control; n = 3. Inset: representative experiment.
Figure 8.
Localization of pp38 in rat renal cortex in response to dietary salt. Immunoreactive pp38 expression was detected at low (×10) (a and b) and higher (×32) power (c and d) in rats maintained on normal chow (a, c, and e) or a salt-deficient diet for 14 days (b, d, and f). Note restriction of pp38 expression to cTALH and tubular expression in the animals on the salt-deficient diet (arrowheads in a and b). Low salt increased pp38 expression in macula densa and surrounding cTALH (arrows in c and d). For comparison, increased macula densa/cTALH COX-2 expression is presented (e and f). (Figure widths: a and b: 700 μm; c–f: 212 μm).
Discussion
Inhibition of renin expression and secretion after administration of COX-2–selective inhibitors suggests a direct role for COX-2–derived prostanoids in renin regulation. PGE2 and PGI2 have been shown to mediate renin secretion in in vitro preparations (15, 16, 18, 36) and in primary cultures of juxtaglomerular cells (37), and inhibition of prostaglandin production with selective COX-2 inhibitors inhibits renin production and secretion (26, 27, 30, 31). In vitro studies in cultured mesangial cells previously demonstrated the existence of a Ca2+-activated Cl– conductance and determined that decreased ambient Cl– attenuated the responsiveness of mesangial cells to vasoactive agonists such as angiotensin II and vasopressin and increased prostaglandin and nitric oxide production (38, 39). However, in normal kidney, extraglomerular mesangial cells do not express COX-2, making it less likely that these cells are the source of the renin-regulating prostanoids. In contrast, COX-2 expression in macula densa and surrounding cTALH increases significantly in high renin states (21, 26–29).
The increase in cTALH/macula densa COX-2 expression in high renin states suggested that the signals mediating increased renin, namely decreased luminal chloride, might also be involved in mediating increased COX-2 expression. In the present studies, decreasing extracellular NaCl or selectively decreasing extracellular Cl– stimulated COX-2 expression in cultured cTALH cells. Increased COX-2 expression was not secondary to alterations in extracellular osmolarity and could be mimicked by administration of bumetanide, suggesting that ion transport via Na+/K+/2Cl– cotransport was involved in regulating COX-2 expression.
Studies with kinase inhibitors indicated complete inhibition of the increased COX-2 expression in response to decreased extracellular NaCl with the p38 inhibitor, PD169316 (40, 41), suggesting a role for p38 in the COX-2 regulation. Our studies further determined that pp38 expression and p38 activity increased in cultured cTALH response to low-NaCl or low-chloride media, that pp38 was localized predominantly to cTALH and macula densa in vivo, and that expression increased in animals on a low-salt diet.
Although we did not observe significant increases in ERK phosphorylation, p98059 did partially inhibit increased COX-2 expression. PD98059 has been reported to be a specific inhibitor of MEK1, which selectively activates ERKs (42). The trend toward partial inhibition with PD98059 suggests either a role for ERK activation or the possibility that in the cultured cTALH cells, PD98059 may also inhibit kinases involved in p38 and/or JNK activation or have other, nonspecific effects (43). As noted, we also observed a transient early increase in pJNK, raising the possibility that JNK may also be involved in the initial signaling to increase COX-2 expression. The lack of specific inhibitors of the JNK pathway will necessitate alternative approaches, such as transfection with dominant negative mutations, to determine definitively the role of JNK in the low Cl–-induced activation.
The mechanism by which low ambient Cl– activates p38 and other MAPKs in cTALH and macula densa has not yet been determined. MAPKs are activated by sequential phosphorylation of an MEK activator and an MEK (MAPK activator), which activates MAPK by dual phosphorylation of tyrosine and threonine residues (44, 45). In general ERKs regulate cell growth and differentiation and are activated by mitogens, whereas JNK and p38 pathways participate in stress responses and are activated by cellular stresses such as ultraviolet light, x-rays, free radicals, heat and osmotic shock, and withdrawal of growth factors, as well as by inflammatory cytokines such as TNF and IL-1β. (46, 47). It is noteworthy that the yeast homologue of p38, HOG, is activated by hyperosmolarity, and in mammalian cells, p38 has been shown to be activated by both hypo- and hyperosmolarity (46–49). The present studies are the first to our knowledge to demonstrate that p38 can be activated in response to altered extracellular Cl–, even under isosmotic conditions. Increased p38 activity was observed when either relatively impermanent (isethianate, gluconate) or permeant (nitrate) anions were substituted for Cl–.
MAPKs have been demonstrated to increase COX-2 expression in other cell types, including activation by ERKs (50, 51), JNK (51–53), and p38 (50–52, 54–56). It has been suggested that MAPKs mediate COX-2 transcription via activation of a cyclic AMP–response element (CREB) in association with either C/EBP (57) or ATF1 sites (58). In addition, p38 has also been shown to increase COX-2 expression by posttranscriptional regulation (mRNA stabilization) (56). Preliminary studies in which cultured cTALH were transiently transfected with a construct containing the mouse COX-2 promoter coupled to GFP indicate increased COX-2 transcription in response to decreased extracellular NaCl (H.-F. Cheng and R.C. Harris, unpublished results), but further studies will be required to determine mechanisms of transcriptional activation and to explore whether posttranscriptional regulation of COX-2 is also involved. Another unanswered question requiring further investigation is whether expression of other proteins important for cTALH and macula densa function, such as nNOS and ion transporters, is also regulated by p38 and/or other MAPKs. It remains possible that the observed increases in COX-2 expression may result from increased production of other mediators of COX-2 activity, such as NO (34), in addition to or instead of direct alteration of COX-2 gene transcription by p38.
Immunoisolation of cTALH cells was originally described by Allen et al. (32) and has been used to attain primary cultured cells that retain characteristics of CTLAH in vivo (26, 33, 34, 59). We have previously determined that these cells express immunoreactive COX-2 (26) and nNOS (34); however, they also express Tamm Horsfall, which is characteristic of cTALH but not macula densa cells. Although both COX-2 and nNOS are expressed by macula densa in vivo (21, 60), the surrounding cTALH are also capable of expressing both enzymes, especially in high renin states (21, 61–63). It is noteworthy that under these conditions, COX-2 expression occurs at the distal end of the loop of Henle, at the point where luminal chloride concentration is lowest. Therefore, the in vitro observation that decreased extracellular Cl– induced increased expression of COX-2 in cTALH offers an explanation why COX-2 expression in vivo occurs at the macula densa and surrounding cTALH, the site of the thick limb with the lowest luminal NaCl concentration, and why macula densa and cTALH COX-2 expression increases with salt depletion and decreases with volume expansion.
In summary, these results suggest that alterations in extracellular chloride may increase COX-2 expression in macula densa and surrounding cTALH at least in part by increasing p38 activity. Increased COX-2 expression in these conditions may be one mechanism by which the macula densa regulates renin expression.
Acknowledgments
This work was supported by the Vanderbilt George O’Brien Kidney and Urologic Diseases Center (NIH grant DK-39261) and by funds from the Department of Veterans Affairs.
References
- 1.Persson AEG, et al. Macula densa function. Kidney Int. 1991;32(Suppl. 32):S39–S44. [PubMed] [Google Scholar]
- 2.Schlatter E, Salomonsson M, Persson AEG, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+2C1-K+ cotransport. Pflugers Arch. 1989;414:286–290. doi: 10.1007/BF00584628. [DOI] [PubMed] [Google Scholar]
- 3.Harris RC. The macula densa: recent developments. J Hypertens. 1996;14:815–822. doi: 10.1097/00004872-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 5.Kotchen TA, Galla JH, Luke RG. Failure of NaHCO3 and KHCO3 to inhibit renin in the rat. Am J Physiol. 1976;231:1050–1056. doi: 10.1152/ajplegacy.1976.231.4.1050. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 7.Scholz H, Gotz KH, Hamann M, Kurtz A. Differential effects of extracellular anions on renin secretion from isolated perfused rat kidneys. Am J Physiol. 1994;267:F1076–F1081. doi: 10.1152/ajprenal.1994.267.6.F1076. [DOI] [PubMed] [Google Scholar]
- 8.He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension. 1995;26:137–142. doi: 10.1161/01.hyp.26.1.137. [DOI] [PubMed] [Google Scholar]
- 9.Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science. 1987;237:1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Characterization of the macula densa stimulus for renin secretion. Am J Physiol. 1990;259:F186–F193. doi: 10.1152/ajprenal.1990.259.1.F186. [DOI] [PubMed] [Google Scholar]
- 11.Salomonsson M, Gonzalez E, Westerlund P, Persson AE. Chloride concentration in macula densa and cortical thick ascending limb cells. Kidney Int. 1991;32(Suppl.):S51–S54. [PubMed] [Google Scholar]
- 12.Greger R. How does the macula densa sense tubule function? Nephrol Dial Transplant. 1997;12:2215–2217. doi: 10.1093/ndt/12.11.2215. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Maldonado M, Gely R, Tapia E, Benabe JE. Role of macula densa in diuretics-induced renin release. Hypertension. 1990;16:261–268. doi: 10.1161/01.hyp.16.3.261. [DOI] [PubMed] [Google Scholar]
- 14.Modena B, et al. Furosemide stimulates renin expression in the kidneys of salt-supplemented rats. Pflugers Arch. 1993;424:403–409. doi: 10.1007/BF00374901. [DOI] [PubMed] [Google Scholar]
- 15.Francisco LJ, Osborn JL, DiBona GF. Prostaglandins in renin release during sodium deprivation. Am J Physiol. 1982;243:261–268. doi: 10.1152/ajprenal.1982.243.6.F537. [DOI] [PubMed] [Google Scholar]
- 16.Linas SL. Role of prostaglandins in renin secretion in the isolated kidney. Am J Physiol. 1984;246:F811–F818. doi: 10.1152/ajprenal.1984.246.6.F811. [DOI] [PubMed] [Google Scholar]
- 17.Frolich JC, et al. Suppression of plasma renin activity by indomethacin in man. Circ Res. 1976;39:447–452. doi: 10.1161/01.res.39.3.447. [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Carretero OA, Abe K, Beierwaltes WH, Yoshinaga K. Effect of prostanoids on renin release from rabbit afferent arterioles with and without macula densa. Kidney Int. 1989;35:1138–1144. doi: 10.1038/ki.1989.102. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SG, Lorenz JN, He XR, Schnermann JB, Briggs JP. Effect of prostaglandin synthesis inhibition on macula densa-stimulated renin secretion. Am J Physiol. 1993;265:F578–F583. doi: 10.1152/ajprenal.1993.265.4.F578. [DOI] [PubMed] [Google Scholar]
- 20.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 21.Harris RC, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komhoff M, Seyberth HW, Nusing RM, Breyer MD. Cyclooxygenase-2 expression is associated with the macula densa in kidneys from patients with Bartter like syndrome. J Am Soc Nephrol. 1999;10:437A. doi: 10.1046/j.1523-1755.2000.00425.x. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 23.Komhoff M, et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 2000;57:414–422. doi: 10.1046/j.1523-1755.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- 24.Guan Y, et al. Cloning, expression, and regulation of rabbit cyclooxygenase-2 in renal medullary interstitial cells. Am J Physiol. 1997;273:F18–F26. doi: 10.1152/ajprenal.1997.273.1.F18. [DOI] [PubMed] [Google Scholar]
- 25.Khan KN, et al. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol Pathol. 1998;26:612–620. doi: 10.1177/019262339802600504. [DOI] [PubMed] [Google Scholar]
- 26.Cheng HF, et al. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 28.Yang T, et al. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 29.Jensen BL, Kurtz A. Differential regulation of renal cyclooxygenase mRNA by dietary salt intake. Kidney Int. 1997;52:1242–1249. doi: 10.1038/ki.1997.449. [DOI] [PubMed] [Google Scholar]
- 30.Harding P, et al. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension. 1997;29:297–302. doi: 10.1161/01.hyp.29.1.297. [DOI] [PubMed] [Google Scholar]
- 31.Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol. 1999;277:F706–F710. doi: 10.1152/ajprenal.1999.277.5.F706. [DOI] [PubMed] [Google Scholar]
- 32.Allen ML, et al. Immunodissection of cortical and medullary thick ascending limb cells from rabbit kidney. Am J Physiol. 1988;255:F704–F710. doi: 10.1152/ajprenal.1988.255.4.F704. [DOI] [PubMed] [Google Scholar]
- 33.Dai LJ, Quamme GA. Intracellular Mg2+ and magnesium depletion in isolated renal thick ascending limb cells. J Clin Invest. 1991;88:1255–1264. doi: 10.1172/JCI115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H-F, Wang J-L, Zhang MZ, McKanna JA, Harris RC. Nitric oxide regulates renal cortical cyclooxygenase-2 expression. Am J Physiol Renal Physiol. 2000;279:F122–F129. doi: 10.1152/ajprenal.2000.279.1.F122. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh-Hamad D, et al. p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J Biol Chem. 1998;273:1832–1837. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 36.Whorton A, et al. Prostaglandins and renin release. I. Stimulation of renin release from rabbit renal cortical slices by PGI2. Prostaglandins. 1977;14:1095–1104. doi: 10.1016/0090-6980(77)90287-8. [DOI] [PubMed] [Google Scholar]
- 37.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol. 1996;271:F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 38.Okuda T, Naruse M, Kurokawa K. Mesangial cell function and chloride ions. Kidney Int. 1991;39(Suppl. 32):S65–S67. [PubMed] [Google Scholar]
- 39.Tsukahara H, Krivenko Y, Moore LC, Goligorsky MS. Decrease in ambient [Cl-] stimulates nitric oxide release from cultured rat mesangial cells. Am J Physiol. 1994;267:F190–F195. doi: 10.1152/ajprenal.1994.267.1.F190. [DOI] [PubMed] [Google Scholar]
- 40.Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, et al. p38 MAP kinase is required for vasopressin-stimulated HSP27 induction in aortic smooth muscle cells. Hypertension. 2000;35:673–678. doi: 10.1161/01.hyp.35.2.673. [DOI] [PubMed] [Google Scholar]
- 42.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 43.Lagaud GJ, Lam E, Lui A, van Breemen C, Laher I. Nonspecific inhibition of myogenic tone by PD98059, a MEK1 inhibitor, in rat middle cerebral arteries. Biochem Biophys Res Commun. 1999;257:523–527. doi: 10.1006/bbrc.1999.0350. [DOI] [PubMed] [Google Scholar]
- 44.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 46.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 47.English J, et al. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 48.Roger F, Martin PY, Rousselot M, Favre H, Feraille E. Cell shrinkage triggers the activation of mitogen-activated protein kinases by hypertonicity in the rat kidney medullary thick ascending limb of the Henle’s loop. Requirement of p38 kinase for the regulatory volume increase response. J Biol Chem. 1999;274:34103–34110. doi: 10.1074/jbc.274.48.34103. [DOI] [PubMed] [Google Scholar]
- 49.Watts BA, III, Di Mari JF, Davis RJ, Good DW. Hypertonicity activates MAP kinases and inhibits HCO-3 absorption via distinct pathways in thick ascending limb. Am J Physiol. 1998;275:F478–F486. doi: 10.1152/ajprenal.1998.275.4.F478. [DOI] [PubMed] [Google Scholar]
- 50.LaPointe MC, Isenovic E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 51.Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2. Evidence for involvement of extracellular signal-regulated kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- 52.Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1beta-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem. 1998;273:28670–28676. doi: 10.1074/jbc.273.44.28670. [DOI] [PubMed] [Google Scholar]
- 53.Xie W, Herschman HR. Transcriptional regulation of prostaglandin synthase 2 gene expression by platelet-derived growth factor and serum. J Biol Chem. 1996;271:31742–31748. doi: 10.1074/jbc.271.49.31742. [DOI] [PubMed] [Google Scholar]
- 54.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 55.Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol (Lond) 1999;520:399–406. doi: 10.1111/j.1469-7793.1999.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley SH, et al. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- 57.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 58.Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J Immunol. 2000;164:3018–3025. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- 59.Pizzonia JH, et al. Immunomagnetic separation, primary culture, and characterization of cortical thick ascending limb plus distal convoluted tubule cells from mouse kidney. In Vitro Cell Dev Biol. 1991;27A:409–416. doi: 10.1007/BF02630961. [DOI] [PubMed] [Google Scholar]
- 60.Wilcox CS, et al. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vio CP, Cespedes C, Gallardo P, Masferrer JL. Renal identification of cyclooxygenase-2 in a subset of thick ascending limb cells. Hypertension. 1997;30:687–692. doi: 10.1161/01.hyp.30.3.687. [DOI] [PubMed] [Google Scholar]
- 62.Bosse HM, Bohm R, Resch S, Bachmann S. Parallel regulation of constitutive NO synthase and renin at JGA of rat kidney under various stimuli. Am J Physiol. 1995;269:F793–F805. doi: 10.1152/ajprenal.1995.269.6.F793. [DOI] [PubMed] [Google Scholar]
- 63.Kihara M, et al. Expression of neuronal type nitric oxide synthase and renin in the juxtaglomerular apparatus of angiotensin type-1a receptor gene-knockout mice. Kidney Int. 1998;53:1585–1593. doi: 10.1046/j.1523-1755.1998.00904.x. [DOI] [PubMed] [Google Scholar]