Abstract
A general C–H functionalization method for the tagging of natural products and pharmaceuticals is described. An azide-containing sulfinate reagent allows the appendage of azidoalkyl chains onto heteroaromatics, the product of which can then be attached to a monoclonal antibody by a “click” reaction. This strategy expands the breadth of bioactive small molecules that can be linked to macromolecules in a manner that is beyond the scope of existing methods in bioconjugation to permit tagging of the “seemingly untaggable.”
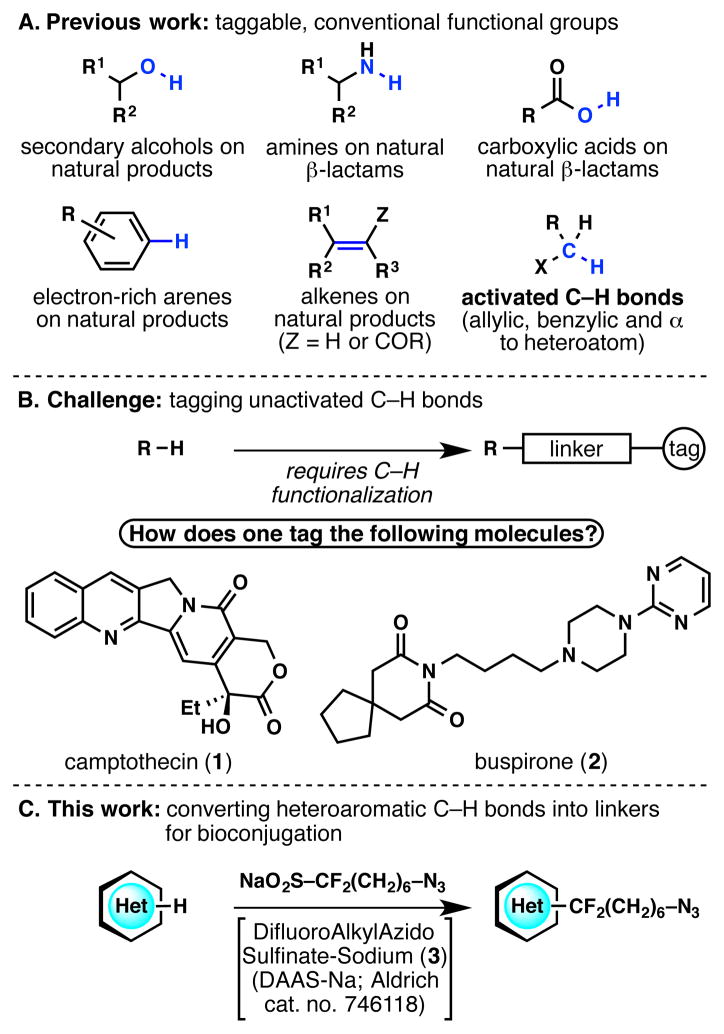
Determining the mode of action of medicinal compounds by bioconjugation is an essential tool in chemical biology.1 Bioconjugation requires a covalent and/or non-covalent linkage of the small-molecule drug with macromolecular tags including antibodies, nucleic acids, and receptor-binding proteins.2 This process of derivatization often involves “click” chemistry,3 with the most popular method arguably being the azide–alkyne cycloaddition:4 a functional group on the lead compound is attached to a linker with an alkyne (or alternatively, an azide), which is then reacted with a macromolecular tag with a pendant azide (or alternatively, an alkyne). Typically, only conventional functional groups can be tagged by linkers with “clickable” groups (Figure 1A),5a–5e but in a recent elegant example, Romo’s group has functionalized activated C–H bonds (allylic C–H, benzylic C–H and C–H α to a heteroatom) to tag natural products.5f Although many medicinal agents contain traditionally “taggable” functional groups such as heteroatom–H bonds5a–5c and π bonds,5e some compounds [such as camptothecin (1) and buspirone (2)] present the challenge of not having any apparent chemical handles (Figure 1B). Herein, we show the invention of a reagent that enables the tagging of unactivated C–H bonds in bioactive heteroarenes for use in bioconjugation. This powerful native chemical tagging technique takes place in water and in the absence of protecting groups.
Figure 1.
A. Literature precedent involving the tagging of conventional functional groups.5 B. Identification of a challenge: the tagging of unactivated C–H bonds. C. Designing an azide-containing fluoroalkylsulfinate salt that converts a heteroaromatic C–H bond into a linker for bioconjugation.
Our laboratory has been interested in assembling a toolkit of (fluoro)alkanesulfinate reagents for heteroaromatic systems.6 Such reagents form radicals in situ via an oxidative process, resulting in a formal C–H functionalization. For the purpose of bioconjugation, an alkyl linker bearing an azide moiety was desired. Considering the mild reaction conditions involved in this chemistry, it was conjectured that the relatively sensitive azide moiety would survive the heterocycle functionalization reaction. Thus, a sulfinate reagent bearing an azide moiety, sodium (difluoroalkylazido)sulfinate (DAAS-Na; 3), was designed (Figure 1C). The synthesis, reactivity and application of this reagent (which has been commercialized through Sigma–Aldrich) are described below.
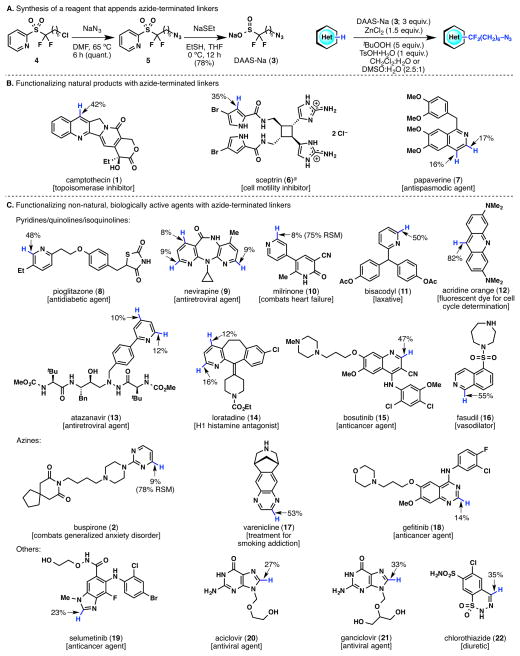
The synthesis of this reagent begins with chlorinated (fluoroalkylsulfonyl)pyridine 4, which was synthesized previously6c in 3 steps from commercially available 2-mercaptopyridine (Figure 2A). Displacement of the chloride with an azide, followed by removal of the pyridine moiety using sodium ethanethiolate, provided DAAS-Na (3) in 78% yield over two steps. Sodium sulfinate reagent 3 was then used in a heteroarene functionalization reaction that involves ZnCl2 and TsOH•H2O as acid additives and tBuOOH as an oxidant. Although various organic solvents have been used previously for sulfinate radical functionalizations,6b either CH2Cl2:H2O or DMSO:H2O (2.5:1) was sufficient for the reaction of DAAS-Na (3). These optimized reaction conditions were used throughout the examination of the substrate scope.
Figure 2.
A. Synthesis and general reaction of DAAS-Na (3). B. Formal C–H functionalization of complex natural products. C. Formal C–H functionalization of non-natural, biologically active agents that contain heteroaromatic moieties. RSM = Recovered starting material. aThe resulting product containing the azide linker has a carbonyl unit in lieu of the difluoromethyl group.
The strength of this chemistry lies in the ability to append azide-terminated linkers to heteroarene moieties in complex natural products bearing sensitive functional groups (Figure 2B). A representative natural product example is depicted with camptothecin (1). While the tertiary alcohol in 1 is likely to be too hindered for chemical derivatization, sulfinate 3 allows for the radical functionalization of the quinoline moiety to give the corresponding azido-containing product in 42% isolated yield. Sceptrin (6), a cell motility inhibitor7 that can be accessed by total synthesis,8 was also functionalized to give a single product in 35% yield. Papaverine (7), an antispasmodic agent, has two sites of reactivity, giving rise to products in 16 and 17% yields, respectively. Known pharmaceutical agents of varying complexity can be equally functionalized using this method (Figure 2C). A range of pyridine-containing drugs (8–16) can be functionalized at their C2 and/or C4 positions, which are the most susceptible sites for nucleophilic radical attack. Azine-based pharmaceuticals (2, 17, 18), and medicinal agents containing other heterocyclic cores (19–22) were also functionalized with DAAS-Na (3). It is notable that alcohol, amine, amide, amidine, hydrazide, sulfonamide, and alkene functional groups were tolerated in this reaction. The multiple sites of C–H activation for many of these compounds allow the exploration of structure-activity relationships for these molecules as well as the opportunity to evaluate optimal linker attachment with respect to bioactivity.
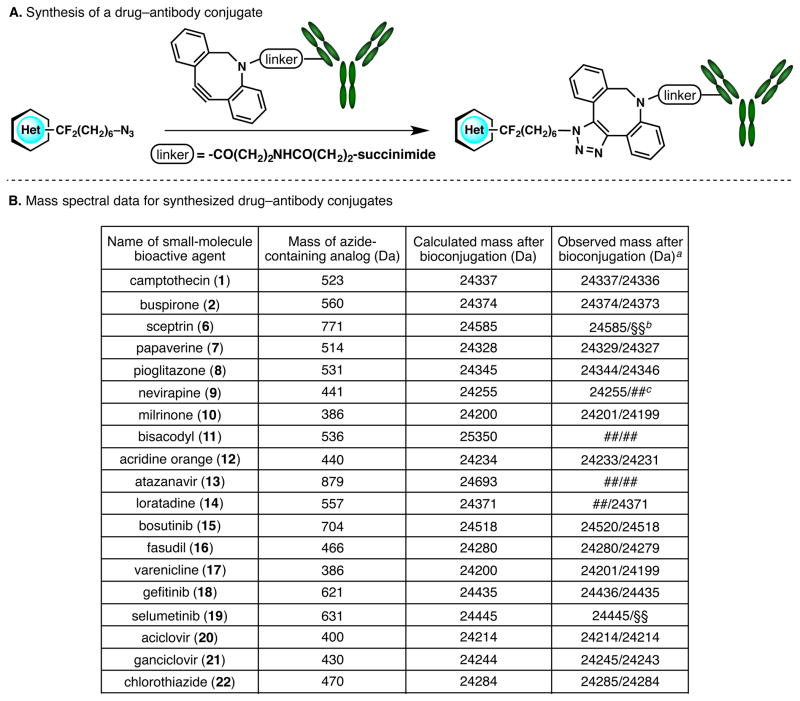
The main goal of this research program is realized by appending macromolecular tags to these bioactive, small-molecule agents. To this end, the synthesized azide-linked medicinal agents were reacted with a diben-zylazacyclooctyne-containing monoclonal antibody in a copper-free azide–alkyne cycloaddition (Figure 3A).9 Two methods of bioconjugation were employed, namely the conventional and high-throughput methods (see Supporting Information for details). The generated drug–antibody conjugates were verified by mass spectrometry, confirming the feasibility of this C–H functionalization approach to bioconjugation (Figure 3B). Future studies will examine whether antibody-drug conjugates generated using this strategy can exert cytotoxicity when armed with potent cytotoxic small molecules.
Figure 3.
A. Appending a monoclonal antibody to an azide-containing bioactive agent. B. Mass spectral data that verifies the formation of drug–antibody conjugates. aThe two indicated values refer to the mass spectral data arising from two different methods of conjugation (please see the Supporting Information for details); the slight differences in mass between the two methods are deconvolution artifacts. bThe symbol §§ indicates that the second method of bioconjugation was not conducted. cThe symbol ## indicates that the bioconjugation was unsuccessful.
Although this method of compound tagging through C–H functionalization extends the scope of existing methods in bioconjugation, it is not without its limitations. Reaction yields can be low and mixtures of regioi-someric products may be obtained, which may render the product purification process difficult. However, the mild (room temperature or 50 °C) and convenient (open air, aqueous solvent) reaction conditions as well as the one-step nature of this chemical tagging method outweigh the occasional drawbacks.
In summary, this work demonstrates an approach to the native chemical tagging of small molecules at seemingly inert C–H bonds. This method holds great potential for labeling and bioconjugation of molecules that do not present functional groups for conventional reactions. Studies demonstrating the enhanced efficacy of these medicinal agents will be reported in due course.
Supplementary Material
Acknowledgments
Financial support for this work was provided by NIH/NIGMS (GM-106210), Morphotek, Eisai, Inc. and SIOC (postdoctoral fellowship to Q. Z. and J. G.). The authors thank J. Eric Carlson for providing materials to demonstrate linker attachments, as well as Ryan Gianatassio, Alessandro Ruffoni, Darryl D. Dixon and Erik Daa Funder for technical contributions to the early stages of this project.
Footnotes
The authors declare no competing financial interest.
Supporting Information. Experimental procedures and analytical data (1H and 13C NMR, MS) for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For an early review in bioconjugation targeting monoclonal antibodies, see: Koppel GA. Bioconjugate Chem. 1990;1:13–23. doi: 10.1021/bc00001a002.For recent reviews in enzyme profiling using bioconjugated chemical probes, see: Evans MJ, Cravatt BF. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g.Carlson EE. ACS Chem Biol. 2010;5:639–653. doi: 10.1021/cb100105c.Böttcher T, Pitscheider M, Sieber SA. Angew Chem Int Ed. 2010;49:2680–2698. doi: 10.1002/anie.200905352.
- 2.For a definition of bioconjugate chemistry, see: Meares C. Bioconjugate Chem. 1990;1:1–1.
- 3.For a definition of “click” chemistry, see: Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.
- 4.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Peddibhotla S, Dang Y, Liu JO, Romo D. J Am Chem Soc. 2007;129:12222–12231. doi: 10.1021/ja0733686. [DOI] [PubMed] [Google Scholar]; (b) Chamni S, He QL, Dang Y, Bhat S, Liu JO, Romo D. ACS Chem Biol. 2011;6:1175–1181. doi: 10.1021/cb2002686. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Staub I, Sieber SA. J Am Chem Soc. 2008;130:13400–13409. doi: 10.1021/ja803349j. [DOI] [PubMed] [Google Scholar]; (d) Zhou CY, Li J, Peddibhotla S, Romo D. Org Lett. 2010;12:2104–2107. doi: 10.1021/ol100587j. [DOI] [PubMed] [Google Scholar]; (e) Robles O, Serna-Saldívar SO, Gutiérrez-Uribe JA, Romo D. Org Lett. 2012;14:1394–1397. doi: 10.1021/ol300105q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li J, Cisar JS, Zhou CY, Vera B, Williams H, Rodríguez AD, Cravatt BF, Romo D. Nat Chem. 2013;5:510–517. doi: 10.1038/nchem.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Fujiwara Y, Dixon JA, Rodriguez RA, Baxter RD, Dixon DD, Collins MR, Blackmond DG, Baran PS. J Am Chem Soc. 2012;134:1494–1497. doi: 10.1021/ja211422g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fujiwara Y, Dixon JA, O’Hara F, Funder ED, Dixon DD, Rodriguez RA, Baxter RD, Herlé B, Sach N, Collins MR, Ishihara Y, Baran PS. Nature. 2012;492:95–99. doi: 10.1038/nature11680. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhou Q, Ruffoni A, Gianatassio R, Fujiwara Y, Sella E, Shabat D, Baran PS. Angew Chem Int Ed. 2013;52:3949–3952. doi: 10.1002/anie.201300763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cipres A, O’Malley DP, Li K, Finlay D, Baran PS, Vuori K. ACS Chem Biol. 2010;5:195–202. doi: 10.1021/cb900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Baran PS, Zografos AL, O’Malley DP. J Am Chem Soc. 2004;126:3726–3727. doi: 10.1021/ja049648s. [DOI] [PubMed] [Google Scholar]; (b) Baran PS, Li K, O’Malley DP, Mitsos C. Angew Chem Int Ed. 2006;45:249–252. doi: 10.1002/anie.200503374. [DOI] [PubMed] [Google Scholar]; (c) O’Malley DP, Li K, Maue M, Zografos AL, Baran PS. J Am Chem Soc. 2007;129:4762–4775. doi: 10.1021/ja069035a. [DOI] [PubMed] [Google Scholar]
- 9.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.