Abstract
Marijuana users consistently demonstrate impairments in attention, executive function and response inhibition, which resemble deficits seen in attention deficit hyperactivity disorder (ADHD). We hypothesized that targeting the cognitive deficits associated with chronic marijuana use through ADHD medications may help identify a therapeutic agent for marijuana dependence.
Thirteen subjects participated in an 11-week open label study to determine the feasibility, safety and tolerability of atomoxetine for individuals seeking treatment for marijuana dependence. The Time-Line Follow-Back measured marijuana use 90 days prior to study entry (p-TLFB) and weekly during the study (s-TLFB) along with weekly qualitative urine drug screen (UDS). For the eight subjects who completed the trial, the TLFB data showed a trend toward reduction in use with an increase in percent days abstinent (p=0.06). Analysis of weekly UDSs did not confirm the TLFB trend with 94% of all possible UDSs positive for THC through out the study.
Marijuana dependent subjects taking atomoxetine experienced an inordinate number of gastrointestinal (GI) adverse events. Overall, 10 of 13 subjects (77%) experienced a mild to moderate GI adverse event defined as nausea, vomiting, dyspepsia, and loose stools. Atomoxetine is of limited utility in the treatment of cannabis dependence and is associated with clinically significant GI adverse events.
Keywords: Marijuana Dependence, Atomoxetine, ADHD
1.0 Introduction
Marijuana has long been the most commonly abused illicit drug in the United States (SAMHSA, 2005). Recent years have seen an increase in the number of medication trials for marijuana dependence. Thus far, clinical trials using psychotropic agents such as valproic acid and buspirone have been conducted with mixed results and no pharmacological intervention has demonstrated efficacy in reducing marijuana use (Levin et al., 2004; McRae et al., 2006). Human laboratory trials of the antidepressants nefazodone and bupropion for amelioration of marijuana withdrawal found nefazodone to be partially effective for control of marijuana withdrawal related anxiety and muscle pain while bupropion was ineffective, and aggravated marijuana withdrawal related mood symptoms (Haney et al., 2003; Haney et al., 2001). Dronabinol, a synthetic cannabinoid agonist, has been used in human laboratory and outpatient studies of chronic marijuana smokers and demonstrated significant improvements in marijuana withdrawal related measures of craving, insomnia, appetite changes and anxiety (Budney et al., 2007; Haney et al., 2004). The CB1 antagonist rimonabant (SR141716) has also been used in marijuana smokers and was found to partially block the physiological response to acute marijuana administration and also demonstrated significant reductions in visual analog measures of drug strength and intoxication (Huestis et al., 2001).
Marijuana users consistently demonstrate time and dose-dependent impairments in attention, memory, executive function and response inhibition that resemble deficits seen in people with attention deficit hyperactivity disorder (ADHD) (Klingberg et al., 2005; Krain and Castellanos, 2006; Pope et al., 1995; Ramaekers et al., 2006; Solowij et al., 2002). Targeting these deficits through ADHD medications may help identify a therapeutic agent for marijuana dependence. Recent publications on the pharmacotherapy of ADHD in adults and adolescents have advocated for the use of medications with low abuse potential (i.e. atomoxetine and bupropion) in persons with comorbid marijuana use (Kratochvil et al., 2006; Wilens, 2006). We hypothesized that the selective norepinephrine reuptake inhibitor atomoxetine may therefore be useful in the treatment of marijuana addicts due to its cognitive enhancement effects and low abuse liability.
2.0 Method
An 11-week open label study was conducted to determine the feasibility, safety and tolerability of atomoxetine for individuals seeking treatment for marijuana dependence. The trial was reviewed and approved by the University of Pennsylvania Institutional Review Board. Subjects were recruited via print ads in local media. All study visits were conducted by a study physician at the clinical research offices of the Center for Studies of Addiction. After complete description of the study to the subjects, written informed consent was obtained prior to study entry. All subjects stated their goal was complete abstinence from marijuana. All subjects met DSM-IV criteria for marijuana dependence. Subjects were excluded for other active DSM-IV mood, anxiety or psychotic disorders, other substance dependence (except nicotine), major medical problems, or a previous diagnosis of ADHD. Subjects received financial reimbursement for completion of weekly study assessments.
Subjects received a flexible dose of atomoxetine (from 25 to 80mg per day) depending upon individual tolerability. By comparison, the maximum recommended daily dose of atomoxetine in adults with ADHD is 100mg/day.. Informed consent and baseline assessments were collected during the first study visit (week 1a). Subjects then returned for a second visit within 48 hours to review laboratory results, a personal feedback report and receive their initial medication dose (week1b). All subjects were started on an initial dose of 25mg atomoxetine. Over the following 2 weeks, medication dose was titrated upward to a target dose of 80mg/day. At the end of study week 3, subjects were maintained on their maximum tolerated dose (25, 40 or 80mg/day). At each study week, subjects had a 15–20 minute visit with the study physician to review adverse events, medication adherence and promote complete abstinence from marijuana. The subjects remained on a stable dose of medication until study week 9 and were tapered off medication over study weeks 10 and 11.
Marijuana use at baseline and at weekly intervals was measured by Time-Line Follow-Back (TLFB, recorded as percent days of abstinence for the data analysis) and by weekly qualitative urine drug screens (UDS, at a standard cutoff of 50ng/dL) (Sobell and Sobell, 1992). No attempt was made to formally measure marijuana withdrawal. The Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) were obtained at baseline and weekly intervals (Beck et al., 1988; Beck and Rickels, 1974). The Addiction Severity Index 5th edition (ASI) was administered at baseline and at the end of study week 11(McLellan, 1992).
All demographic and descriptive statistics are presented as percentages and means (±SD). Differences between means of BDI, BAI and ASI were analyzed using two-tailed t-tests with SPSS version 14.0 for Windows (SPSS Inc., 2005). A random effects model ANOVA was used to assess change from baseline TLFB over time with subject as the random effect.
3.0 Results
During the eight week period of recruitment, twenty-one individuals signed informed consent for the trial and fourteen subjects started medication. One subject who started medication was excluded from the analysis because he had no marijuana use on weekly TLFB and negative UDSs at baseline and throughout the trial. Eight subjects (8/13) completed the eleven-week trial.
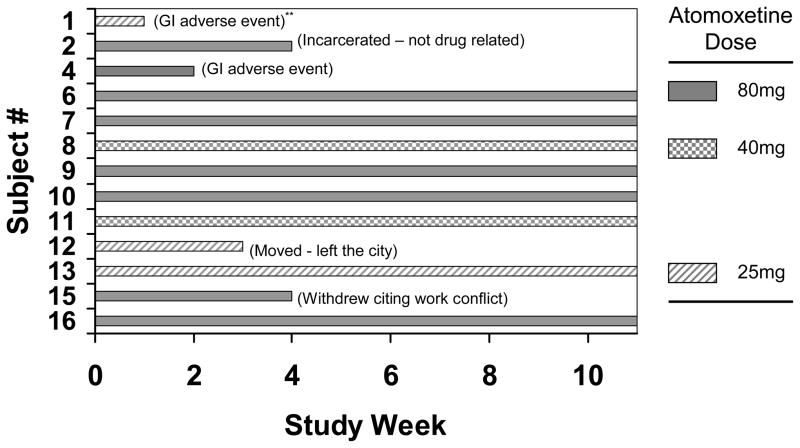
The subjects in the medication group were predominantly male (77%), average age 34.5 yrs (SD=11.3), and used marijuana sixteen (SD=11.8) out of the last 30 days. Figure 1 illustrates subject retention by week, reason for early withdrawal (in parenthesis), and final atomoxetine dose for each subject in the medication group. The mean final dose of atomoxetine was 62.3 mg per day (SD = 23.8).
Figure 1.
Subject retention by week for the 13 subjects who started medication. The reason for subject drop is in parenthesis by subject bar.
Mixed effects analysis of the TLFB data showed a trend toward reduction in use that was not statistically significant. Mixed effects ANOVA of the eight study completers found a numerical increase in proportion of days abstinent and a trend toward significance (p=0.06).
Using last observation carried forward to account for early subject withdrawal, analysis of weekly UDSs in the medication group did not confirm the TLFB trend. Ninety four percent of all possible UDSs (146 out of 156) were positive for THC over the 11 week trial. This discrepancy between UDS and TLFB may be explained in part by the long half-life of THC metabolites making UDSs an insensitive measure of reduction in MJ use. Despite no significant reduction in use, subjects experienced an approximate two-fold reduction in Beck Anxiety and Depression Inventory scores, and in ASI Family Problem Severity and Psychiatric Problem Severity composite scores (Table 1).
Table 1.
Comparison of mean baseline and end of study (EOS) ASI composite, BDI and BAI scores
| Variable (N=8) | Baseline (SD) | EOS (SD) | p value |
|---|---|---|---|
|
| |||
| ASI Composite | |||
|
| |||
| Medical | 0.21 (0.31) | 0.50 (0.34) | 0.15 |
| Family | 0.35 (0.3) | 0.18 (0.19) | 0.09 |
| Psychiatric | 0.29 (0.19) | 0.17 (0.19) | 0.2 |
| Drug | 0.19 (0.11) | 0.20 (0.08) | 0.8 |
| Alcohol | 0.13 (0.02) | 0.06 (0.09) | 0.16 |
| Employment | 0.73 (0.25) | 0.77 (0.23) | 0.40 |
| Legal | 0.02 (0.04) | 0.00 (0.00) | 0.18 |
|
| |||
| BDI | 12.6 (9.7) | 7.3 (4.4) | 0.107 |
|
| |||
| BAI | 13.3 (13.5) | 5.8 (8.9) | 0.71 |
Note: ASI = Addiction Severity Index; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; EOS = End of Study; SD= Standard Deviation
Of particular concern, marijuana dependent subjects taking atomoxetine experienced an inordinate number of gastrointestinal (GI) adverse events. Overall, 10 of 13 subjects (77%) experienced a mild to moderate GI adverse event defined as nausea, vomiting, dyspepsia, and loose stools. Two subjects (Fig. 1: subjects 1 and 4) withdrew from the trial at weeks 2 and 3 respectively citing discomfort from dyspepsia and loose stools as the reason for discontinuation. Only 7 of 13 subjects were able to tolerate the target dose of 80mg and only 2 of 13 subjects tolerated the target dose of 80mg with no reported AEs. Dry mouth was reported in 5 of 13 subjects. Medication related increase in anxiety was reported in 2 of 13 subjects. Other physical adverse events such as headache, insomnia, arthralgia and urogenital complaints occurred in 7 of 13 subjects. Along with self reports of adverse events, the mean ASI Medical Problem Severity composite score increased two-fold (Table 1), but was not significantly correlated with total dose of medication taken during the trial (Spearman’s rho = 0.335, p = 0.416, n=8).
4.0 Discussion
This open-label study suggests atomoxetine is of limited to no utility in the treatment of marijuana dependence and is associated with clinically significant GI adverse events. This is of particular relevance since recent clinical case and review articles in peer review publications have advocated for the use of atomoxetine for the treatment of ADHD in persons dependent on marijuana and other drugs of abuse (Kratochvil et al., 2006; Wilens, 2006). Taken with a prior negative study of bupropion for marijuana withdrawal, it would seem that non-stimulant cognitive and mood enhancing agents are not promising compounds for the treatment of marijuana dependence.
Although abstinence was the goal for all subjects entering the trial, the absence of additional psychosocial or behavioral interventions beyond a brief visit with a study physician may have contributed to the absence of a significant reduction in marijuana use. This is not surprising as previous psychosocial and behavioral intervention trials have demonstrated small to moderate effectiveness in achieving sustained abstinence from marijuana (Budney et al., 2000; Dennis et al., 2004; Stephens et al., 2000). Although, there is no control group for comparison, the lack of significant self-reported reduction in use and high percentage of THC positive urine throughout the trial is indicative of limited efficacy under control conditions unless coupled with a robust psychosocial or behavioral treatment.
This pilot study demonstrates a clear interest in receiving treatment for primary marijuana dependence in a large urban population and that subject recruitment and retention are adequate for conducting pharmacologic trials for marijuana dependence. On the other hand, the recruitment window of this trial was only eight weeks, so it is difficult to know if acceptable levels of recruitment can be maintained over an extended period of time. Approximately twenty-two percent of eligible participants started medication, which could signal ambivalence over the use of medications to control marijuana use. Although there was a demonstrable trend toward self reported reduction in marijuana use, sustained reduction in use was not observed or verified by UDS. The eight subjects who completed the trial did experience reductions in anxiety symptoms and improvement in family-social functioning as well as overall reduction in depressive symptoms compared to baseline.
The unexpectedly high rate of GI adverse events in these subjects is concerning. Since marijuana withdrawal was not formally measured in this study, it is certainly possible that subjects were experiencing symptoms of marijuana withdrawal (which include GI discomfort) during the trial when they tried to stop or cut down (Budney et al., 2004) Although marijuana use did not significantly decline during the trial, any attempt to cut down or stop marijuana use may have resulted in withdrawal symptoms. It is known that marijuana (through CB1 activity) can reduce gut motility and thereby prolong exposure of atomoxetine to gastrointestinal mucosa, possibly leading to GI irritation that could further compound withdrawal related GI symptoms. (Croci et al., 1998; Izzo et al., 2000).
The observation of significant GI adverse events may suggest a previously undescribed interaction between atomoxetine and cannabinoid metabolites or direct irritation of bowel lumen by atomoxetine resulting in unanticipated GI symptoms in chronic marijuana users. These findings warrant further investigation and vigilance on the part of clinicians using atomoxetine in patients with known or subclinical marijuana dependence and unexplained gastrointestinal symptoms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rickels K. Assessment of depression: the depression inventory. Modern problems of pharmacopsychiatry. 1974;7:151. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Croci T, Manara L, Aureggi G, Guagnini F, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Mukenge S, Ferla G. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br J Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials.[see comment] J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology (Berl) 2003;165:157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716.[see comment] Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Capasso F. Forgotten target for marijuana: the endocannabinoid system in the gut.[comment] Trends Pharmacol Sci. 2000;21:372–373. doi: 10.1016/s0165-6147(00)01543-1. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Wilens TE, Upadhyaya H. Pharmacological management of a youth with ADHD, marijuana use, and mood symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1138–1141. doi: 10.1097/01.chi.0000228126.34085.79. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McRae AL, Brady KT, Carter RE. Buspirone for treatment of marijuana dependence: a pilot study. Am J Addict. 2006;15:404. doi: 10.1080/10550490600860635. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber A, Yurgelun-Todd D. The residual neuropsychological Effects of cannabis: the current status of research. Drug Alcohol Depend. 1995;38:25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- SAMHSA. DHHS Publication No SMA 05-4062 NSDUH Series H-28. 2005. Results from the 2004 National Survey on Drug Use and Mental Health. [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen LA, editor. Measuring Alcohol Consumption. The Humana Press; 1992. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000:898–908. [PubMed] [Google Scholar]
- Wilens TE. Attention Deficit Hyperactivity Disorder and Substance Use Disorders. Am J Psychiatry. 2006;163:2059–2063. doi: 10.1176/ajp.2006.163.12.2059. [DOI] [PubMed] [Google Scholar]