Abstract
Long chain acyl-CoA's (LCACoA) are the activated form of long chain fatty acids and serve as key lipid metabolites. Excess accumulation of intracellular LCACoA, diacylglycerols (DAGs) and ceramides may create insulin resistance with respect to glucose metabolism. We present a new method to measure LCACoA concentrations and isotopic enrichment of palmitoyl-CoA ([U-13C]16-CoA) and oleoyl-CoA ([U-13C]18:1-CoA) using UPLC/MS/MS to quantitate 7 different LCACoA (C14-CoA, C16-CoA, C16:1-CoA, C18-CoA, C18:1-CoA, C18:2-CoA, C20-CoA). The molecules are separated on reverse-phase UPLC column using a binary gradient with ammonium hydroxide (NH4OH) in water and NH4OH in ACN. The LCACoA are quantified using selected reaction monitoring (SRM) on a triple quadrupole mass spectrometer in positive electrospray ionization (ESI) mode. All LCACoA ions except enriched palmitate and oleate were monitored as [M+2+H]+ and the [U13C]16-CoA and [U13C]18:1-CoA were monitored as [M+16+H]+ and [M+18+H]+ respectively. The method is simple, sensitive and efficient (run time as short as 5 min) and allowed us to measure the concentration and detect enrichment in intramyocellular [U13C]16-CoA and [U13C]18:1-CoA during a low dose intravenous infusion of [U13C]palmitate and [U13C]oleate in adults undergoing either a saline control experiment or an insulin/glucose infusion experiment. This technique should allow investigators to measure the trafficking of extracellular fatty acids to the intracellular LCACoA pool.
Keywords: ultra-pressure liquid chromatography, muscle, free fatty acids, tandem mass spectrometry, stable isotopes
Introduction
Circulating free fatty acids (FFA) must be converted to their active form – fatty acyl coenzyme A - after entering the cell in order to participate in further metabolic processes. Their production is catalyzed by the action of acyl-CoA synthetase (ACS) enzymes. Insulin resistance in muscle is associated with excess intramyocellular lipid accumulation,[1-5] suggesting that aberrant storage of fatty acids in these lipid intermediates plays a direct role in insulin resistance. The main groups of lipid that are considered to be responsible for induction of insulin resistance in skeletal muscle are: long-chain acyl-CoA's (LCACoA), diacylglycerols (DAG) and ceramide (Cer) [1] [2] [4] [6] [5] [7]. Moreover LCACoA are obligate substrates in the de novo synthesis both ceramide and DAG.
LCACoA have been shown to decrease insulin signaling by a number of mechanisms,[3] including inhibition of hexokinase,[8] pyruvate dehydrogenase and glycogen synthase activities.[9] LCACoA can also influence muscle glucose utilization through activation of PKC isoenzymes that promote serine/threonine phosphorylation of the insulin receptor or insulin receptor substrate 1 (IRS1).[7] The serine/threonine phosphorylation prevents the tyrosine phosphorylation of IRS1 that is necessary for the proper function of insulin signaling cascade. Moreover, palmitoyl-CoA has been shown to inhibit mitochondrial adenine nucleotide translocator (ANT), resulting in greater formation of reactive oxygen species [10].
Methods used to measure LCACoA concentrations include HPLC with UV or fluorometric detection,[11-18] GC-FID or GC-EI/MS[19, 20] (each requiring a time-consuming derivitization step), HPLC/MS[21] and LC/MS/MS with[22] or without[23-25] online solid-phase extraction. In addition to measuring concentration, however, it can be helpful to understand the relative contribution of circulating FFA to intracellular LCACoA content.[21]
By giving an intravenous infusion of a stable isotope FFA tracer and performing tissue biopsies it is possible to better understand the intracellular the fate of plasma FFA.[26-28] Sun et al[21] described an HPLC/MS method using selected ion monitoring (SIM) to measure tissue LCACoA concentration and [U13C]-palmitoyl-CoA enrichments are being monitored (∼ 1-2%). By employing UPLC using a more polar (C8) column, which utilizes a smaller particle size (∼1.7μm vs 3.0μm of conventional HPLC columns) we can improve peak shape and resolution, reduce signal to noise and achieve faster run times. Moreover, the triple quadrupole with selected reaction monitoring (SRM) improves selectivity and sensitivity compared to SIM. This is achieved by monitoring a fragment in the third quadrupole of the mass spectrometer which is derived from the molecular ion of the compound of interest by collision induced dissociation in the reaction cell (second quadrupole). A fragment for the U13C- tracer is produced in the same manner. These product ion fragments are specific to the compounds being measured and the chances of similar transitions occurring from other compounds are much reduced. This leads to higher specificity and lower baseline levels and thus ultimately greater signal-to-noise ratios. Herein we report a UPLC/MS/MS approach to measure intramyocellular LCACoA concentrations that can accurately measure much lower [U13C]-palmitoyl-CoA and [U13C]18:1-CoA enrichment, in the range of 0.01 – 0.05%. These low level enrichment measurements thus enable intravenous infusions of relatively small quantities of [U13C]-FFA to be utilized with the added advantage that a smaller level of albumin is required as a carrier. We believe that using a UPLC system with tandem mass spectrometer in SRM mode to measure concentration and enrichment offers significant advantages over current approaches.
Materials and Methods
Chemicals
Standards
Myristoyl coenzyme A (C14-CoA), palmitoyl coenzyme A (C16-CoA), palmitoleoyl coenzyme A (C16:1-CoA), stearoyl coenzyme A (C18-CoA), oleoyl coenzyme A (C18:1-CoA), linoleoyl coenzyme A (C18:2-CoA), arachidonoyl coenzyme A (C20-CoA) as well as internal standard – heptadecanoyl coenzyme A (C17-CoA) were purchased from Avanti Polar Lipids (Alabaster, Al, USA). [U13C]16-CoA and [U13C]18:1-CoA were obtained from Isotec. The HPLC grade Acetonitrile and HPLC grade water was obtained from Fisher Chemical (Pittsburg, PA). An ammonium hydroxide was obtained from Sigma (St. Louis, MO).
Experimental design, tissue homogenization and lipids extraction
These studies were designed to study the relationship between plasma FFA and intramyocellular lipids as well as the effects of insulin on this process. The studies were approved by the Mayo Clinic IRB and the participants signed an approved consent form. Six volunteers underwent measures of body fat and fat free mass (FFM) using dual energy x-ray absorptiometry and were subsequently admitted to the Mayo Clinical Studies Unit. Three of the volunteers underwent euglycemic, hyperinsulinemic clamp studies, 2 volunteers underwent saline control studies and 1 volunteer underwent both studies. The evening prior to the muscle biopsy study they received a standard evening meal at 1800 h and then remained fasting except for water until the completion of the studies. Before beginning the tracer infusions, baseline blood samples were collected for measurement of background plasma FFA enrichment, which was also used to estimate the background enrichment of intramyocellular fatty acids. An intravenous catheter was placed in a forearm vein and kept patent with a continuous infusion of 0.45 % NaCl. To study the relationship between plasma FFA and intramyocellular fatty acid metabolites we used a pulse-chase approach similar to that previously reported [29].
For the saline control studies we pre-labeled (pulsed) the imTG pool using a continuous infusion of albumin bound [U-13C]palmitate (6 nmol•kg FFM−1 • min−1) as described by Kanaley et al [27]. In brief, the infusion was initiated at 0300 h and continued until the first muscle biopsy at 0900 h, which we refer to as the “1st” biopsy. Blood samples were collected at 10 min intervals between 0830 and 0900 h using the heated hand vein technique from a retrograde intravenous catheter. The [U-13C]palmitate infusion was discontinued at 0900 h. At 0800 h, a continuous infusion of albumin bound [U-13C]oleate (6 nmol•kg FFM−1 • min−1) was started to act as the “chase” isotope and continued until 1400 h. Between 1330 and 1400 h blood samples were collected at 10 min intervals for measuring FFA enrichment. This was immediately followed by a second muscle biopsy taken from the contra-lateral leg that we refer to herein as the 2nd biopsy. Muscle biopsies were taken using sterile technique from the vastus lateralis under local anesthesia using a Bergstrom needle.
For the insulin clamp studies the same initial dose of [U-13C]palmitate was begun at 0300 h, but at 0800 h, the start of the insulin clamp (1.0 mU•kg FFM−1 • min−1), the palmitate infusion rate was decreased to (0.6 nmol•kg FFM−1 • min−1) in order to prevent the plasma palmitate enrichment from increasing beyond the ideal range for the mass spectrometer. The [U-13C]oleate infusion rate for the insulin clamp studies was 0.6 nmol•kg FFM−1 • min−1. During the insulin clamp blood samples were collected every 10 min to guide the glucose infusion rate needed to maintain euglycemia.
The volunteers remained in bed until the completion of the 2nd biopsy, but were asked to move both legs every 15 min to avoid complete immobility.
The muscle tissue (∼300 mg) was immediately washed of blood using an ice cold normal saline solution, dissected of all visible adipose tissue, further rinsed of lipid droplets, and saved immediately in liquid N2. Samples were stored at −80° C until analysis.
Materials
[U-13C]palmitate and [U-13C]oleate were obtained from Isotec (Miamisburg, OH), as were custom synthesized standards for [U-13C]palmitoyl-CoA and [U-13C]oleoyl-CoA. The palmitate and oleate tracers were prepared for intravenous infusion as a solution of 0.3 % albumin in 0.9 % NaCl.
Processing of muscle samples
The LCACoA tissue extraction was performed as previously described [21] with modifications as described below. The extraction procedure requires considerable care at each step because of unstable nature of LCACoA. Briefly, ∼40 mg of frozen muscle tissue is placed in 0.5 ml of freshly prepared 100 mM potassium phosphate monobasic (KH2PO4, pH 4.9) and 0.5 ml of ACN:2-propanol:methanol (3:1:1) with 20 ng of the heptadecanoyl CoA internal standard (IS). This sample is homogenized twice on ice using an Omni TH homogenizer. The homogenate is vortexed for 2 min, sonicated for 3 min and centrifuged at 16000 g at 4°C for 10 min. The supernatant is collected and the pellet again re-extracted using the same volumes of ACN:2-propanol:methanol (3:1:1). After a second centrifugation the two supernatants are combined and dried under nitrogen (N2). The dry extract is re-suspended in 50 μl of methanol:water 1:1 (v/v) and centrifuged at 14000 g for 10 min at 4°C. This supernatant is used for UPLC/MS/MS analysis.
The extraction efficiency can vary between LCACoA species depending upon chain length and the saturation of the fatty acid.[21, 25] To measure the efficiency of our modified method we performed two experiments. For the first experiment we used a known concentration standard mix solution: 100 ng of each of the following: C14-CoA, C16-CoA, C16:1-CoA, C18-CoA, C18:1-CoA, C18:2-CoA, C20-CoA in albumin and performed the extraction using both our modification and the previously described method used to measure concentration and enrichment.[21] For the second experiment we prepared a muscle homogenate depleted of LCACoA. This was achieved by keeping the muscle homogenate at room temperature for one day. We confirmed there were no detectable LCACoA in the homogenate using the LC/MS/MS system. We then added to the LCACoA –depleted muscle homogenate an LCACoA standard mix (the same concentration as above mentioned) and performed the extractions using the same approach as for the albumin LCACoA solution. Using this modified approach the LCACoA recovery was 70-80 % for all the LCACoA that we measured, which provides some advantages over approaches that have larger variations in efficiency between shorter chain/unsaturated vs. longer chain/saturated LCACoA.
Preparation of calibration standards
The IS solution was prepared in the methanol:water (1:1). In each 10 μl of the solution was 50 ng of C17-CoA. The standard curves were prepared in the range from 1.56 -100 ng per 50μl. The stock solution of LCACoA standards for standard curve preparation was dissolved in methanol:water (1:1). Each 10 μl of this stock contained 200 ng of C14-CoA, C16-CoA, C16:1-CoA, C18-CoA, C18:1-CoA, C18:2-CoA, C20-CoA. After preparation of the standard curve, 10 μl of ISTD solution was added to each aliquot and all aliquots underwent the extraction procedure.
UPLC/MS/MS condition
The UPLC/MS/MS analysis was performed using Waters ACQUITY UPLC system connected to Thermo TSQ Quantum Ultra mass spectrometer (Waltham, MA).
UPLC separation
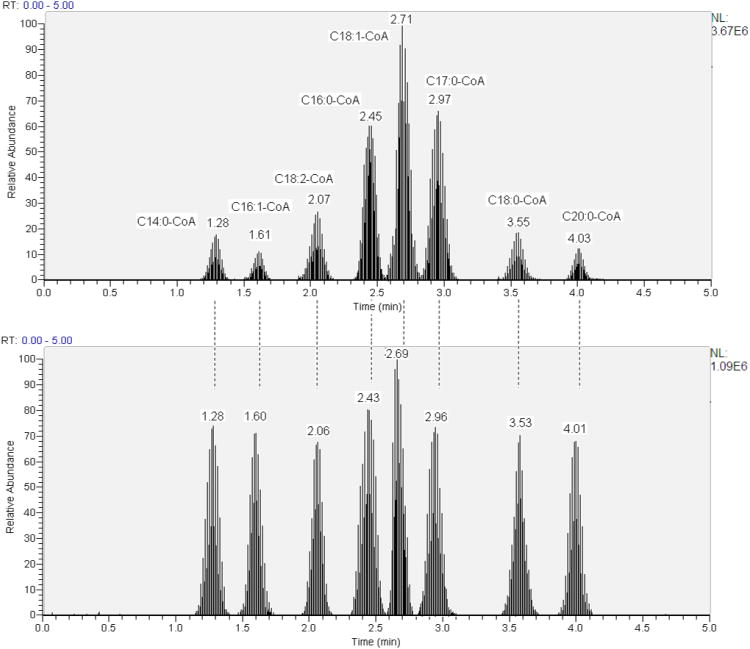
The chromatographic conditions were optimized for the separation of LCACoA in a standard solution using a reverse-phase Acquity 1.7 μm C8 UPLC BEH analytical column 2.1 × 150 mm (Waters, Milford, MA). The chromatographic separation was achieved using a binary gradient with 15 mM ammonium hydroxide (NH4OH) in water (solvent A) and 15 mM NH4OH in ACN (solvent B) at the flow rate 0.4 ml/min. The gradient started at 20 % B, increasing to 45 % B over 2.8 min, then decreased to 25 % B over 0.2 min, followed by an increase to 65 % B over 1 min and finally a decrease to 20 % B over 0.5 min. The column temperature was set at 35°C and auto sampler temperature was 4°C. We diverted the flow to waste for the first 30 s and the last 50 s to prevent early and late eluting impurities from entering the mass spectrometer. Figure 1 shows the total ion chromatogram of LCACoA standards (lower panel) and lipid extracts from skeletal muscle (upper panel). The 8 LCACoA species showed good peak shape and resolution. The UPLC system enables measurement of the LCACoA in only 5 minutes.
Figure 1.
Upper panel - total ion chromatogram (TIC) of LCACoA extract from skeletal muscle; lower panel - total ion chromatogram of LCACoA standards using LC-ESI-MS/MS. The dashed lines between the upper and lower panel indicate the peaks containing the same compounds are the same for both the standards and muscle extract.
Mass spectrometry analytical condition
To establish the optimal parameters for MS and MS/MS, mixtures of LCACoA standards (0.5 ng/μl each in methanol:water) were infused directly to mass spectrometer. The positive full scan spectrum allowed us to identify individual [M+H]+ molecular ions for each LCACoA species. Moreover we established the collision energy and collision gas pressure for individual LCACoA species that allow us to analyze the acyl-pantetheine fragment [23], of LCACoA as the product ion, which contains the fatty acid necessary for enrichment measurement. To achieve our goal of measuring the LCACoA concentrations and isotopic enrichment in one run we monitored the [M+2+H]+ rather than [M+H]+ for all LCACoA species except [U13C]16-CoA and [U13C]18:1-CoA. This tactic increases the relative abundance of [M+16+H]+ and [M+18+H]+ species, allowing accurate measures of enrichment at much lower levels than possible using the [M+H]+ species [30-32]. We used the electrospray ionization (ESI) source in positive electrospray mode to achieve ionization. The parameters for the source were as follows: spray voltage at 3.5 kV, sheath gas at 45 a.u., sweep gas at 2 a.u. and capillary temperature 275°C, the collision energy was the same for all compounds − 30 eV and the collision gas pressure was 1.2 mTorr. The precursor and product ions masses we monitored are provided in Table 1.
Table 1.
Quantitative parameters for LCACoA analysis.
| Compound | Enrichment and concentration | |
|---|---|---|
| Precursor ion | Product ion | |
| C14-CoA | 978.6 | 471.3 |
| C16-CoA | 1006.6 | 499.3 |
| C16:1-CoA | 1004.6 | 497.2 |
| C17-CoA | 1020.6 | 513.3 |
| C18-CoA | 1034.6 | 527.3 |
| C18:1-CoA | 1032.6 | 525.5 |
| C18:2-CoA | 1030.6 | 523.1 |
| C20-CoA | 1062.6 | 555.6 |
| [U13C]16-CoA | 1022.6 | 515.6 |
| [U13C]18:1-CoA | 1050.5 | 543.4 |
C14-CoA – myristoyl coenzyme A, C16-CoA – palmitoyl coenzyme A, C16:1-CoA – palmitoleoyl coenzyme A, C18-CoA – stearoyl coenzyme A, C18:1-CoA – oleoyl coenzyme A, C18:2-CoA – linoleoyl coenzyme A, C20-CoA – arachidonoyl coenzyme A, C17-CoA – heptadecanoyl coenzyme A (internal standard). Collision energy and collision gas pressure was 30 eV and 1.2 mTorr respectively.
Results and discussion
The aim of the present study was to develop a quantitative UPLC/MS/MS method for simultaneous analysis of tissue LCACoA concentration and relatively low levels of [U13C]16-CoA and [U13C]18:1-CoA enrichment. We built upon the only previously published method for measuring LCACoA concentrations and isotopic enrichment using LC/MS in SIM mode [21]. In order to minimize impact of sample impurities and further decrease baseline noise we used the SRM mode. To permit accurate measures of low enrichment levels while simultaneously measuring concentration, we employed an approach that monitors the [M+2+H]+ (the protonated isotopomer 2 mass units greater than the molecular ion), rather than [M+H]+ of the LCACoA. This tactic increases the relative abundance of [M+16+H]+ and [M+18+H]+ species of palmitoyl-CoA and oleoyl-CoA, respectively, similar to the approach used to measure low levels of enrichment in amino acids [30-32], plasma FFA during a low dose [U-13C]palmitate infusion [33] and intracellular long-chain acyl-carnitine[27,34] enrichments during low dose [U-13C]FFA infusions.
Measurement of LCACoA concentrations
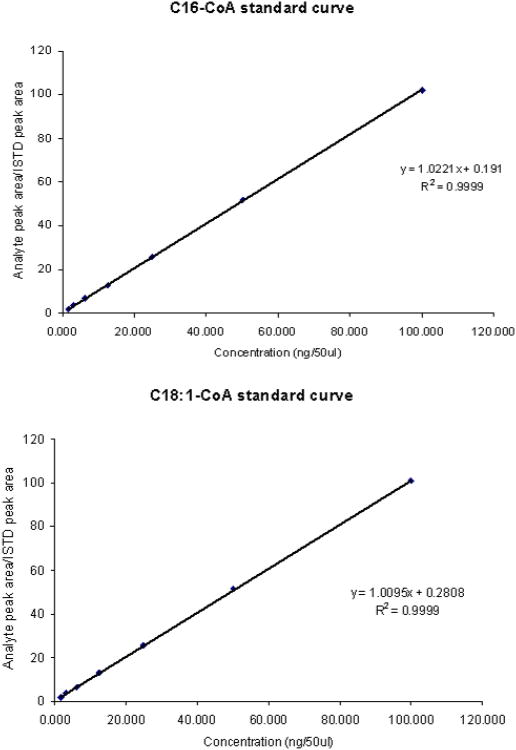
The concentration of each LCACoA species was measured against a seven point standard curve in a range for each compound from 1.56 ng to 100 ng per 50 μl. The concentrations of individual LCACoA were calculated based on the peak area ratio of peak of interest and the IS peak. Figure 2 shows the standard curve of C16-CoA and C18:1-CoA. The measured peak area ratio and theoretical concentration ratio of each LCACoA to IS showed excellent linear relationship (Figure 2). The R2 for each of individual LCACoA standard curves was > 0.999.
Figure 2.
Standard curves of C16-CoA and C18:1-CoA concentration obtained on UPLC/MS/MS.
The validation of the reproducibility of current method was performed using human skeletal muscle samples (40 mg). Twelve different samples were taken for extraction and each of the samples was run on UPLC/MS/MS system (Table 2). The results were obtained with three replicate human muscle samples analyzed over 3 days. The concentrations for all long-chain fatty acyl-CoA's showed good sample-to-sample and day-to-day reproducibility. The concentration results showed very good analytical precision for the seven individual fatty acyl-CoA esters analyzed. The inter-assay CV was 5-6 % for the concentration of each component. The intra-assay CV ranged from an average of 5 % for C18:1-CoA to 10 % for C16:1-CoA. These results demonstrate the reliability of this method to determine the concentration of fatty acyl-CoA species in biological samples. Of interest, the concentrations of the long-chain fatty acyl-CoA were not dramatically different between 1st and 2nd biopsies or between insulin clamp and saline control conditions. This is surprising because hyperinsulinemia dramatically suppresses plasma FFA concentrations, which we anticipated would lower intramyocellular LCACoA concentrations. The ability to measure the enrichment in both plasma and intramyocellular LCACoA should help us understand the relative contribution of plasma FFA vs. non-plasma sources, such as intramyocellular triglycerides, to LCACoA.
Table 2.
Long-chain acyl-CoA concentrations in human skeletal muscle.
| Analyte (ng/10mg muscle) | 1st biopsy/2nd biopsy | Saline control/Insulin clamp | C14-CoA | C16:1-CoA | C18:2-CoA | C16-CoA | C18:1-CoA | C18-CoA | C20-CoA | Total LCACoA |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | 1st | Saline control | 3.7 | 4.7 | 9.6 | 6.4 | 12.5 | 2.2 | 3.7 | 42.6 |
| Subject 2 | 1st | Saline control | 2.9 | 2.9 | 8.2 | 5.3 | 10.3 | 2.4 | 2.9 | 34.8 |
| Subject 3 | 1st | Saline control | 3.9 | 3.3 | 9.7 | 6.3 | 11.8 | 3.4 | 3.9 | 42.2 |
| Average | 3.5 | 3.6 | 9.2 | 6.0 | 11.5 | 2.7 | 3.5 | 39.9 | ||
| Subject 4 | 1st | Insulin clamp | 3.3 | 4.2 | 8.6 | 5.5 | 15.3 | 2.3 | 3.3 | 42.5 |
| Subject 5 | 1st | Insulin clamp | 2.8 | 3.8 | 7.5 | 5.0 | 11.4 | 2.2 | 2.8 | 35.4 |
| Subject 6 | 1st | Insulin clamp | 3.3 | 2.7 | 9.4 | 6.7 | 11.3 | 3.0 | 3.3 | 39.6 |
| Average | 3.1 | 3.6 | 8.5 | 5.7 | 12.7 | 2.5 | 3.1 | 39.2 | ||
| Subject 1 | 2nd | Saline control | 3.5 | 4.8 | 9.5 | 6.1 | 13.1 | 2.5 | 3.4 | 42.9 |
| Subject 3 | 2nd | Saline control | 3.3 | 3.2 | 9.6 | 5.8 | 12.4 | 3.7 | 3.3 | 41.2 |
| Average | 3.6 | 3.6 | 9.2 | 6.2 | 12.4 | 3.1 | 3.6 | 41.7 | ||
| Subject 2 | 2nd | Insulin clamp | 3.1 | 3.2 | 8.6 | 5.9 | 10.5 | 2.0 | 3.1 | 36.3 |
| Subject 4 | 2nd | Insulin clamp | 4.0 | 4.2 | 8.9 | 5.3 | 14.9 | 3.1 | 4.0 | 44.4 |
| Subject 5 | 2nd | Insulin clamp | 3.1 | 3.3 | 8.1 | 5.3 | 12.2 | 2.3 | 3.1 | 37.3 |
| Subject 6 | 2nd | Insulin clamp | 4.1 | 3.1 | 9.1 | 7.0 | 11.8 | 3.4 | 4.1 | 42.5 |
| Average | 3.6 | 3.4 | 8.7 | 5.9 | 12.3 | 2.7 | 3.6 | 40.1 | ||
For a description of the experimental design see the text. 1st biopsy refers to the muscle biopsy performed in the morning after a 6 hour [U-13C]palmitate and 1 hour [U-13C]oleate intravenous infusion; the 2nd biopsy refers to the muscle biopsy performed 6 h after beginning the infusion of [U-13C]oleate (5 h after discontinuing the [U-13C]palmitate). Insulin clamp refers to the intravenous infusion of insulin while maintaining plasma glucose concentrations in the normal range with intravenous glucose; saline control refers to the control condition for comparison with the insulin clamp. C14-CoA – myristoyl coenzyme A, C16-CoA – palmitoyl coenzyme A, C16:1-CoA – palmitoleoyl coenzyme A, C18-CoA – stearoyl coenzyme A, C18:1-CoA – oleoyl coenzyme A, C18:2-CoA – linoleoyl coenzyme A, C20-CoA – arachidonoyl coenzyme A.
LCACoA isotopic enrichment measurement
To prepare a standard enrichment curve for [U13C]16-CoA and [U13C]18:1-CoA, we first calculated the isotope purity of the standard using regression analysis. The calculated purity of [U13C]16-CoA and [U13C]18:1-CoA standards were 87.5 % and 85.65 % respectively.
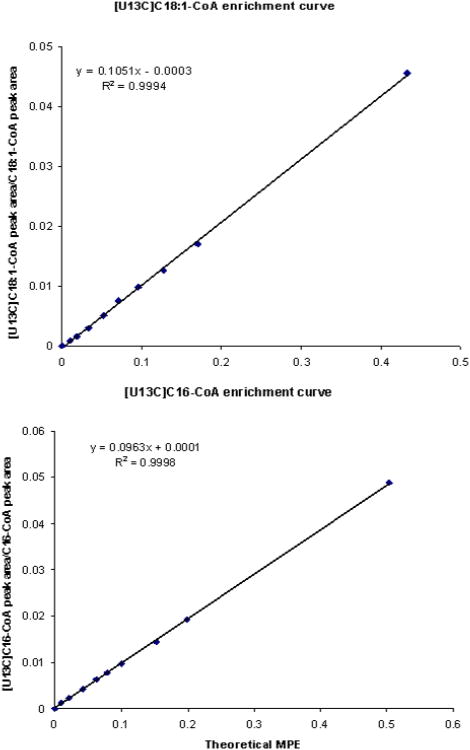
Ten point standard enrichment curves (Figure 3) were prepared by mixing constant amount of unlabeled standards and different amounts of labeled standards for each point. The standard samples contain [U-13C]16-CoA or [U13C]18:1-CoA and C16-CoA or C18:-CoA in the mole percentage excess (MPE) range from 0.01 % to 0.5 %. The calculated [U13C]16-CoA/C16-CoA and [U13C]18:1-CoA/C18:1-CoA peak area ratio and theoretical MPE of [13C]16-CoA and [U13C]18:1-CoA were taken to construct the enrichment standard curve. We found excellent linear relationships with R2 of 0.9997 and 0.9994 for palmitoyl-CoA and oleoyl-CoA respectively.
Figure 3.
Standard curve of [13C]16-CoA and [13C]18:1-CoA enrichment obtained on UPLC/MS/MS.
To measure the enrichment in biological samples, human skeletal muscle samples were taken 6 hours after beginning the continuous infusion of [U-13C]palmitate (1 hour after beginning the [U-13C]oleate) and again six hours after beginning the [U-13C]oleate (5 hours after discontinuing the [U-13C]palmitate infusion). The intra-assay and inter-assay CV's for [U13C]16-CoA enrichment averaged 5% and 6%, respectively (range 2-8%). The intra-assay and inter-assay CV's for [U13C]18:1-CoA enrichment also averaged 5% and 6%, respectively (range 2-9%).
[U13C]-palmitate and [U13C]oleate enrichment in different lipid classes (plasma FFA, muscle acyl-CoA, muscle acyl-carnitine and muscle TAG) are provided in Table 3. Plasma [U13C]-palmitate and [U13C]oleate enrichment was measured according to Persson et al.[33] Muscle [U13C]16-carnitine and [U13C]18:1-carnitine enrichment was measured by LC/MS/MS.[27] Enrichment in muscle triacylglycerol fraction coming from [U13C]-palmitate and [U13C]-oleate was quantified as previously described.[26] When examining the samples obtained after 6 h of [U13C]-palmitate (1st biopsy) and 6 h of [U13C]oleate (2nd biopsy), we found that the enrichment in intramyocellular palmitoyl-CoA (M+16) and oleyl-CoA (M+18) was 5-10 times that in the corresponding intramyocellular triglyceride and 13-92 % that of plasma FFA enrichment. Of note, in the saline control condition the intramyocellular palmitoyl-CoA (M+16) enrichment was ∼20% of plasma enrichment at the time of the 1st biopsy (after 6.5 h of [U13C]palmitate infusion) while the oleoyl-CoA (M+18) enrichment, after only 1 h of [U13C]oleate infusion, was ∼28% that of plasma oleate. This implies that only one forth of intramyocellular LCACoA originate from plasma FFA under overnight postabsorptive conditions, and that the equilibration between plasma and intramyocellular LCACoA is relatively fast. In contrast, under insulin clamp conditions, the enrichment of intramyocellular palmitoyl-CoA (M+16) and oleoyl-CoA (M+18) were 45-60% that of plasma palmitate and oleate enrichment. The contribution of non-plasma FFA sources to the intramyocellular LCACoA pool appears to be reduced under hyperinsulinemic conditions.
Table 3.
Plasma and intramyocellular fatty acid compound enrichment.
| 1st biopsy/2nd biopsy | Saline control/Insulin clamp | Plasma [U13C] 16:0 | Muscle [U13C]16-CoA | Muscle [U13C]16-carmitine | Muscle [U13C]16:0TAG | |
|---|---|---|---|---|---|---|
| Subject 1 | 1st | Saline | 0.459 | 0.093 | 0.032 | 0.0219 |
| Subject 2 | 1st | Saline | 0.641 | 0.083 | 0.034 | 0.0296 |
| Subject 3 | 1st | Saline | 0.324 | 0.079 | 0.017 | 0.0189 |
| Subject 4 | 1st | Clamp | 0.188 | 0.076 | 0.018 | 0.017 |
| Subject 5 | 1st | Clamp | 0.189 | 0.068 | 0.012 | 0.017 |
| Subject 6 | 1st | Clamp | 0.149 | 0.082 | 0.014 | 0.024 |
| Subject 1 | 2nd | Saline | 0.03 | 0.023 | 0.02 | 0.021 |
| Subject 3 | 2nd | Saline | 0.02 | 0.025 | 0.01 | 0.022 |
| Subject 2 | 2nd | Clamp | 0.02 | 0.018 | 0.02 | 0.024 |
| Subject 4 | 2nd | Clamp | 0.024 | 0.018 | 0.022 | 0.022 |
| Subject 5 | 2nd | Clamp | 0.030 | 0.013 | 0.011 | 0.018 |
| Subject 6 | 2nd | Clamp | 0.040 | 0.023 | 0.014 | 0.024 |
| 1st biopsy/2nd biopsy | Saline control/Insulin clamp | Plasma [U13C] 18:1 | Muscle [U13C]18:1 -CoA | Muscle [U13C]18:1 -carmitine | Muscle [U13C]18:1 TAG | |
| Subject 1 | 1st | Saline | 0.273 | 0.075 | 0.000 | 0.010 |
| Subject 2 | 1st | Saline | 0.386 | 0.126 | 0.001 | 0.012 |
| Subject 3 | 1st | Saline | 0.156 | 0.039 | 0.003 | 0.011 |
| Subject 5 | 1st | Clamp | 0.167 | 0.062 | 0.001 | 0.008 |
| Subject 6 | 1st | Clamp | 0.089 | 0.048 | 0.001 | 0.008 |
| Subject 1 | 2nd | Saline | 0.31 | 0.109 | 0.014 | 0.012 |
| Subject 3 | 2nd | Saline | 0.126 | 0.022 | 0.020 | 0.0237 |
| Subject 2 | 2nd | Clamp | 0.17 | 0.157 | 0.019 | 0.0269 |
| Subject 4 | 2nd | Clamp | 0.084 | 0.040 | 0.010 | 0.006 |
| Subject 5 | 2nd | Clamp | 0.424 | 0.156 | 0.010 | 0.010 |
| Subject 6 | 2nd | Clamp | 0.142 | 0.096 | 0.010 | 0.008 |
For a description of the experimental design see the text. 1st biopsy refers to the muscle biopsy performed in the morning after a 6 hour [U-13C]palmitate and 1 hour [U-13C]oleate intravenous infusion; the 2nd biopsy refers to the muscle biopsy performed 6 h after beginning the infusion of [U-13C]oleate (5 h after discontinuing the [U-13C]palmitate). Insulin clamp refers to the intravenous infusion of insulin while maintaining plasma glucose concentrations in the normal range with intravenous glucose; saline control refers to the control condition for comparison with the insulin clamp. [U13C]palmitate and [U13C]oleate enrichment and [U13C]16-CoA, [U13C]18:1-CoA, [U13C]16-carmitine, [U13C]18:1-carmitine, TAG [U13C]palmitate and [U13C]oleate fractions enrichment in human skeletal muscle after [U13C]palmitate and [U13C]oleate infusion.
Conclusion
We describe a new UPLC/MS/MS method for simultaneous measurement of intramyocellular LCACoA concentrations and [U-13C]16-CoA and [U13C]18:1-CoA enrichment. We detected enrichment in both compounds during an intravenous infusion of low doses of [U-13C]palmitate and [U-13C]oleate. The method is a robust, rapid, and reliable. Our method use ultra performance liquid chromatography - electrospray ionization, tandem quadrupole mass spectrometry in the SRM mode to minimize impact of sample impurities and decrease baseline noise. Monitoring the [M+2+H]+ species permits accurate concentration measurement and simultaneously improves the sensitivity of enrichment measurement of [M+16+H]+ and [M+18+H]+ species. The ability to examine the inter-relationships between enrichments in plasma FFA and intracellular fatty acid pools (triglycerides, long chain acyl-carnitines and LCACoA) should greatly advance our understanding of cellular fatty acid trafficking in health and disease.
Acknowledgments
This work was supported by 1 UL1 RR024150 from the National Center for Research Resources (NCRR), by grants DK40484, DK45343, and DK50456 from the U.S. Public Health Service, 7-07-DCS-03 from the American Diabetes Association, and by the Mayo Foundation. Dr. Blachnio-Zabielska was supported by an educational grant from sanofi-aventis. Our thanks to Mai Persson and Charles Ford for helpful suggestions in method development.
Abbreviations
- LCACoA
long chain acyl-coenzyme A
- DAG
diacylglycerol
- UPLC
ultra performance liquid chromatograph
- SRM
selected reaction monitoring
- ESI
electrospray ionization
- FFA
free fatty acids
- ACS
acyl-CoA synthetase
- Cer
ceramides
- IRS1
insulin receptor substrate 1
- HPLC
high performance liquid chromatography
- GC
gas chromatography
- MS
mass spectrometer
- SIM
selected ion monitoring
- UPLC
ultra performance liquid chromatography
References
- 1.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab. 2000;279:E554. doi: 10.1152/ajpendo.2000.279.3.E554. [DOI] [PubMed] [Google Scholar]
- 3.Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW. Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol. 1999;277:E1070. doi: 10.1152/ajpendo.1999.277.6.E1070. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz-Peiffer C. Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Annal N Y Acad Sci. 2002;967:146. doi: 10.1111/j.1749-6632.2002.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 5.Turinsky J, O'Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880. [PubMed] [Google Scholar]
- 6.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 7.Yu Ch, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 8.Thompson AL, Cooney GJ. Acyl-CoA inhibition of hexokinase in rat and human skeletal muscle is a potential mechanism of lipid-induced insulin resistance. Diabetes. 2000;49:1761. doi: 10.2337/diabetes.49.11.1761. [DOI] [PubMed] [Google Scholar]
- 9.Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997;323:1. doi: 10.1042/bj3230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciapaite J, Bakker SJL, Diamant M, van Eikenhorst G, Heine RJ, Westerhoff HV, Krab K. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects. Implications for a mechanism linking obesity and type 2 diabetes. FEBS J. 2006;273:5288. doi: 10.1111/j.1742-4658.2006.05523.x. [DOI] [PubMed] [Google Scholar]
- 11.Baker FC, Schooley DA. Analysis and purification of acyl coenzyme A thioesters by reversed-phase ion-pair liquid chromatography. Anal Biochem. 1979;94:417. doi: 10.1016/0003-2697(79)90384-1. [DOI] [PubMed] [Google Scholar]
- 12.Corkey BE. Analysis of acyl-coenzyme A esters in biological samples. Methods Enzymol. 1988;166:55. doi: 10.1016/s0076-6879(88)66011-3. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain Acyl-coenzyme A Esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- 14.Larson TR, Graham IA. Application of a new method for the sensitive detection and quantification of acyl-CoA esters in Arabidopsis thaliana seedlings and mature leaves. Biochem Soc Trans. 2000;28:575. [PubMed] [Google Scholar]
- 15.Larson TR, Graham IA. Technical Advance: a novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 2001;25:115. doi: 10.1046/j.1365-313x.2001.00929.x. [DOI] [PubMed] [Google Scholar]
- 16.Mangino MJ, Zografakis J, Murphy MK, Anderson CB. Improved and simplified tissue extraction method for quantitating long-chain acyl-coenzyme A thioesters with picomolar detection using high-performance liquid chromatography. J Chromatogr. 1992;A. 577:157. doi: 10.1016/0378-4347(92)80612-t. [DOI] [PubMed] [Google Scholar]
- 17.Rosendal J, Knudsen J. A fast and versatile method for extraction and quantitation of long-chain acyl-CoA esters from tissue: content of individual long-chain acyl-CoA esters in various tissues from fed rat. Anal Biochem. 1992;207:63. doi: 10.1016/0003-2697(92)90500-7. [DOI] [PubMed] [Google Scholar]
- 18.Woldegiorgis G, Spennetta T, Corkey BE, Williamson JR, Shrago E. Extraction of tissue long-chain acyl-CoA esters and measurement by reverse-phase high-performance liquid chromatography. Anal Biochem. 1985;150:8. doi: 10.1016/0003-2697(85)90434-8. [DOI] [PubMed] [Google Scholar]
- 19.Kopka J, Ohlrogge JB, Jaworski JG. Analysis of in vivo levels of acyl-thioesters with gas chromatography/mass spectrometry of the butylamide derivative. Anal Biochem. 1995;224:51. doi: 10.1006/abio.1995.1007. [DOI] [PubMed] [Google Scholar]
- 20.Prasad MR, Sauter J, Lands WE. Quantitative determination of acyl chain composition of subnanomole amounts of cellular long-chain acyl-coenzyme A esters. Anal Biochem. 1987;162:202. doi: 10.1016/0003-2697(87)90028-5. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, Cree MG, Wolfe RR. Quantification of the concentration and 13C tracer enrichment of long-chain fatty acyl-coenzyme A in muscle by liquid chromatography/mass spectrometry. Anal Biochem. 2006;349:87. doi: 10.1016/j.ab.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Magnes C, Suppan M, Pieber TR, Moustafa T, Trauner M, Haemmerle G, Sinner FM. Validated comprehensive analytical method for quantification of coenzyme A activated compounds in biological tissues by online solid-phase extrction LC/MS/MS. Anal Chem. 2008;80:5736. doi: 10.1021/ac800031u. [DOI] [PubMed] [Google Scholar]
- 23.Magnes C, Sinner FM, Regittnig W, Pieber TR. LC/MS/MS method for quantitative determination of long-chain fatty acyl-CoAs. Anal Chem. 2005;77:2889. doi: 10.1021/ac048314i. [DOI] [PubMed] [Google Scholar]
- 24.Mauriala T, Herzig KH, Heinonen M, Idziak J, Auriola S. Determination of long-chain fatty acid acyl-coenzyme A compounds using liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;808:263. doi: 10.1016/j.jchromb.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Haynes CA, Allegood JC, Sims K, Wang EW, Sullards MC, Merrill AH., Jr Quantitation of fatty acyl-coenzyme As in mammalian cells by liquid chromatography-electrospray ionization tandem mass spectrometry. J Lipid Res. 2008;49:1113. doi: 10.1194/jlr.D800001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Jensen MD. Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol. 1998;84:1674. doi: 10.1152/jappl.1998.84.5.1674. [DOI] [PubMed] [Google Scholar]
- 27.Kanaley JA, Shadid S, Sheehan MT, Guo ZK, Jensen MD. Relationship between plasma FFA, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol. 2009;587:5939. doi: 10.1113/jphysiol.2009.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacchetti M, Saltin B, Olsen DB, van Hall G. High triacylglycerol turnover rate in human skeletal muscle. J Physiol. 2004;561:883. doi: 10.1113/jphysiol.2004.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol. 2000;89:2057. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- 30.Slater C, Preston T, McMillan DC, Stuart Falconer J, Fearon CH. GC/MS analysis of [2H5]phenylalanine at very low enrichment: measurement of protein synthesis in health and disease. J Mass Spectrom. 1995;30:1325. [Google Scholar]
- 31.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 32.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Comm Mass Spectrom. 1992;6:421. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 33.Persson XMT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761. doi: 10.1194/jlr.M008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo ZK, Yarasheski K, Jensen MD. High precision isotopic analysis of palmitoylcarnitine by liquid chromatography/electrospray ion-trap tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3365. doi: 10.1002/rcm.2753. [DOI] [PubMed] [Google Scholar]