Abstract
Pulmonary fibrosis is characterized by epithelial cell injury, accumulation of myofibroblasts, and excessive deposition of collagen and other extracellular matrix elements, leading to loss of pulmonary function. Studies in both humans and animal models strongly suggest that TGF-β1 plays a pivotal role in the pathogenesis of pulmonary fibrosis. This review will first give an overview of TGF-β signaling and the effects of its inhibition on lung fibrogenesis. This overview includes information on TGF-β signal transduction pathways, the importance of TGF-β in the accumulation of myofibroblasts, the role of TGF-β in epithelial injury and apoptosis, the role of TGF-β in extracellular matrix remodeling, and the effects of inhibiting TGF-β signaling in animal models of lung fibrosis. Subsequently this review will highlight recent advances in two areas of particular interest to our research group: (1) TGF-β and proteoglycans; (2) TGF-β and histone deacetylases. Although our understanding of the role of TGF-β and its mechanisms of action in lung fibrogenesis has increased dramatically in recent years, there is still much to be learned about this important molecule, especially how TGF-β function is modulated in vivo, and its complex interactions with other factors expressed during lung injury and repair. Research in these areas will help identify novel therapeutic targets for the treatment of pulmonary fibrosis that will hopefully improve the prognosis of this devastating illness.
Keywords: TGF-β, pulmonary fibrosis, myofibroblasts, epithelial-mesenchymal transition, apoptosis, integrin, reactive oxygen species, proteoglycan, sulf, histone deacetylase
1. INTRODUCTION
Pulmonary fibrosis is the final pathway of many interstitial lung diseases, including those induced by autoimmune diseases, exposure to organic and inorganic dusts, and radiation or chemotherapy [1]. However, the cause of one of the most common form of pulmonary fibrotic conditions, idiopathic pulmonary fibrosis (IPF), is still not clear. Pulmonary fibrosis is characterized by injury and loss of lung epithelial cells, abnormal accumulation of myofibroblasts, and excessive deposition of collagen and other extracellular matrix (ECM) elements, resulting in a progressive loss of pulmonary function. The prognosis of IPF is poor with a median survival of 3 years after diagnosis, and currently there are no effective medicinal therapies [2, 3].
The role of TGF-β in the development of lung fibrosis was first recognized 20 years ago [4]. TGF-β is one of the most potent inducers of ECM production, including collagen and other matrix proteins. TGF-β expression is elevated in both animal models of lung fibrosis and in fibrotic human lungs. In animal models of lung fibrosis, elevation of TGF-β expression precedes collagen synthesis and deposition [5–7]. The pro-fibrotic role of TGF-β in lung fibrosis was confirmed by the finding that adenoviral-mediated gene transfer of active TGF-β1 was sufficient to induce severe fibrosis in rodent lungs [8, 9] and further supported by observations in a transgenic mouse model that over-expresses TGF-β1 using a lung specific promoter [10]. In addition, deficiency in Smad3, a key TGF-β specific intracellular signaling molecule, attenuates lung fibrosis in response to bleomycin and to lung specific TGF-β1 over-expression [11, 12].
The major cellular sources of TGF-β over-production during the development of lung fibrosis have been identified as alveolar macrophages, bronchial epithelium and hyper-plastic type II alveolar epithelial cells (AECs) [13–16]. In addition, TGF-β has also been found in eosinophils and myofibroblasts at sites of active fibrosis [17]. Although all three TGF-β isoforms stimulate fibroblast procollagen production in vitro, TGF-β1 is the predominant isoform expressed during lung fibrosis [18–20]. It is worth mentioning that the role of TGF-β1 in lung fibrosis could not be studied using TGF-β1 knockout mice as these mice develop multifocal inflammatory disease and die shortly after birth [21].
This review will first summarize established as well as recent findings on TGF-β signal transduction, TGF-β’s role in myofibroblast differentiation, AEC apoptosis and ECM remodeling, TGF-β activation and approaches to target TGF-β to abrogate lung fibrosis in animal models as well as in clinical trials. Then it will focus on the two areas of our research interests: TGF-β and proteoglycans; and TGF-β and histone deacetylases.
2. OVERVIEW OF TGF-β SIGNALING AND ITS INHIBITION IN LUNG FIBROGENESIS
2.1. TGF-β Signal Transduction
TGF-β signals are transduced by transmembrane serine/threonine kinase type II (TβRII) and type I (TβRI, also known as activin receptor-like kinase 5, ALK5) receptors [22]. TGF-β ligands first bind to TβRII, then TβRI is recruited into the complex where it is phosphorylated and activated by TβRII. The intracellular signaling pathway downstream of TGF-β receptors is mediated by a family of transcription factors, known as the Smad proteins. The receptor-regulated Smads (R-Smads) 2 and 3 are phosphorylated by TβRI and transported in a complex with Smad4 into the nucleus where they interact with other transcription factors to activate or suppress the transcription of TGF-β target genes. The inhibitory Smads, Smad6 and Smad7, function by binding to TβRI and prevent the recruitment and phosphorylation of the R-Smads [23, 24]. Detailed reviews on TGF-β signal transduction have been published elsewhere [22, 25].
Smad signaling is essential for most, but not all, TGF-β responses. TGF-β has been shown to activate other signaling pathways including mitogen-activated protein kinases (MAPKs), Jun N-terminal kinase (JNK), p38, PI3K and Rho family members [26]. Pro-fibrogenic TGF-β signaling is mediated predominantly via Smad-dependent pathways, although non-Smad signaling pathways have been implicated for certain TGF-β activities.
2.2. TGF-β and the Myofibroblasts
Myofibroblasts are the primary effector cells for lung fibrogenesis [27, 28]. Indeed increased numbers of fibroblastic foci are associated with disease progression and a worse prognosis in IPF [29]. Myofibroblasts can be distinguished from fibroblasts by their expression of α;-smooth muscle actin (α-SMA), which confers a contractile phenotype upon them. In addition, myofibroblasts actively synthesize ECM components including collagen. Myofibroblasts may be derived from three cellular sources: 1) proliferation and activation of resident intrapulmonary fibroblasts; 2) AECs via epithelial-mesenchymal transition (EMT); 3) recruitment of circulating fibrocytes (bone marrow-derived progenitor cells). TGF-β1 plays critical roles in the activation of resident lung fibroblasts and in the process of AEC EMT.
TGF-β1 induces fibroblast proliferation via the release of extracellular FGF-2 [30]. Importantly, TGF-β1 induces the differentiation of fibroblasts to myofibroblasts, which is exemplified by the de novo expression of α-SMA. The canonical SMAD pathway is essential for activation of α-SMA as the SMAD3 binding element in the α-SMA promoter is required for transcriptional activation by TGF-β1 [31]. In addition, TGF-β1-induced inhibition of GSK-3β and nuclear β-catenin translocation via ERK1/2 activation has also been shown to be involved in the differentiation of human lung fibroblasts into myofibroblasts [32]. The expression of α-SMA confers the myofibroblast a contractile capacity which contributes to the distortion of normal lung architecture [33]. Studies in both patients and animal models have also shown that myofibroblasts are the primary source of ECM gene expression in the active fibrotic sites [34, 35]. In addition, compared to fibroblasts, myofibroblasts have reduced motility and proliferative capacity [36] and TGF-β1 protects myofibroblasts against apoptosis [37].
EMT is a process by which epithelial cells lose cell-cell contact, polarity and epithelial cell markers, undergo cytoskeletal remodeling, and gain a mesenchymal phenotype. TGF-β1 induces AEC EMT both in vitro and in vivo [38]. Both epithelial and mesenchymal markers have been localized to hyperplastic type II AECs in human IPF tissues, suggesting that EMT contributes to myofibroblast accumulation in human lung fibrosis [39]. A direct role of TGF-β in AEC EMT was recently demonstrated by over-expression of TGF- β 1 using an adenoviral vector in triple transgenic mice in which type II AECs permanently express β-galactosidase [39]. In this model, β-gal-positive cells that expressed the myofibroblast marker α-SMA were identified in injured lungs and were shown to contribute significantly to the expanded numbers of fibroblasts/myofibroblasts.
Detailed reviews of TGF-β function on myofibroblast differentiation and AEC EMT have recently been published [27, 28, 38].
2.3. TGF-β and AEC Apoptosis
The alveolar epithelium is suggested to mediate pulmonary fibrosis via two apparently paradoxical responses to injury, EMT and apoptosis [40]. TGF-β1 is a potent inducer of apoptosis in ACEs. Transgenic expression of TGF-β1 in lung epithelial cells leads to AEC apoptosis and fibrosis in mice [10]. AECs isolated from biopsies derived from IPF patients display features of apoptosis, which is further confirmed by localization of apoptotic AECs adjacent to fibroblastic foci [41]. Moreover, blocking the apoptotic pathways has protective effects against pulmonary fibrosis in mice insulted with bleomycin [42]. What dictates AEC’s response to TGF-β1 is not clear. A recent study suggests that provisional extracellular matrix is a determinant of the two disparate responses. Primary cultures of murine AECs respond to TGF-β1 with EMT when cultured on a fibronectin matrix, but with apoptosis when cultured on Matrigel which is predominantly composed of laminin, Type IV collagen and heparan sulfate proteoglycans [39].
2.4. TGF-β and ECM Remodeling
TGF-β1 plays an essential role in modulating ECM gene expression during lung fibrogenesis. Collagen I is the major fibrous collagen synthesized by the fibroblasts/myofibroblasts and is composed of two α1 (COL1A1) and one α2 (COL1A2) chains. TGF-β1 potently stimulates the transcription of both COL1A1 and COL1A2. TGF-β1 activates the COL1A1 promoter via the TGF-β1 response element [43], whereas the transcription of the COL1A2 is mediated by the Smad3 pathway [44]. Recently it was shown that Smad2 could bind in association with Sp1 to the CC(GG)-rich TGF- β1 response element of the COL1A1 promoter that lacks the classical Smad recognition element, thus enhancing the binding of Sp1 and in this manner activating COL1A1 promoter [45].
TGF-β1 induces other components of the ECM as well, most notably fibronectins (FNs) and tenacin C. FNs are present in abnormally large quantities in fibrotic lungs and localize to areas of active fibrogenesis [34,46,47]. TGF-β1 enhances the expression of the extra type III domain A (EDA)-containing FN [48, 49]. The synthesis and deposition of EDA-FN precede α-SMA expression and appear to be required for the induction of the myofibroblastic phenotype [49]. Consequently, EDA-null mice failed to develop significant fibrosis after bleomycin challenge [50]. In the case of tenacin C, TGF-β1 tends to induce most strongly the expression of a low molecular weight alternatively spliced variant of tenascin-C that lacks the so-called ‘variable domain’ [51]. Tenacin C expression is upregulated in bleomyin-induced lung fibrosis in mice [52, 53]. In IPF, tenacin C is abundant in the fibroblast foci of active fibrosis and in the basement membrane regions beneath the metaplastic epithelium lining honeycomb cysts, and may function in both fibroblast migration and the adhesion of metaplastic bronchial-type epithelium [54].
In addition to enhancing ECM synthesis, TGF-β reduces the breakdown of ECM by inhibiting the generation of members of the matrix metalloproteases (interstitial collagenases) and plasminogen activators, as well as by enhancing the expression of tissue inhibitors of metalloproteases and plasminogen activator inhibitors [55, 56].
2.5. TGF-β Activation
TGF-β genes encode a C-terminal TGF-β sequence and an N-terminal prodomain called latency associated peptide (LAP) [57]. TGF-β and LAP are produced as a single protein, which is cleaved by furin-like proteases during secretion. The secreted TGF-β/LAP complex is inactive and contains a 25-kDa TGF-β homodimer non-covalently associated with a 75-kDa LAP homodimer. The release of TGF-β from LAP is required for TGF-β activation. In addition, the LAP component of this small latent complex is frequently linked by disulfide bonds to a separately coded latent TGF-β binding protein (LTBP) to form a large latent TGF-β complex, which facilitates its sequestration within the ECM.
2.5.1. Thrombospondin-1-Mediated TGF-β Activation
Thrombospondin-1 (TSP-1) is a large homotrimeric matricellular protein involved in a variety of biological processes [58]. TSP-1 is an important natural activator of TGF-β in the lung [59]. Thromospondin-1 can bind to both the small (TGF-β/LAP) and the large latent TGF-β complexes (TGF- β/LAP/LTBP). A specific peptide sequence within thrombospondin-1, KRFK, mediates TGF-β activation through binding to the LSKL sequence near the N-terminus of LAP. This association induces a conformational change in LAP resulting in the release of active TGF-β.
2.5.2. Integrin-Mediated TGF-β Activation
Integrin-mediated activation of TGF-β was first demonstrated in vivo using integrin β6 knockout mice which display enhanced inflammation but dramatically reduced fibrosis following bleomycin treatment [60]. The propeptide of TGF-β1 and TGF-β3, LAP-β1 and LAP-β3, contains a RGD motif that is recognized by a subset of integrins sharing in common the promiscuous αv integrin subunit. TGF-β2 lacks the critical RGD sequence and thus is not activated by the integrins. The epithelial specific integrin αvβ6 interacts with the RGD domain of LAP, which induces a conformation change in the LAP resulting in the presentation of the mature active TGF-β to target cells. The association of αvβ6 with the actin cytoskeleton is required for αvβ6-mediated TGF-β activation [60]. The importance of RGD-binding integrin in TGF-β activation was recently demonstrated in mice heterozygous for a TGF-β1 mutation, which encodes a nonfunctional integrin-binding site (RGE instead of RGD). Mice expressing this mutation are protected from radiation-induced fibrosis [61]. The integrin αvβ6 can also activate TGF-β in the large latent TGF-β complex through a fibronectin-dependent mechanism [62]. Matrix targeting of the complex mediated by fibronectin binding to LTBP in addition to LAP-mediated interaction with the cell surface αvβ6 is believed to create the traction necessary for the release of active TGF-β.
In contrast to αvβ6, proteolytic activity is necessary for TGF-β activation by αvβ8 [63]. Latent TGF-β is sequestered to the epithelial cell surface through binding to integrin αvβ8. This binding then results in membrane-type I matrix metalloproteinase (MT1-MMP)-dependent cleavage of LAP and the release of active TGF-β. In contrast to αvβ6 mediated TGF-β activation in which direct cell-cell contact is required [60], αvβ8 mediated TGF-β activation does not depend on direct cell-cell contact and could release free TGF-β, which could function on cells at a distance from the αvβ8 expressing cells.
Other integrins, including αvβ3 and αvβ5, have been shown to contribute to TGF-β activation in fibroblasts in vitro [64–66]. Currently, however, there is no direct evidence for αvβ3- or αvβ5-mediated TGF-β activation in lung fibrosis in vivo.
2.5.3. Protease-Mediated TGF-β Activation
The serine protease plasmin was one of the first molecules found to activate TGF-β [67]. The proteolytic cleavage of LAP releases mature TGF-β, which is then free to activate its cell surface receptors. Plasmin can also release the large latent TGF-β complex from the ECM by cleaving LTBP [68]. Plasmin has been shown to regulate the activation of cell-associated latent TGF-β1 secreted by rat alveolar macrophages after in vivo bleomycin injury [69].
In addition to plasmin, many other proteases including thrombin, mast cell chymase, leukocyte elastase, and MMP-2 and -9 have been linked to the release or activation of TGF-β [70]. It is relevant that higher levels of MMP-9 have been linked to a more rapid demise in patients afflicted with IPF [71] and that the NIH-sponsored IPFnet is launching a trial involving anticoagulant therapy for the treatment of IPF (ACE-IPF).
2.5.4. Reactive Oxygen Species-Mediated TGF-β Activation
Increased oxidative stress has been reported in lung fibrosis, with increased release of oxidants from neutrophils, macrophages and fibroblasts, and decreased levels of anti-oxidants [72]. Reactive oxygen species (ROS)-mediated TGF-β activation is specific for the TGF-β1 isoform, and the unique methionine residue at amino acid position 253 in the LAP-β1 functions as a redox switch center [73]. Elevated levels of ROS oxidize LAP, which triggers a conformational change leading to the release of mature TGF-β [73, 74].
TGF-β itself induces ROS production as part of its signal-transduction pathway in lung fibroblasts [75, 76]. TGF- β1 induces intracellular ROS production through the NADPH oxidase isoform 4, NOX-4. NOX-4-dependent generation of hydrogen peroxide is required for TGF-β1-induced myofibroblast differentiation, ECM production and contractility [77]. In addition, NOX-4 is upregulated in murine lungs subjected to bleomycin and in cases of human IPF [77]. Genetic or pharmacologic targeting of NOX-4 has been shown to abrogate fibrogenesis in two murine models of lung injury [77].
Excellent reviews on different modes of TGF-β activation have been published recently [78–80].
2.6. Targeting TGF-β in experimental Lung Fibrosis
Given the central role of TGF-β in lung fibrosis, various anti-TGF-β approaches have been investigated in animal models to prevent or reduce pulmonary fibrosis. Inhibition of integrin αvβ6 mediated TGF-β activation using an inhibitory & agr;vβ6 monoclonal antibody, as well as direct inhibition of mature TGF-β using an TGF-β antibody diminish both bleomycin- and radiation-induced lung fibrosis [61, 81–83]. Intratracheal instillation of recombinant soluble TβRII (extracellular domain of TβRII fused to human IgG Fc) and gene transfer of soluble TβRII successfully reduced bleomycin-induced lung fibrosis in hamsters and mice, respectively [84, 85]. Recombinant soluble TβRII also prevented radiation-induced lung fibrosis in mice [61]. SD-208, an orally active small molecular weight inhibitor of TβRI (ALK5) was able to block both the initiation and progression phase of TGF-β1-induced lung fibrosis in mice [86]. Another small molecular ALK5 inhibitor, SM16, was also shown to prevent radiation-induced lung injury in a rat model [87].
Transient gene transfer and expression of Smad7, an intracellular antagonist of TGF-β signaling, has been shown to prevent bleomycin-induced lung fibrosis in mice [88]. Oligo decoys targeting Smads or TGF-β response elements in the collagen promoter have also been used successfully to inhibit fibrogenesis [89].
2.7. Clinical Trials
Genzyme corporation has recently completed a phase I clinical trial to investigate the safety, pharmacokinetics and potential clinical benefit of GC1008, an antibody that neutralizes all three mammalian TGF-β isoforms, in patients with IPF.
Imatinib mesylate (Gleevec) is a protein kinase inhibitor that blocks both PDGF receptor and c-Abl, a tyrosine kinase downstream of TGF-β [90]. In bleomycin treated mice, imatinib mesylate has been shown to significantly inhibit lung fibrosis [90]. A recent phase II trial using Gleevec for the treatment of IPF, however, did not demonstrate efficacy (in press).
Pirfenidone is an anti-fibrotic agent that inhibits TGF-β1, decreases lung fibroblasts proliferation and down regulates pro-fibrotic cytokines [91]. In a phase II open-label study in IPF patients who previously had shown disease progression on immunosuppressive therapy, 29 of 54 patients showed evidence of stability or improvement of lung function at 6 month [92]. A double-blind, randomized, placebo controlled trial showed that treatment with pirfenidone diminished the decline in vital capacity and prevented acute exacerbation of IPF during the 9 month follow-up, though a premature end to this trial prevented the trial from achieving its primary endpoint [93]. Pirfenidone for IPF has been studied in three subsequent Phase III clinical trials, however, we cannot comment further on these trials because these studies have not completed the peer review process.
Borok et al. have shown that administration of aerosolized glutathione in IPF patients restored glutathione levels in the lower respiratory tract, while reducing superoxide anion release by alveolar macrophages, suggesting that anti-oxidant treatment could be biologically efficacious [94]. The antioxidant glutathione precursor N-acetylcysteine (NAC) also restores levels of the epithelial lining fluid glutathione in patients with IPF [95]. In vitro, NAC reduces dimeric active TGF-β into inactive monomers and thus blocks intracellular signaling [96]. NAC has also been shown to inhibit TGF-β-induced AEC EMT [97]. An aerosolized NAC study showed that although NAC did not influence pulmonary function or quality of life, it may delay disease progression [98]. Two studies have examined the role of NAC therapy in combination with prednisone and azathioprine in patients with IPF, and showed better preservation of vital capacity and diffusion capacity at one year [99, 100], albeit a dropout rate of 30% has compelled the IFPnet to embark on a trial employing NAC with the inclusion of a placebo arm (PANTHER).
3. INTERESTS IN OUR GROUP
3.1. TGF-β and Proteoglycans
Proteoglycans (PGs) are a heterogeneous family of genetically unrelated proteins with covalently attached glycosaminoglycan (GAG) side chains [101]. They constitute major components of the cell surface, basement membrane and the ECM, performing diverse functions including cell-cell, cell-matrix interactions, matrix assembly and tissue homeostasis. Chondroitin sulfate/dermatan sulfate (CS/DS) PGs and heparan sulfate (HS) PGs are two major PG families in the lung, and the expression of both the core proteins and the GAG side chains of these two PG families have been shown to be altered during the development of pulmonary fibrosis [102–104].
3.1.1. CS/DS PGs
Versican is a large CS-containing PG that binds specifically with hyaluronic acid forming macromolecular aggregates in the interstitial matrix [105]. Versican expression is increased in human IPF lungs [102] as well as in bleomycin-induced lung fibrosis in rodents, with peak expression occurring at 14 days following bleomycin treatment [103]. Lung fibroblasts isolated from bleomycin-exposed rat lung exhibit increased production of versican [106]. This overproduction of versican is due to TGF-β as a neutralizing TGF-β antibody reduced versican expression. Increased versican expression may be important in providing provisional matrix for cell migration and proliferation, as well as for subsequent collagen deposition.
Decorin and biglycan are small CS/DS PGs (with core protein of ~ 40 kDa) residing in the ECM. Decorin binds to collagen and plays a key role in regulating collagen fibril formation and the spatial arrangement of collagen fibers in the matrix [107]. Both decorin and biglycan bind to TGF-β through their core proteins, with the dissociation constant (Kd) for the high affinity binding sites ranging from 1 to 20 nM [108, 109]. The leucine-rich repeats that characterize decorin and biglycan may be the site of TGF-β binding. Importantly only the activated form of TGF-β binds to decorin and biglycan [108]. Decorin and biglycan have been shown to neutralize TGF-β activity in vitro [109].
TGF-β stimulates the synthesis of decorin and biglycan in a cell-type specific manner, thus the ability of both decorin and biglycan to neutralize TGF-β activity suggests that these PGs serve as negative-feedback regulators of TGF- β. In lung fibroblasts, TGF-β selectively induces the expression of biglycan, whereas the level of decorin remains unaltered [110]. In type II AECs, TGF-β1 induces decorin [111]. In bleomycin-induced lung fibrosis in rats, biglycan mRNA expression is increased following the increase in TGF-β mRNA expression, whereas the expression of decorin was decreased [103, 104]. Primary lung fibroblasts isolated from bleomycin-exposed rat lung also exhibit increased expression of biglycan which was inhibited using a neutralizing TGF-β antibody, suggesting that antocrine TGF-β is responsible for increased biglycan expression [106].
Because of their TGF-β blocking effects in vitro, both biglycan and decorin have been targeted in animal models of lung fibrosis. Intratracheal instillation of recombinant decorin reduced lung hydroxyproline levels, and neutrophil infiltration along with neutrophil-derived myeloperoxidase activity in the bronchoalveolar lavage fluid in hamsters treated with bleomycin [112]. Transient transgene expression of decorin via delivery of a replication-deficient adenovirus into the lung also reduces both bleomycin-induced and TGF- β-induced lung fibrosis in mice [113–115]. Although bigly-can was effective in vitro to inhibit TGF-β activity, it failed to reduce TGF-β-induced fibrogenesis in mice [113]. In fact, adenovirus-mediated gene transfer of biglycan induces fibroblastic responses in the lung [116]. Variant tissue localization of these two PGs might account for the differences in their biological effect in vivo [117]. Decorin is bound to collagen and is probably able to bind TGF-β and prohibit its interaction with cellular receptors. In contrast, biglycan is more closely associated with the pericellular space and cell surface, and thus still allows for bound TGF-β to interact with its cell surface receptors, similar to betaglycan. Beta-glycan, a transmembrane PG, is the TGF-β type III receptor which binds and stores TGF-β and eventually presents it to the TGF-β signal transducing TβRI and TβRII [118]. Although introduction of decorin successfully reduces lung fibrosis in animal models, no human study has been reported.
In addition to inducing the expression of the core proteins, TGF-β also increases the synthesis of CS/DS side chains of decorin and biglycan. The backbone of CS/DS GAG chains is composed of alternating N-acetyl-D-galactosamine (GalNAc) and D-glucuronic acid (GlcA) residues. Some of the GlcA residues are epimerized to L-iduronic acids (IdoA). CS chains are linear polymers constructed from 40 to more than 100 repeating disaccharide units comprising GlcA and GalNAc, whereas DS chains contain varying proportions of IdoA in place of GlcA [119]. In addition, the CS/DS chains can be further modified by sulfation at various positions. CS/DS comprise 52% of total GAG in adult lung parenchyma [120]. Using metabolic labeling, TGF-β1 increase 35SO4 incorporation into CS/DS chains in both cultured type II AECs and lung fibroblasts [111, 121]. As mentioned previously, both decorin and biglycan bind to TGF-β through their core proteins, not the GAG chains. In fact, removal of the CS/DS side chains increased the inhibitory activity of decorin and biglycan, suggesting the GAG chains may hinder the interaction of the core proteins with TGF-β [108].
The fine structures of CS/DS are also altered by TGF-β1. In human fetal lung fibroblasts, TGF-β1 treatment reduced the IdoA content in decorin and biglycan DS chains by 50%, whereas 4-O-sulfation is increased 2-fold in versican [122]. In human lung fibroblasts, DS GAG chains exhibit anti-proliferative effects, and the higher the IdoA content, the higher the anti-proliferative activity [123]. A recent report showed that treatment with chondroitinase ABC, which degrades the CS/DS GAG chains, alleviates bleomycin-induced lung fibrosis in mice [124].
3.1.2. HSPGs
The expression of HSPGs are also increased in bleomyin-induced lung fibrosis [103]. In both the asbestos and bleomycin murine models of pulmonary fibrosis, both the expression of syndecan-1 and the shedding of syndecan-1 into the bronchoalveolar lavage fluid are increased [125]. Similar findings were reported in human IPF lungs compared with normal controls [125]. Immunohistochemistry localized syndecan-1 expression to the abluminal surface of alveolar epithelial cells, terminal bronchiolar epithelial cells and within areas of fibrosis [125]. Syndecan-1 shedding may promote the development and progression of lung fibrosis, as shed syndecan-1 or recombinant syndecan-1 ectodomain promotes neutrophil chemotaxis, inhibits alveolar re-epithelialization, and increases proliferation as well as TGF- β1 release in cultured lung fibroblasts [125]. All three TGF-β isoforms can induce syndecan-1 expression in A549 cells, adenocarcinoma cells derived from the type II AECs [126].
HS comprise 38% of total GAG in the adult lung parenchyma [120]. In contrast to CS/DS, 35SO4 incorporation into HS chains was either unchanged or even decreased by TGF- β1 [111]. HS polysaccharide chains contain repeating disaccharide units of uronic acid (IdoA or GlcA) linked to N-acetylglucosamine. During HS biosynthesis in the Golgi, these disaccharides are further modified by sulfation at the N-, 6-O- and 3-O-positions of the glucosamine and the 2-O-position of the uronic acid residues [127]. Importantly, these modifications are incomplete resulting in HS chains with highly distinct saccharide sequences and sulfation patterns [127]. Research from our laboratory has shown that TGF-β1 alters the fine structure of HS. Specifically, TGF-β1 reduces the 6-O-sulfation level of HS by inducing either Sulf1 or Sulf2. Sulf1 and Sulf2 are HS specific 6-O-endosulfatases located at the cell surface and the ECM. By removing 6-O-sulfate from specific HS intra-chain sites, Sulf1 and Sulf2 have been shown to modulate the function of many growth factors and morphorgens including FGF and Wnt [128]. We now know that TGF-β1 induces Sulf1 in normal human lung fibroblasts [129] and Sulf2 in type II AECs (submitted for publication). Interestingly, the feedback mechanisms of Sulf1 and Sulf2 on TGF-β1 function are different in these two types of cells. In lung fibroblasts, Sulf1 negatively regulates TGF-β1 function, whereas Sulf2 is required for maximum TGF-β1 responses in type II AECs. Both Sulf1 and Sulf2 are induced in murine models of lung fibrosis [129] as well as in human IPF lungs (unpublished data). Defining the role of Sulf1 and Sulf2 in the development of lung fibrosis in vivo will require further studies. As Sulf1 and Sulf2 are extracellular enzymes readily amendable by therapeutic agents such as inhibitors or antibodies, the Sulfs could serve as new therapeutic targets for the treatment of IPF.
Compared to HS, heparin (a mast cell product) is highly and uniformed sulfated. It was shown that at low concentration, heparin stimulates lung fibroblast proliferation, whereas higher concentrations exert an inhibitory effect [130]. In mice and rabbits, heparin was shown to attenuate bleomycin-induced pulmonary fibrosis [131, 132]. In patients with IPF, one small study showed that low-molecular-weight heparin has a beneficial effect on patient survival, albeit this study requires confirmation using a more rigorous study protocol [133]. In the above studies, the effects of heparin on reducing fibrosis have been attributed to its anti-coagulant properties, as increased local pro-coagulant activity is a characteristic feature of lung fibrosis [134]. However, heparin could also serve as a competitive substrate for Sulf1 and Sulf2, and therefore may be functioning as a sulfatase inhibitor in these studies.
3.2. TGF-β and Histone Deacetylases
Lysine acetylation transfers an acetyl moiety from acetyl-coenzyme A (CoA) to the ε-amino group of a lysine residue. This covalent modification was initially discovered as one of the histone codes for epigenetic regulation of gene expression. During the past decade, lysine acetylation has been identified in hundreds of non-histone peptides. Substrate and functional diversity of acetylation revealed by proteomic surveys highlights lysine acetylation as a key component of the cellular signaling network that coordinates fundamental cellular processes [135]. Reversible and dynamic lysine acetylation is tightly controlled by the two families of opposing enzymes, histone deacetylatases (HDACs) that remove acetyl moieties and histone acetyltransferases (HAT) that add acetyl moieties upon physiological and pathological stimuli [136]. Investigations from our group and others have shown that HDAC4 and HDAC6 play important roles in TGF-β1-induced fibrogenic processes. Both HDAC4 and HDAC6 are class II HDAC members [137].
3.2.1. HDAC4
Like other Class II HDACs, HDAC4 possesses intrinsic nuclear-cytoplasmic shuttling signals and thereby regulates signaling pathways in the nucleus as well as in the cytoplasm [137]. Recent studies in our laboratory and others indicate a requirement of HDAC4 in TGF-β1-induced fibroblast to myofibroblast differentiation [138, 139]. Trichostatin A (TSA), a global inhibitor of HDACs, prevented α-SMA transcript and protein expression and morphological changes mediated by TGF-β1 in cultured primary normal human lung fibroblasts [138]. The inhibition of α-SMA expression by TSA was associated with reduced phosphorylation of Akt. It is noteworthy that TGF-β1-mediated Akt phosphorylation is also an event that is crucial for inhibiting myofibroblast apoptosis [140]. The effect of TSA is mainly mediated by inhibition of HDAC4 as small interference RNA medicated HDAC4 knockdown was as effective in inhibiting TGF-β1-induced α-SMA expression as well as the phosphorylation of Akt. Our unpublished work also shows that TGF-β1-induced ROS could modulate nucleus to cytoplasm translocation of HDAC4. The cytoplasmic substrate(s) for HDAC4 in lung fibroblasts, however, have not been identified. The above findings strongly suggest that HDAC4 is an integral component of the signaling network that modulates TGF-β-induced fibroblast to myofibroblast differentiation.
3.2.2. HDAC6
HDAC6 predominantly modulates acetylation of non-histone proteins in the cytoplasm and regulates many important biological processes including cell migration, immune synapse formation, viral infection and the degradation of misfolded proteins [141]. HDAC6 deacetylates α-tubulin and over-expression of HDAC6 causes hypoacetylation of α-tubulin as well as increased cell motility [142]. We showed in a previous report that HDAC6 tubulin deacetylase activity is up-regulated post-translationally by TGF-β1, which coincides with TGF-β1-induced EMT in A549 cells [143]. Importantly, employment of the HDAC6 specific inhibitor, tubacin, or RNA interference mediated HDAC6 knockdown revealed that HDAC6 is required for the activation of Smad3 as well as the maximal expression of a panel of EMT markers induced by TGF-β1. These findings strongly suggest that HDAC6-dependent deacetylation of & agr;-tubulin is an integral component of TGF-β1-induced EMT. HDAC6 has been shown to deacetylate tubulin in the lung in vivo [144]. Our unpublished work also showed that HDAC6 appears to be catalytically active in the murine lung. Lung extracts from HDAC6 knockout mice exhibited hyperacetylation in multiple proteins relative to that from the wild-type animals.
Broad HDAC inhibitors have been used to target HDACs in the treatment of cancer, and have been shown to be well tolerated [145]. Defining the role of class II HDACs in the process of pulmonary fibrosis may lead to the novel employment of a more focused class II HDAC inhibitor as an effective therapy for pulmonary fibrosis.
4. CONCLUSION
This review did not cover the role of TGF-β in airway fibrosis associated with asthma [146, 147]. Recently TGF-β1 has been shown to modulate microRNA expression, most notably miR-155 in lung fibroblasts [148, 149]. One of the target genes of miR-155 in lung fibroblasts was identified as keratinocyte growth factor, implicating this miRNA in the regulation by mesenchymal cells of the surrounding lung epithelium [148]. Interestingly miR-155 expression level correlated with the degree of lung fibrosis in mice [148]. Research in these areas will likely yield interesting findings.
The crucial role of TGF-β in the pathogenesis of lung fibrosis denotes it as an attractive therapeutic target. Each step along the pathway of synthesis, activation, and signaling of TGF-β represents a potential target for regulating the activity of TGF-β. Fig. (1) summarizes the TGF-β signaling pathway in the pathobiology of lung fibrosis, and the reagents that have been or could be used to inhibit TGF-β function at different steps along its signaling pathway. As summarized in this review, our understandings of the role of TGF-β and its mechanisms of action have increased dramatically in recent years. However, there is still much to be learned about this important and highly-regulated molecule, especially how TGF-β functions are modulated in vivo and the complex interaction between TGF-β and other signaling pathways. Research in these areas could help identify novel therapeutic strategies for the treatment of lung fibrosis, and have the potential to improve the prognosis of patients afflicted with this devastating illness in the near future.
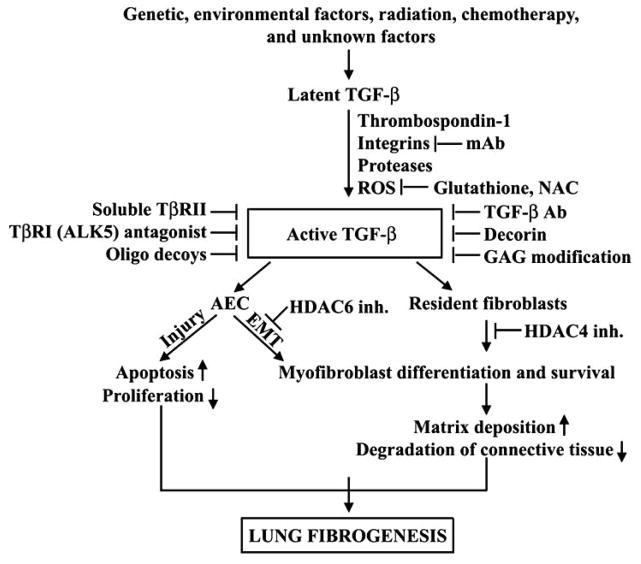
Fig. 1.
TGF-β signaling and inhibition in lung fibrogenesis. TGF-β plays pivotal roles in the key events of lung fibrogenesis: fibroblast to myofibroblast differentiation, AEC apoptosis and EMT. Each step along the pathway of activation and signaling of TGF-β represents a potential target for regulating the activity of TGF-β. Inh.: inhibition. Please see text for details.
Acknowledgments
This work was supported by NIH R03 HL096949 to X.Y. and NIH R01 HL083480 to J.A.L.
References
- 1.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2:319–338. doi: 10.1177/1753465808096948. [DOI] [PubMed] [Google Scholar]
- 3.Rogliani P, Mura M, Assunta Porretta M, Saltini C. New perspectives in the treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2008;2:75–93. doi: 10.1177/1753465808089363. [DOI] [PubMed] [Google Scholar]
- 4.Khalil N, Greenberg AH. The role of TGF-beta in pulmonary fibrosis. Ciba Found Symp. 1991;157:194–207. doi: 10.1002/9780470514061.ch13. discussion 207–111. [DOI] [PubMed] [Google Scholar]
- 5.Hoyt DG, Lazo JS. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther. 1988;246:765–771. [PubMed] [Google Scholar]
- 6.Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- 7.Yi ES, Bedoya A, Lee H, Chin E, Saunders W, Kim SJ, Danielpour D, Remick DG, Yin S, Ulich TR. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996;20:339–352. doi: 10.1007/BF01486737. [DOI] [PubMed] [Google Scholar]
- 8.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshamana GS, Pociask DA, Fisher KJ, Liu JY, Sime PJ, Brody AR. Titration of non-replicating adenovirus as a vector for transducing active TGF-beta1 gene expression causing inflammation and fibrogenesis in the lungs of C57BL/6 mice. Int J Exp Pathol. 2002;83:183–201. doi: 10.1046/j.1365-2613.2002.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 13.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170:727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limper AH, Broekelmann TJ, Colby TV, Malizia G, McDonald JA. Analysis of local mRNA expression for extracellular matrix proteins and growth factors using in situ hybridization in fibroproliferative lung disorders. Chest. 1991;99(3 Suppl):55S–56S. doi: 10.1378/chest.99.3_supplement.55s. [DOI] [PubMed] [Google Scholar]
- 16.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]
- 18.Baecher-Allan CM, Barth RK. PCR analysis of cytokine induction profiles associated with mouse strain variation in susceptibility to pulmonary fibrosis. Reg Immunol. 1993;5:207–217. [PubMed] [Google Scholar]
- 19.Khalil N, O’Connor RN, Flanders KC, Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 20.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150:981–991. [PMC free article] [PubMed] [Google Scholar]
- 21.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 24.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 27.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 28.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 29.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 30.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–43009. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 31.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 32.Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A, Vancheri C. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–537. [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 36.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 37.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 38.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corvol H, Flamein F, Epaud R, Clement A, Guillot L. Lung alveolar epithelium and interstitial lung disease. Int J Biochem Cell Biol. 2009;41:1643–1651. doi: 10.1016/j.biocel.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275(6 Pt 1):L1192–1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 42.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bell-meyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, Chandel NS. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA. 2006;103:4604–4609. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jimenez SA, Varga J, Olsen A, Li L, Diaz A, Herhal J, Koch J. Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J Biol Chem. 1994;269:12684–12691. [PubMed] [Google Scholar]
- 44.Chen SJ, Yuan W, Lo S, Trojanowska M, Varga J. Interaction of smad3 with a proximal smad-binding element of the human alpha2(I) procollagen gene promoter required for transcriptional activation by TGF-beta. J Cell Physiol. 2000;183:381–392. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Sysa P, Potter JJ, Liu X, Mezey E. Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors. DNA Cell Biol. 2009;28:425–434. doi: 10.1089/dna.2009.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn C, 3rd, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 47.Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J. 1992;5:404–410. [PubMed] [Google Scholar]
- 48.Borsi L, Castellani P, Risso AM, Leprini A, Zardi L. Transforming growth factor-beta regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Lett. 1990;261:175–178. doi: 10.1016/0014-5793(90)80664-5. [DOI] [PubMed] [Google Scholar]
- 49.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta. 2009;1793:888–892. doi: 10.1016/j.bbamcr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, Bernatchez PN, Sessa WC, Silver RM, Hoffman S. Anti-fibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–861. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 53.Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174:420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- 54.Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147:1759–1769. [PMC free article] [PubMed] [Google Scholar]
- 55.Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal. 2008;10:303–319. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eickelberg O, Kohler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am J Physiol. 1999;276(5 Pt 1):L814–824. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- 57.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 58.Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 60.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 61.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 63.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 65.Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–1769. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 66.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khalil N, Corne S, Whitman C, Yacyshyn H. Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol. 1996;15:252–259. doi: 10.1165/ajrcmb.15.2.8703482. [DOI] [PubMed] [Google Scholar]
- 70.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 71.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J. 2009;33:77–84. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 72.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166:839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 74.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest. 2004;84:1013–1023. doi: 10.1038/labinvest.3700109. [DOI] [PubMed] [Google Scholar]
- 75.Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- 76.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 77.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10:333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 79.Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37(Pt 4):849–854. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- 80.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 83.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 84.Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST, Giri SN. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax. 1999;54:805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamada M, Kuwano K, Maeyama T, Yoshimi M, Hamada N, Fukumoto J, Egashira K, Hiasa K, Takayama K, Nakanishi Y. Gene transfer of soluble transforming growth factor type II receptor by in vivo electroporation attenuates lung injury and fibrosis. J Clin Pathol. 2007;60:916–920. doi: 10.1136/jcp.2006.039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 87.Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, Sun L, Lee WC, Ling LE, Vujaskovic Z. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 88.Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cutroneo KR. TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen. 2007;15(Suppl 1):S54–60. doi: 10.1111/j.1524-475X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 90.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 92.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 93.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 94.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 95.Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 96.Lichtenberger FJ, Montague C, Hunter M, Frambach G, Marsh CB. NAC and DTT promote TGF-beta1 monomer formation: demonstration of competitive binding. J Inflamm (Lond) 2006;3:7. doi: 10.1186/1476-9255-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Felton VM, Borok Z, Willis BC. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L805–812. doi: 10.1152/ajplung.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10:449–455. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 99.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immuno-suppression. Am J Respir Crit Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 100.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 101.Perrimon N, Bernfield M. Cellular functions of proteoglycans--an overview. Semin Cell Dev Biol. 2001;12(2):65–67. doi: 10.1006/scdb.2000.0237. [DOI] [PubMed] [Google Scholar]
- 102.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteo-glycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1819–1828. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 103.Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS. Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2000;161:2066–2073. doi: 10.1164/ajrccm.161.6.9909098. [DOI] [PubMed] [Google Scholar]
- 104.Westergren-Thorsson G, Hernnas J, Sarnstrand B, Oldberg A, Heinegard D, Malmstrom A. Altered expression of small proteo-glycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 106.Venkatesan N, Roughley PJ, Ludwig MS. Proteoglycan expression in bleomycin lung fibroblasts: role of transforming growth factor-beta(1) and interferon-gamma. Am J Physiol Lung Cell Mol Physiol. 2002;283:L806–814. doi: 10.1152/ajplung.00061.2002. [DOI] [PubMed] [Google Scholar]
- 107.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 108.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 110.Romaris M, Heredia A, Molist A, Bassols A. Differential effect of transforming growth factor beta on proteoglycan synthesis in human embryonic lung fibroblasts. Biochim Biophys Acta. 1991;1093:229–233. doi: 10.1016/0167-4889(91)90127-j. [DOI] [PubMed] [Google Scholar]
- 111.Maniscalco WM, Campbell MH. Transforming growth factor-beta induces a chondroitin sulfate/dermatan sulfate proteoglycan in alveolar type II cells. Am J Physiol. 1994;266(6 Pt 1):L672–680. doi: 10.1152/ajplung.1994.266.6.L672. [DOI] [PubMed] [Google Scholar]
- 112.Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54:1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- 113.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1327–1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 114.Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, Gauldie J. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163(3 Pt 1):770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 115.Shimizukawa M, Ebina M, Narumi K, Kikuchi T, Munakata H, Nukiwa T. Intratracheal gene transfer of decorin reduces sub-pleural fibroproliferation induced by bleomycin. Am J Physiol Lung Cell Mol Physiol. 2003;284:L526–532. doi: 10.1152/ajplung.00131.2002. [DOI] [PubMed] [Google Scholar]
- 116.Sime PJ, Sarnstrand B, Xing Z, Graham F, Fisher L, Gauldie J. Adenovirus-mediated gene transfer of the proteoglycan biglycan induces fibroblastic responses in the lung. Chest. 1997;111(6 Suppl):137S. doi: 10.1378/chest.111.6_supplement.137s. [DOI] [PubMed] [Google Scholar]
- 117.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 118.Kaname S, Ruoslahti E. Betaglycan has multiple binding sites for transforming growth factor-beta 1. Biochem J. 1996;315(Pt 3):815–820. doi: 10.1042/bj3150815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 120.Hance AJ, Horwitz AL, Cowan MJ, Elson NA, Collins JF, Bienkowski RS, Bradley KH, McConnell-Breul S, Wagner WM, Crystal RG. Biochemical approaches to the investigation of fibrotic lung disease. Chest. 1976;69(2 Suppl):257–261. doi: 10.1378/chest.69.2_supplement.257. [DOI] [PubMed] [Google Scholar]
- 121.Romaris M, Bassols A, David G. Effect of transforming growth factor-beta 1 and basic fibroblast growth factor on the expression of cell surface proteoglycans in human lung fibroblasts. Enhanced glycanation and fibronectin-binding of CD44 proteoglycan, and down-regulation of glypican. Biochem J. 1995;310(Pt 1):73–81. doi: 10.1042/bj3100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tiedemann K, Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, Malmstrom A. Regulation of the chondroitin/dermatan fine structure by transforming growth factor-beta1 through effects on polymer-modifying enzymes. Glycobiology. 2005;15:1277–1285. doi: 10.1093/glycob/cwj027. [DOI] [PubMed] [Google Scholar]
- 123.Westergren-Thorsson G, Onnervik PO, Fransson LA, Malmstrom A. Proliferation of cultured fibroblasts is inhibited by L-iduronate-containing glycosaminoglycans. J Cell Physiol. 1991;147(3):523–530. doi: 10.1002/jcp.1041470319. [DOI] [PubMed] [Google Scholar]
- 124.Kai Y, Yoneyama H, Koyama J, Hamada K, Kimura H, Matsushima K. Treatment with chondroitinase ABC alleviates bleomycin-induced pulmonary fibrosis. Med Mol Morphol. 2007;40:128–140. doi: 10.1007/s00795-007-0370-y. [DOI] [PubMed] [Google Scholar]
- 125.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284(6):3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hayashida K, Johnston DR, Goldberger O, Park PW. Synde-can-1 expression in epithelial cells is induced by transforming growth factor beta through a PKA-dependent pathway. J Biol Chem. 2006;281:24365–24374. doi: 10.1074/jbc.M509320200. [DOI] [PubMed] [Google Scholar]
- 127.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 128.Lamanna WC, Kalus I, Padva M, Baldwin RJ, Merry CL, Dierks T. The heparanome-The enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol. 2007;129:290–307. doi: 10.1016/j.jbiotec.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 129.Yue X, Li X, Nguyen HT, Chin DR, Sullivan DE, Lasky JA. Transforming growth factor-beta1 induces heparan sulfate 6- O-endosulfatase 1 expression in vitro and in vivo. J Biol Chem. 2008;283:20397–20407. doi: 10.1074/jbc.M802850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamashita Y, Nakagomi K, Takeda T, Hasegawa S, Mitsui Y. Effect of heparin on pulmonary fibroblasts and vascular cells. Thorax. 1992;47:634–639. doi: 10.1136/thx.47.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, Grimminger F, Seeger W. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168:1358–1365. doi: 10.1164/rccm.2201082. [DOI] [PubMed] [Google Scholar]
- 132.Piguet PF, Van GY, Guo J. Heparin attenuates bleomycin but not silica-induced pulmonary fibrosis in mice: possible relationship with involvement of myofibroblasts in bleomycin, and fibroblasts in silica-induced fibrosis. Int J Exp Pathol. 1996;77:155–161. doi: 10.1046/j.1365-2613.1996.d01-214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, Sasaki H. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 134.Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153(Suppl 1):S367–378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 136.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 137.Yang XJ, Gregoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol. 2005;25:2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 140.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a micro-tubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 143.Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem. 2008;283:21065–21073. doi: 10.1074/jbc.M802786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40:76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mottet D, Castronovo V. Histone deacetylases: target enzymes for cancer therapy. Clin Exp Metastasis. 2008;25:183–189. doi: 10.1007/s10585-007-9131-5. [DOI] [PubMed] [Google Scholar]
- 146.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta inairway remodeling in asthma. Immunol Cell Biol. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 147.Bosse Y, Rola-Pleszczynski M. Controversy surrounding the increased expression of TGF beta 1 in asthma. Respir Res. 2007;8:66. doi: 10.1186/1465-9921-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pottier N, Maurin T, Chevalier B, Puissegur MP, Lebrigand K, Robbe-Sermesant K, Bertero T, Lino Cardenas CL, Courcot E, Rios G, Fourre S, Lo-Guidice JM, Marcet B, Cardinaud B, Barbry P, Mari B. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS ONE. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006;281:18277–18284. doi: 10.1074/jbc.M601496200. [DOI] [PubMed] [Google Scholar]