Abstract
The vesicular adenosine triphosphatase (ATPase) acidifies intracellular compartments, including synaptic vesicles and secretory granules. A controversy about a second function of this ATPase in exocytosis has been fuelled by questions about multiple putative roles of acidification in the exocytic process. Now, Poëa-Guyon et al. (2013. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201303104) present new evidence that the vesicular ATPase performs separate acidification and exocytosis roles and propose a mechanism for how these two functions are causally linked.
Vesicular H+-ATPases (V-ATPases) function as ATP-driven proton pumps in intracellular compartments, such as endosomes, Golgi-derived vesicles, secretory vesicles, synaptic vesicles, lysosomes, and vacuoles (Forgac, 2007). Acidification is important for a plethora of cell biological processes ranging from endosomal ligand–receptor dissociation to lysosomal degradation (Yan et al., 2009; Williamson et al., 2010; Zoncu et al., 2011). Consequently, interfering with V-ATPase function leads to direct and indirect defects that are difficult to tease apart. In addition, acidification-independent roles of the V-ATPase in secretion and membrane fusion have been proposed (Israël et al., 1986; Peters et al., 2001; Morel et al., 2003; Hiesinger et al., 2005; Liégeois et al., 2006; Sun-Wada et al., 2006; Peri and Nüsslein-Volhard, 2008). The difficulty to distinguish consequences of an acidification-independent mechanism from indirect effects of acidification defects is exacerbated by an unclear dependence of secretion on acidification (Cousin and Nicholls, 1997; Ungermann et al., 1999; Hiesinger et al., 2005).
In synaptic vesicles, the V-ATPase generates a proton gradient that is used by an antiporter to fill synaptic vesicles with neurotransmitter. Hence, loss of acidification leads to “empty” synaptic vesicles and loss of neurotransmitter release. Can such vesicles still fuse and thereby “shoot blanks”? The V-ATPase comprises of two sectors that can reversibly dissociate: the cytosolic V1 sector and the membrane-bound V0 sector. Loss of the neuronal a1 subunit of the V0 sector (V0a1) leads to almost complete loss of neurotransmission in Drosophila melanogaster—a phenotype that may result from a defect in neurotransmitter loading or exocytosis (Hiesinger et al., 2005). Single vesicle release events in the V0a1 mutant revealed a quantal postsynaptic response, suggesting that at least some vesicles are loaded. In addition, loss of V0a1 impairs synaptic vesicle cycling in an FM1-43 dye uptake assay, whereas pharmacological block of the V-ATPase with bafilomycin causes no significant defect in this assay (Hiesinger et al., 2005). These findings supported previous studies of an acidification-independent role of the yeast V0 sector and specifically the V0a1 orthologue vph1 in vacuole fusion (Peters et al., 2001; Bayer et al., 2003). They also support earlier controversial implications of the V-ATPase V0 sector in neurotransmitter release (Israël et al., 1986). More recently, numerous studies have added evidence in worm, fish, fly, and mouse for possible acidification-independent roles of various V-ATPase V0 subunits in secretion or membrane fusion (Bayer et al., 2003; Lee et al., 2006; Liégeois et al., 2006; Sun-Wada et al., 2006; Peri and Nüsslein-Volhard, 2008; Di Giovanni et al., 2010; Williamson et al., 2010; Strasser et al., 2011). However, questions about the relationship of V-ATPase–dependent acidification and the observed secretion or membrane fusion defects remained. How can one cleanly separate between two protein functions if one potentially depends on the other? The study of V0a1 has been complicated by the finding that it is not only a synaptic vesicle protein but also localizes to other organelles. Consequently, specific disruption of the acidification function of V0a1 led to endolysosomal acidification defects, even though it partially restored neurotransmission (Williamson et al., 2010). A better dissection of the two possible functions is needed.
In this issue of JCB, Poëa-Guyon et al. provide compelling evidence for two separable functions of V0a1 in acidification and exocytosis. Instead of a genetic dissection, they opted for an elegant temporal dissection with the idea that acute inactivation of a function of V0a1 in exocytosis should instantly block neurotransmission, whereas acute inactivation of the proton pump should leave neurotransmission functional as long as loaded vesicles are available. Indeed, Poëa-Guyon et al. (2013) found a fast disruption of secretion in both primary rat neuronal culture and chromaffin cells when they acutely inactivated V0a1 using chromophore-assisted light inactivation. In contrast, inactivation of the reversibly associated V1 sector revealed very different effects than what would be expected from a loss of the proton pump.
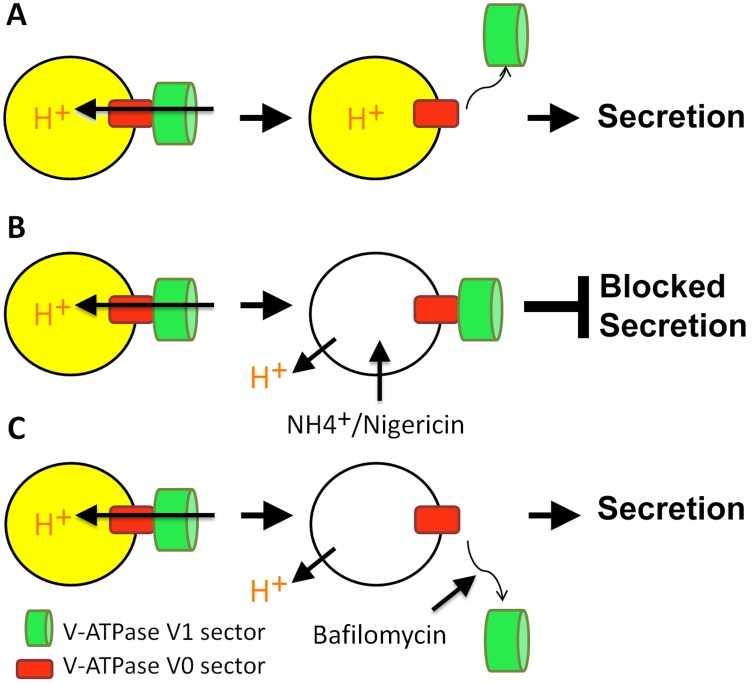
How about the dependence of exocytosis on acidification? Poëa-Guyon et al. (2013) developed an assay based on granule exocytosis in neurosecretory PC12 cells. Compartmental proton gradients can be abolished by a variety of means, including pharmacological inhibition of the V-ATPase with bafilomycin or concanamycin. A more acute destruction of intracompartmental proton gradients can be achieved through addition of alkalizing ammonium chloride or the potassium ionophore nigericin, which exchanges intracompartmental protons with potassium ions. Interestingly, Poëa-Guyon et al. (2013) found that only the acute disruption of the intracompartmental proton gradients with ammonium chloride or nigericin leads to an impairment of secretion but not block of the V-ATPase. What happens to the V-ATPase and its acidification-independent function under these different conditions? The authors show that both ammonium chloride and nigericin not only abolish the proton gradient but also lead to increased association of the V0 and V1 sectors (Fig. 1). This association is required to form a functional pump. A straightforward explanation for this observation is that the cell attempts to activate the proton pump to reacidify vesicles that lost their proton gradient. In contrast, the pharmacological block of the V-ATPase itself leads to increased free V0 sectors, consistent with loss of proton pump function. In a key experiment, Poëa-Guyon et al. (2013) show that this pharmacological inhibition of the V-ATPase with bafilomycin can override the effects of ammonium chloride or nigericin and restore secretion. If the pharmacological block of the V-ATPase prevents V0–V1 association, no functional pumps assemble even in the presence of ammonium chloride or nigericin. This result implies that the V0–V1 association itself prevents exocytosis. Pharmacological V0–V1 dissociation seems sufficient to expose V0 and exert a V1-independent function in exocytosis. This conclusion is consistent with previous findings in which the same pharmacological inhibition of the V-ATPase was found to leave exocytosis and endocytosis intact (Cousin and Nicholls, 1997; Hiesinger et al., 2005). However, the interpretation of the data changes: according to the new findings, exocytosis does not depend on vesicle acidification, per se, but on V0–V1 association that results from lack of acidification. Acidification and V-ATPase assembly thereby become a checkpoint for vesicle loading, and the assembled V-ATPase becomes a no-go signal for fusion (Fig. 1). The model is elegant and leads Poëa-Guyon et al. (2013) to suggest the V-ATPase as an acidification sensor, similar to previous observations (Hurtado-Lorenzo et al., 2006). However, whether it is really the V-ATPase itself that senses the proton gradient is not directly assessed in this study.
Figure 1.
V-ATPase V0–V1 association blocks secretion. (A) Poëa-Guyon et al. (2013) suggest that dissociation of V0 (red boxes) and V1 (green cylinders) sectors follows vesicle acidification (yellow) and frees the V0 sector for an acute, acidification-independent function in secretion.(B) V-ATPase–independent pharmacological disruption of vesicular acidification causes increased V0–V1 assembly of the functional proton pump, which in turn blocks secretion.(C) Pharmacological disruption of the V-ATPase disrupts both vesicle acidification and V0–V1 assembly, thereby permitting V0-dependent secretion. This mechanism can override disruption of acidification shown in B and restores secretion of nonacidified vesicles.
How general is the V-ATPase checkpoint, and what is the mechanism of V0-mediated, acidification-independent exocytosis? Both questions remain unanswered. The checkpoint idea is beautiful and does not obviously contradict current ideas on exocytic regulation. However, potential mechanisms for V0-mediated membrane fusion remain controversial (Saw et al., 2011; Ernstrom et al., 2012). In yeast, V0 proteolipid expansion in the membrane has been proposed to play a direct role in lipid mixing during vacuole fusion based on a thorough genetic dissection of fusion and acidification functions of the V0 sector (Strasser et al., 2011). No such role has hitherto been shown for neurotransmitter release, which comprises numerous different forms of vesicle release that are differentially regulated. A better genetic or pharmacological dissection is needed to reveal when, where, and how V0 meddles with membrane fusion.
Acknowledgments
We would like to thank all members of the Hiesinger laboratory for discussion.
Work in our laboratory is supported by grants from the National Institutes of Health (RO1EY018884) and the Welch Foundation to P.R. Hiesinger (I-1657). P.R. Hiesinger is a Eugene McDermott Scholar in Biomedical Research.
References
- Bayer M.J., Reese C., Buhler S., Peters C., Mayer A. 2003. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J. Cell Biol. 162:211–222 10.1083/jcb.200212004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin M.A., Nicholls D.G. 1997. Synaptic vesicle recycling in cultured cerebellar granule cells: role of vesicular acidification and refilling. J. Neurochem. 69:1927–1935 10.1046/j.1471-4159.1997.69051927.x [DOI] [PubMed] [Google Scholar]
- Di Giovanni J., Boudkkazi S., Mochida S., Bialowas A., Samari N., Lévêque C., Youssouf F., Brechet A., Iborra C., Maulet Y., et al. 2010. V-ATPase membrane sector associates with synaptobrevin to modulate neurotransmitter release. Neuron. 67:268–279 10.1016/j.neuron.2010.06.024 [DOI] [PubMed] [Google Scholar]
- Ernstrom G.G., Weimer R., Pawar D.R., Watanabe S., Hobson R.J., Greenstein D., Jorgensen E.M. 2012. V-ATPase V1 sector is required for corpse clearance and neurotransmission in Caenorhabditis elegans. Genetics. 191:461–475 10.1534/genetics.112.139667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. 2007. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8:917–929 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- Hiesinger P.R., Fayyazuddin A., Mehta S.Q., Rosenmund T., Schulze K.L., Zhai R.G., Verstreken P., Cao Y., Zhou Y., Kunz J., Bellen H.J. 2005. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell. 121:607–620 10.1016/j.cell.2005.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A., Skinner M., El Annan J., Futai M., Sun-Wada G.H., Bourgoin S., Casanova J., Wildeman A., Bechoua S., Ausiello D.A., et al. 2006. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8:124–136 10.1038/ncb1348 [DOI] [PubMed] [Google Scholar]
- Israël M., Morel N., Lesbats B., Birman S., Manaranche R. 1986. Purification of a presynaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc. Natl. Acad. Sci. USA. 83:9226–9230 10.1073/pnas.83.23.9226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Rho J., Jeong D., Sul J.Y., Kim T., Kim N., Kang J.S., Miyamoto T., Suda T., Lee S.K., et al. 2006. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 12:1403–1409 10.1038/nm1514 [DOI] [PubMed] [Google Scholar]
- Liégeois S., Benedetto A., Garnier J.M., Schwab Y., Labouesse M. 2006. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 173:949–961 10.1083/jcb.200511072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Dedieu J.C., Philippe J.M. 2003. Specific sorting of the a1 isoform of the V-H+ATPase a subunit to nerve terminals where it associates with both synaptic vesicles and the presynaptic plasma membrane. J. Cell Sci. 116:4751–4762 10.1242/jcs.00791 [DOI] [PubMed] [Google Scholar]
- Peri F., Nüsslein-Volhard C. 2008. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 133:916–927 10.1016/j.cell.2008.04.037 [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer M.J., Bühler S., Andersen J.S., Mann M., Mayer A. 2001. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 409:581–588 10.1038/35054500 [DOI] [PubMed] [Google Scholar]
- Poëa-Guyon S., Raafet Ammar M., Erard M., Amar M., Moreau A.W., Fossier P., Gleize V., Vitale N., Morel N. 2013. The V-ATPase membrane domain is a sensor of granular pH that controls the exocytotic machinery. J. Cell Biol. 203:283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw N.M., Kang S.Y., Parsaud L., Han G.A., Jiang T., Grzegorczyk K., Surkont M., Sun-Wada G.H., Wada Y., Li L., Sugita S. 2011. Vacuolar H(+)-ATPase subunits Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake, and storage. Mol. Biol. Cell. 22:3394–3409 10.1091/mbc.E11-02-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B., Iwaszkiewicz J., Michielin O., Mayer A. 2011. The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J. 30:4126–4141 10.1038/emboj.2011.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun-Wada G.H., Toyomura T., Murata Y., Yamamoto A., Futai M., Wada Y. 2006. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J. Cell Sci. 119:4531–4540 10.1242/jcs.03234 [DOI] [PubMed] [Google Scholar]
- Ungermann C., Wickner W., Xu Z. 1999. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl. Acad. Sci. USA. 96:11194–11199 10.1073/pnas.96.20.11194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson W.R., Wang D., Haberman A.S., Hiesinger P.R. 2010. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J. Cell Biol. 189:885–899 10.1083/jcb.201003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Denef N., Schüpbach T. 2009. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev. Cell. 17:387–402 10.1016/j.devcel.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D.M. 2011. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 334:678–683 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]