Abstract
Fragile X Syndrome (FXS) is the most common single-gene inherited form of intellectual disability with behaviors characteristic of autism. People with FXS display childhood seizures, hyperactivity, anxiety, developmental delay, attention deficits, and visual-spatial memory impairment, as well as a propensity for obsessive-compulsive disorder (OCD). Several of these aberrant behaviors and FXS-associated synaptic irregularities also occur in “fragile X mental retardation gene” knock-out (Fmr1 KO) mice. We previously reported that minocycline promotes the maturation of dendritic spines - postsynaptic sites for excitatory synapses - in the developing hippocampus of Fmr1 KO mice, which may underlie the beneficial effects of minocycline on anxiolytic behavior in young Fmr1 KO mice. In this study, we compared the effectiveness of minocycline treatment in young and adult Fmr1 KO mice, and determined the dependence of behavioral improvements on short-term versus long-term minocycline administration. We found that 4 and 8 week long treatments significantly reduced locomotor activity in both young and adult Fmr1 KO mice. Some behavioral improvements persisted in young mice post-treatment, but in adults the beneficial effects were lost soon after minocycline treatment was stopped. We also show, for the first time, that minocycline treatment partially attenuates the number and severity of audiogenic seizures in Fmr1 KO mice. This report provides further evidence that minocycline treatment has immediate and long-lasting benefits on FXS-associated behaviors in the Fmr1 KO mouse model.
Keywords: Fragile X Syndrome, minocycline, anxiety, hyperactivity, perseverance, audiogenic seizures
1. Introduction
Fragile X Syndrome (FXS) is the most common, inherited, single-gene cause of intellectual disability and the largest known genetic cause of autistic behaviors. Individuals with FXS display childhood seizures, hyperactivity, anxiety, developmental delay, attention deficits, and visual-spatial memory impairment, as well as a propensity for repetitive behavior (Musumeci et al., 1999; Hagerman and Hagerman, 2002; Hagerman et al., 2010). Hypermethylation of long CGG repeats in the promoter of the Fragile X Mental Retardation 1 (FMR1) gene leads to gene silencing and deficiency of FMR1 protein (FMRP) (Brown et al., 1998; Khandjian, 1999). In mice, FMRP has been shown to regulate protein synthesis by binding and transporting mRNAs encoding key post-synaptic proteins that are translated in dendritic spines; and FMRP can stall ribosomal translocation of mRNAs linked to synaptic function (Khandjian, 1999; Greenough et al., 2001; Hou et al., 2006; Zalfa et al., 2006; Pfeiffer and Huber, 2009; Darnell et al., 2011).
FMRP deficiency impairs dendritic spine development and maintenance, defects that may underlie some behavioral aspects of FXS. Dendritic spines are small protrusions from the surface of dendrites that host the majority of post-synaptic excitatory contacts in the brain (Harris, 1999; Yuste and Bonhoeffer, 2001; Carlisle and Kennedy, 2005; Ethell and Pasquale, 2005). Changes in dendritic spine shape and number directly correlate with synaptic development and plasticity, and play a central role in learning, memory and cognition. Abnormal dendritic spine morphology is a hallmark of several neurodevelopmental disorders, including FXS, Rett syndrome and Down syndrome (Rudelli et al., 1985; Kaufmann and Moser, 2000; Fiala et al., 2002). Fmr1 KO mice, an animal model for FXS, exhibit dendritic spine abnormalities similar to the human condition (Grossman et al., 2006) and display FXS-associated behavioral impairments such as hyperactivity, anxiety, repetitive behavior and a susceptibility to audiogenic seizures (Yan et al., 2005; Dolen et al., 2007; Bilousova et al., 2009). We previously reported that minocycline treatment restored dendritic spine maturation in the hippocampus and improved behavior in young Fmr1 KO mice (Bilousova et al., 2009).
Our early results indicated that minocycline could be beneficial for the treatment of FXS-associated behaviors and those findings provided an impetus for successful clinical trials (Paribello et al., 2010; Utari et al., 2010). In one study, more than half (54%) of FXS subjects treated with minocycline showed improvements in language use. Those improvements included the use of more intelligible and expressive language, as well as an overall increase in verbal output (Utari et al., 2010). Minocycline treatment also improved attention span, social communication, attention deficit, perseveration, anxiety, self-injurious behavior, abnormal vocalizations, mood swings, social avoidance, and repetitive behavior in FXS subjects (Paribello et al., 2010). A case report on two FXS siblings who were given minocycline at a young age reported improvement in anxiety and aggression as well as academic aptitude in mathematics (Winarni et al., 2012). In addition, a randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with FXS resulted in greater global improvement compared to placebo (Leigh et al., 2013). These studies demonstrate that minocycline is well tolerated and provides significant functional benefits to people with FXS.
Major issues to be resolved for the use of this drug in treating FXS-associated behaviors are: 1) the relative effectiveness of minocycline at different ages, particularly young versus adult; 2) the dependence of behavioral improvements on the continuous administration of minocycline. That is, which behavioral improvements might revert after the drug is discontinued? To clarify these issues we assessed the short- and long-term effectiveness, and lasting benefits of minocycline treatment on FXS-like behaviors in young and adult Fmr1 KO mice.
2. Experimental Procedures
2.1 Ethics statement
All animal care protocols and procedures were approved by the UC Riverside Animal Care & Use Program, which is accredited by AAALAC International, and animal welfare assurance number A3439-01 is on file with the Office of Laboratory Animal Welfare (OLAW).
2.2 Animal care
The FVB.129P2-Fmr1tm1Cgr/J (Fmr1 KO) and their control strain FVB.129P2-Pde6b+Tyrc-ch/AntJ (WT) were obtained from the Jackson Laboratories. These mice do not suffer from retinal degeneration due to restoration of the pde6b allele and do not develop blindness, making them a suitable model for behavioral analysis. Mice were maintained in an AAALAC accredited facility under a 12 hour light/dark cycle with standard mouse chow. All studies were done in accordance with NIH and Institutional Animal Care and Use Committee guidelines. All mice were weaned at 28 days and were group housed with a maximum of 4 mice per cage. For behavioral studies mice were tested in the morning and returned to the vivarium at the completion of each set of daily tests. All behavioral experiments were performed in a designated testing room. Mice were monitored during testing by the observer using video monitor. Any mouse showing unexpected signs of distress, such as lethargy or poor grooming, were excluded from the studies and either treated or euthanized following recommendations of the campus veterinarian.
Minocycline was administered to neonatal WT and Fmr1 KO mice by adding it to the nursing mother’s drinking water at 30 mg/kg/day in dark, amber-colored bottles every day until the pups were weaned, as previously described (Bilousova et al., 2009). Minocycline was administered to weaned pups and adult mice through their drinking water at 30 mg/kg/day every day in dark, amber-colored bottles for 4 or 8 wks. This method of minocycline administration has been previously shown to yield detectable concentrations of minocycline in the blood of adult mice (Lee et al., 2006) and in the breast milk of lactating dams (Lin et al., 2005; Luzi et al., 2009). The volume of consumed water was monitored daily and was comparable between study groups (not shown). Young male WT and Fmr1 KO mice were born to and raised by WT or Fmr1 KO mothers, respectively, and either not treated (untreated group 1, 16 WT and 23 Fmr1 KO mice; untreated group 2, 17 WT and 18 Fmr1 KO mice) or treated with minocycline from birth for 4 wks (group 3, 17 WT and 16 Fmr1 KO mice) or 8 wks (group 4, 14 WT and 13 Fmr1 KO mice). Groups 1 and 3 were behaviorally tested prior to weaning at 4 wks of age. Group 4 was treated with minocycline from birth for 4 wks and continued to receive minocycline for an additional 4 wks after weaning (8 wks of treatment). Groups 2 and 4 were first tested at 8 wks of age. All groups were tested a second time 4 wks after treatment was stopped (4+4 wks or 8+4 wks, respectively). Age-matched untreated WT and Fmr1 KO mice (groups 1 and 2) received only water.
Adult 2 month old male WT and Fmr1 KO mice were tested prior to treatment. Control group 1 (15 WT and 19 Fmr1 KO mice) and group 2 (18 WT and 14 Fmr1 KO mice) received only water. Group 3 (14 WT and 14 Fmr1 KO mice) was treated with minocycline for 4 wks (4 wks of treatment) and group 4 (16 WT and 16 Fmr1 KO mice) was treated with minocycline for 8 wks (8 wks of treatment). All groups were tested immediately following treatment and 4 wks after treatment was stopped (4+4 wks or 8+4 wks, respectively). To eliminate differences in behavior attributable to retesting, all groups within a specific treatment regimen (WT untreated, Fmr1 KO untreated, WT treated and Fmr1 KO treated) experienced each of the behavioral tasks the same number of times. Overall, re-testing did not have a significant impact on the performance of young animals. Although re-testing affected the behavioral performance of adult mice in the open field, re-testing did not favor either genotype. Behavioral assessments at all ages included the elevated plus maze, open field test, and marble burying assay.
2.3 Elevated Plus Maze
The elevated plus maze consisted of 4 arms in a plus configuration. Two opposing arms had 15 cm tall walls (closed arms), and 2 arms were without walls (open arms). The entire maze sat on a stand 1 meter above the floor. Each arm measured 30 cm long and 10 cm wide. Mice were allowed to explore the maze for 5 min while being recorded by digital video from above. The examiner left the room during testing. The maze was wiped with 2–3% acetic acid, 70% ethanol and water between each test to eliminate odor trails. This test was always done following the open field test. TopScan Lite software was used to measure the percent of time spent in open arms and velocity. The time spent in open arm was used to evaluate exploratory behavior, specifically it was expected that animals with higher anxiety spend less time in the open arms (Carobrez and Bertoglio, 2005). The velocity and total arm entries were measured to evaluate overall locomotor activity. Assessments of the digital recordings were done by blinded observers. This test was performed in the afternoon on the same day as the open field analysis.
2.4 Open Field Test
Mice were tested for exploratory behavior by evaluating their tendency to travel to the center of an open field (Yan et al., 2004; Yan et al., 2005). A 72×72 cm open field arena with 50 cm high walls was constructed from opaque acrylic sheets with a clear acrylic sheet for the bottom. The open field arena was placed in a room that was brightly lit with fluorescent lights. One mouse at a time was placed in a corner of the open field and allowed to explore for 5 min while being recorded with digital video from above. The examiner left the room during testing. The floor was cleaned with 2–3% acetic acid, 70% ethanol, and water between tests to eliminate odor trails. This test was always performed prior to the elevated plus maze. Locomotor activity was scored as described previously with some modifications (Brown et al., 1999; Yan et al., 2005) using TopScan Lite software (Clever Sys., Inc, Reston, VA 20190). The arena was subdivided into a 4×4 grid of squares with the middle of the grid defined as the center. A line 4 cm from each wall was added to measure thigmotaxis. To score locomotor activity the following measures were used: total horizontal and vertical line crosses, average velocity, number of entries into the center, time spent in the center, velocity within the center, and velocity along the walls (thigmotaxis). Average velocity and total line crosses were measured to score locomotor activity. Assessments of the digital recordings were done by blinded observers. This test was always performed on animals in the morning.
2.5 Marble Burying Test
Following the open field and elevated plus behavior tests, mice were individually housed in a 28×17.5×12 cm transparent, plastic cage with 3–4 cm of bedding overnight. The next day each animal was tested within that same cage for marble burying activity: a test of anxiety and task perseverance (Njung’e and Handley, 1991; Pan et al., 2008). Specifically, 15 blue marbles, each 1.4 cm in diameter were evenly spaced within the cage: 4.5 cm apart, 3.5 cm from the long edge and 4.5 cm from the short edge. Marbles were cleaned with 2–3% acetic acid, then rinsed with water and thoroughly dried between trials. During testing, cages were covered with a clear acrylic sheet. Animals were given 30 min of exposure to the marbles and recorded by digital video from above. Marble burying was assessed as the number of individual marbles that the animal actively buried resulting in >90% coverage of the marble during the test. Assessments were done by a blinded observer who watched the digital recordings.
2.6 Audiogenic Seizure Susceptibility
Male WT and Fmr1 KO mice were either untreated or treated with minocycline from birth for 28–30 days. 15 WT and 23 Fmr1 KO mice were tested immediately following minocycline treatment and prior to weaning, together with age-matched untreated 21 WT and 26 Fmr1 KO mice. All mice were exposed to a high intensity siren generated by alternating 500 msec upward frequency modulated (FM) sweeps (2–6 kHz) and 495 msec downward FM sweeps (6–2 kHz) at an average sound pressure level of 110 dB at 19.5 cm for 15 min in an empty, transparent plastic cage with an open grid lid (28×17.5×19.5 cm). The high intensity siren was generated using a custom program (Batlab, Dr. Don Gans, Kent State University), a Microstar digital signal processing board and Tucker Davis System programmable attenuators (PA5). The sounds were further amplified with a power amplifier (Parasound) and presented through a speaker (Fostex FF165K, Madisound, WI) that was mounted on top of the open grid lid of the plastic cage. The experiment was performed on one cage of mice at a time (maximum of 5 mice per cage) that was placed in an 8×8 sq ft sound-proof chamber lined with anechoic foam (Gretch-Ken Industries Inc), with digital video recording from the long side of the cage. A similar method has been previously shown to trigger seizures in Fmr1 KO mice of the same age (Yan et al., 2004; Yan et al., 2005). Audiogenic seizures were scored by a blinded observer who watched videos of the experiments. Periods of wild running and jumping (WRJ), as well as seizures, were scored by the time of occurrence, length and type: tonic or status epilepticus. Clonic seizures were omitted from scoring since they can be subject to the observer’s interpretation. This test was performed on a separate group of animals that did not undergo any of the other testing paradigms. Any mouse showing unexpected signs of distress, such as lethargy or poor grooming, were either treated or euthanized following the recommendations of the campus veterinarian.
2.7 Statistical Analysis
For analysis of the effects of genotype and treatment two-way ANOVA was used to compare four groups (WT untreated, Fmr1 KO untreated, WT treated and Fmr1 KO treated mice) within a specific experiment (4 wks, 8wks, 4+4 wks, or 8+4 wks). All four groups of animals within a specific experiment were age-matched and experienced each of the behavioral tasks the same number of times. For open field, elevated plus maze and marble burying behavior analyses, following ANOVA post-hoc pair-by-pair differences between groups were resolved with the least significant difference (LSD) using Dunnett’s method and Hsu’s Multiple Comparison with the Best (MCB) treatment. For audiogenic seizure behavior analysis when ANOVA was applicable post-hoc pair-by-pair differences between groups were resolved with the LSD using Dunnett’s method and Hsu’s MCB treatment (Fig. 5B and 5C), otherwise differences between groups were resolved using Fisher’s exact test, the effects of likelihood chi-square ratio and the odds ratio comparison (Fig. 5A and 5D).
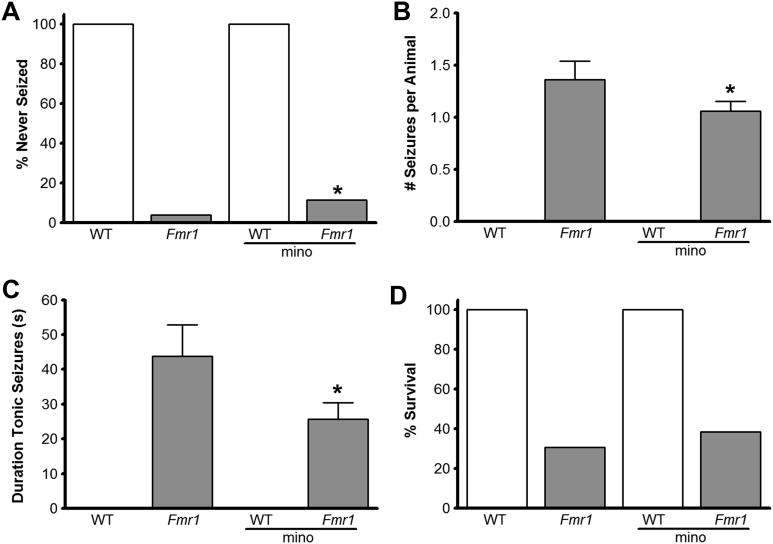
Figure 5. Minocycline moderately reduced susceptibility of P28–30 Fmr1 KO mice to audiogenic seizures and increased the odds of survival.
(A) The percent of animals that never seized. (B) The average number of seizures per animal. (C) The total duration of tonic seizures. (D) The percent of animals that survived the test. Minocycline treatment provided a 2-fold increase in the rate of survival in the Fmr1 KO mice (D) and caused a 7-fold reduction in the seizure activity of Fmr1 KO mice (A). When applicable, statistical analysis was performed using one-way ANOVA and post-hoc pair-by-pair differences were resolved using Dunnett’s comparisons with control and Hsu’s multiple comparisons with the best (B,C). For all other measures, statistical differences were resolved using Fisher’s Exact Test, effect of likelihood Chi-square test and the odds ratio comparison (A,D). Vertical bars indicate SEM (n = 21 WT H2O, 26 Fmr1 KO H2O, 15 WT Mino, 23 Fmr1 KO Mino mice;*, p<0.05; **, p<0.01; ***, p<0.001 indicate significant differences between treated and untreated Fmr1 KO mice).
2.8 Measuring IgG Levels
Blood was collected from the minocycline-treated and age-matched untreated young Fmr1 KO mice immediately following the 4 week regimen beginning from birth as well as 6 weeks after administration had ceased. The blood was coagulated for 15 minutes at room temperature, spun down at 16 × g for 15 minutes at 4°C after which the plasma was saved and used for analysis. Plasma blood samples were diluted 1:4000 and 1:8000 and analyzed for total levels of IgG following the protocol for the IgG ELISA (Bethyl Laboratories, E99–131). Differences between treated and untreated mice were evaluated using student’s t-test.
2.9 Multi-Kinase ELISA Array
Hippocampi from 4 week minocycline-treated (from birth) or age-matched Fmr1 KO mice were dissected, lysed and analyzed for relative activation states of various kinases through analysis of their phosphorylation levels. The phosphorylation of serine (pS), tyrosine (pY) or threonine (pT) residues of Akt-1 (pS473), Akt-2 (pS474), ERK1/2 (ERK1: pT202, pY204; ERK2: pT185, pY187), p38 MAPK (pT180, pY182), GSK3α (pS21), and GSK3β (pS9) were detected following the protocol for the multi-kinase ELISA array (Symansis, MKA001). Briefly, hippocampi were collected and lysed in a buffer containing 6M urea, 10mM Tris-HCl (pH 7.4), 150mM NaCl, 1mM EDTA, 1mM EGTA, 1mM sodium pervanadate, 0.5% Triton X-100 and 1% protease inhibitor cocktail. Lysates were diluted 1:2 for analysis of Akt-1/2, ERK1/2, and GSKα/β and were diluted 1:5 for analysis of p38 MAPK. Total protein concentrations were evaluated in hippocampal lysates using the protocol for the BCA colorimetric protein assay (Pierce, catalog #23235). Three mice were assayed per genotype and treatment. Statistical analysis was performed using one-way ANOVA following which post-hoc pair-by-pair differences between groups were resolved with the Tukey-Kramer method.
3. Results
3.1 Behavioral performance in the elevated-plus maze
Young Fmr1 KO mice were tested for locomotor activity and anxiety in an elevated-plus maze by measuring time spent in open arms and total number of entries, respectively (Fig. 1). Young Fmr1 KO mice showed increased locomotor activity by making significantly more total arm entries than WT mice at 4 and 8 wks of age (p = 0.0075 and 0.0350; Fig. 1A), but spent less time in open arm per entry (p = 0.0311 and 0.0310; Fig. 1C). However, Fmr1 KO mice spent a higher percentage of time in open arms than WT mice (p = 0.0425 and 0.0025; Fig. 1E), most likely due to an increase in total number of entries. Although re-testing affected the behavioral performance of mice in the elevated plus maze, re-testing did not favor either genotype. Both WT and Fmr1 KO mice that experienced the test for the second time at 8 weeks of age (4+4 weeks; Fig. 1D, F) spent less time in the open arm than mice that experienced the test for the first time at 8 weeks of age (8 weeks; Fig. 1C, E). Interestingly, age also affected the performance of mice in the elevated plus maze. The differences between untreated WT and Fmr1 KO mice were less pronounced at 8 wks of age as compared to 4 wks of age (Fig. 1A, 1C, 1E) and diminished as the mice became older (> 3 months of age, not shown), suggesting that the elevated plus maze becomes a less reliable indicator in adult mice.
Figure 1. Minocycline treatment altered open arm exploration behavior in young Fmr1 KO mice.
(A–D) Graphs demonstrate the performance of young Fmr1 KO mice in the elevated plus maze as measured by the total number of arm entries (A, B) the total amount of time spent in the open arm per entry (C, D), and the percent of time spent in the open arms (E, F). Young WT and Fmr1 KO mice were first tested after 4 or 8 wks of treatment (A, C). All mice were tested a second time 4 wks after the treatment was stopped (4+4 wks or 8+4 wks, B, D). Vertical bars indicate SEM (n = 16 WT H2O, 23 Fmr1 KO H2O, 17 WT Mino, 16 Fmr1 KO Mino mice; *, p<0.05; **, p<0.01; ***, p<0.001).
Fmr1 KO mice treated with minocycline for 4 or 8 wks showed less locomotor activity by making significantly fewer total arm entries (p = 0.0285 and 0.0127; Fig. 1A) and spending more time in open arm per entry (p = 0.0270 and 0.0144; Fig. 1C), but less total time in the open arms (p = 0.0357 and 0.0392; Fig. 1E) than untreated Fmr1 KO mice, similar to untreated WT mice. Minocycline-treated mice showed no visible indications of distress or lethargy as compared to their untreated counterparts, suggesting that the reduction in locomotor activity was unlikely to be the result of poor drug tolerance. The effects of minocycline were maintained in young Fmr1 KO mice when the mice were tested again 4 wks after minocycline treatment was stopped (4+4 wks and 8+4 wks) with greater differences in total arm entries (p = 0.0063 and 0.0205; Fig. 1B). Minocycline-treated Fmr1 KO mice (4+4 wks and 8+4 wks) also spent significantly more time in open arm per entry (p = 0.0495 and 0.0089; Fig. 1D), but less total time in the open arms (p = 0.0197 and 0.0350; Fig. 1F) than untreated Fmr1 KO mice.
These findings establish that minocycline treatment reduces locomotor activity and anxiety in young Fmr1 KO mice, as evaluated in the elevated-plus maze, and demonstrate long lasting benefits of minocycline in young Fmr1 KO mice.
3.2 Minocycline has lasting effects on the behaviors of young Fmr1 KO mice in the open field test
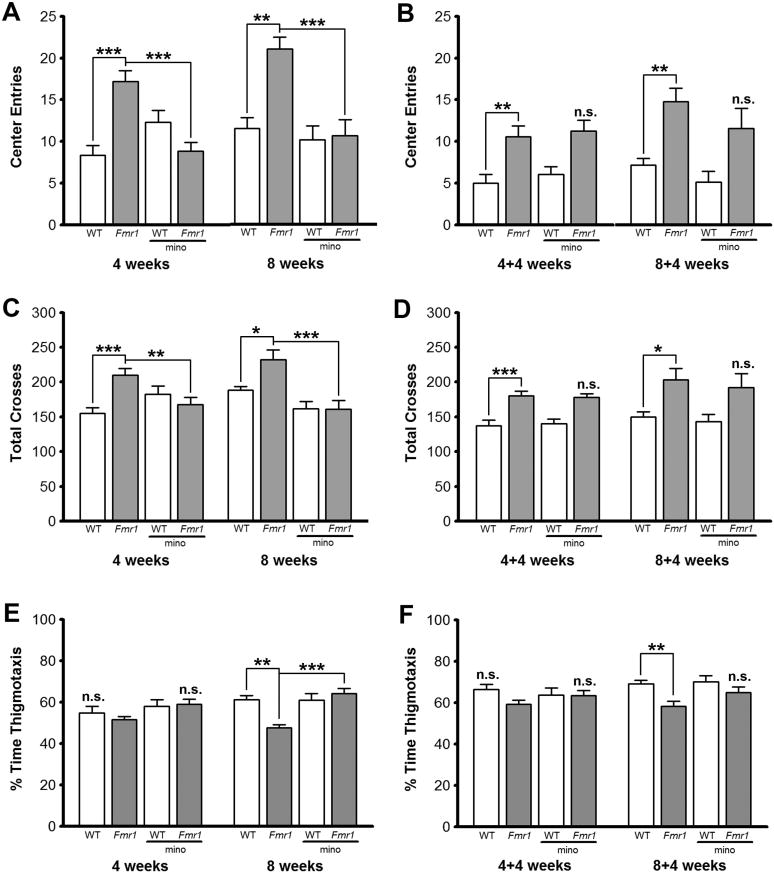
We used an open field test as another gauge of anxiety and locomotor activity, by determining the tendency of mice to travel through the center of an open field and the total number of lines crossed, respectively. Both young and adult Fmr1 KO mice showed increased locomotor activity with significantly more line crosses (p < 0.001 for all groups; Fig. 2 and 3). Interestingly, this activity correlated with an increased tendency to travel to the center of the open field. Young Fmr1 KO mice made significantly more center entries than WT mice at 4 wks and 8 wks of age (p = 0.0001 and p = 0.0015, respectively, Fig. 2A) and spent significantly less time in thigmotaxis at 4 wks and 8 wks of age (p = 0.0124 and p = 0.0134, respectively, Fig. 2E). Similar to the performance in the elevated plus maze, re-testing affected the behavior of mice in the open field, but did not favor either genotype. Both WT and Fmr1 KO mice that experienced the test for the second time at 8 weeks of age (4+4 weeks; Fig. 2B, D) made fewer line crosses and fewer center entries than mice that experienced the test for the first time at 8 weeks of age (8 weeks; Fig. 2A, C). Minocycline treatment for 4 wks improved the behavior of young Fmr1 KO mice, as they made significantly fewer lines crosses (p = 0.0181), fewer entries into the center of the open field (p = 0.0070), and spent more time in thigmotaxis (p = 0.0131) than untreated Fmr1 KO mice (4 wks in Fig. 2A, 2C, 2E). Indeed, young Fmr1 KO mice treated with minocycline for 4 wks behaved similar to age-matched WT mice in the open field test.
Figure 2. Minocycline alters the performance of young Fmr1 KO mice in open field test and the effects are maintained 4 wks post-treatment.
(A–D) Graphs demonstrate the performance of young Fmr1 KO mice in the open field as measured by the number of center entries (A, B), the total number of line crosses (C, D) and the percent time spent in thigmotaxis (E, F). WT and Fmr1 KO mice were first tested immediately after 4 or 8 wks of treatment (A, C). All mice were tested a second time 4 wks after the treatment was stopped (4+4 wks or 8+4 wks, B, D). Vertical bars indicate SEM (n = 16 WT H2O, 23 Fmr1 KO H2O, 17 WT Mino, 16 Fmr1 KO Mino mice; *, p<0.05; **, p<0.01; ***, p<0.001).
Figure 3. Minocycline treated adult Fmr1 KO mice demonstrated reduced hyperactivity and tendency to travel to the center of an open field, but these effects were not maintained 4 wks post-treatment.
(A–F) Graphs demonstrate the performance of adult Fmr1 KO mice in the open field as measured by the number of center entries (A, B), the total line crosses (C, D) and the percent time spent in thigmotaxis (E, F). WT and Fmr1 KO mice were tested immediately after 4 or 8 wks of treatment (A, C). All mice were tested again 4 wks after the treatment was stopped (4+4 wks or 8+4 wks, B, D). Vertical bars indicate SEM (n = 15 WT H2O, 19 Fmr1 KO H2O, 14 WT Mino, 14 Fmr1 KO Mino mice; *, p<0.05; **, p<0.01; ***, p<0.001).
Beneficial effects of minocycline were maintained in young Fmr1 KO mice 4 wks after treatment was stopped. Minocycline treated Fmr1 KO mice made fewer center entries (p = 0.0346 for 4+4 wks and p = 0.0122 for 8+4 wks; Fig. 2B) and spend more time in thigmotaxis (p = 0.0288 for 4+4 wks and p = 0.0385 for 8+4 wks; Fig. 2F) than untreated Fmr1 KO mice. Minocycline treated Fmr1 KO mice exhibited avoidance of the open field similar to untreated and treated WT mice. However, increased locomotor activity returned as no significant differences were found in numbers of total line crosses between minocycline treated and untreated young Fmr1 KO mice (Fig. 2D). Therefore, minocycline had long-lasting effects on the tendency of young Fmr1 KO mice to travel through the center of the open field, but not overall locomotor activity.
3.3 Minocycline effects on the behaviors of adult Fmr1 KO mice in the open field test
The behaviors of adult Fmr1 KO mice in the open field test were also significantly different than age-matched WT controls with Fmr1 KO mice making significantly more center entries and more line crosses (Fig. 3). Both 4 and 8 week treatments with minocycline significantly improved the behavior of adult Fmr1 KO mice in the open field test. That is, the behaviors of Fmr1 KO mice were similar to WT mice while they were receiving minocycline. However, the lasting effects of minocycline on center entry, that were also seen in young Fmr1 KO mice (Fig. 2B, 2F), were lost 4 wks after minocycline treatment was stopped (Fig. 3B). Minocycline treatment of adult Fmr1 KO mice also reduced their total line crosses after 4 wks and 8 wks of treatment (p = 0.0006 and p = 0.0203, respectively, Fig. 3C), and increased their time spent in thigmotaxis after 8 wks of treatment (p = 0.0005, Fig. 3E), but these effects were lost 4 wks after treatment was stopped (Fig. 3D, 3F). In most cases both young and adult Fmr1 KO mice made fewer line crosses after 4 wks and 8 wks of minocycline-treatment, but the effects were not maintained after treatment was stopped, indicating that minocycline effects on locomotor activity require continuous treatment in adult Fmr1 KO mice.
3.4 Minocycline reduces marble burying behavior in Fmr1 KO mice
A reduced ability to shift focus from one task or object to another is a common trait in FXS subjects that often manifests as OCD or perseverance. In mice, we analyzed the tendency of mice to focus on the task of burying marbles placed into their cage. Fmr1 KO mice buried significantly more marbles than WT mice at 4 and 8 wks of age (p = 0.0337 and p = 0.0057, respectively; Fig. 4A), and as adults (>2 months; Fig. 4C). Young Fmr1 KO mice treated with minocycline for 4 or 8 wks buried significantly fewer marbles than untreated Fmr1 KO mice (p = 0.0168 and 0.0257, Fig. 4A). Untreated adult Fmr1 KO mice buried 10.69 ± 0.40 marbles, compared to only 8.87 ± 0.73 for WT mice (p = 0.0146). A significant reduction in marble burying activity was also observed in adult Fmr1 KO mice following 4 wks and 8 wks of minocycline treatment (p = 0.0229 and 0.0384, respectively; Fig. 4C). The reduction in marble burying behavior was lost when adult Fmr1 KO mice were re-tested 4 wks after treatment was stopped (4+4 wks and 8+4 wks; Fig. 4D). By contrast, the beneficial effects of an 8 wk treatment on young Fmr1 KO mice lasted at least 4 wks after minocycline treatment was stopped (p = 0.0117, 8+4 wks in Fig. 4B). These results demonstrate that the ability to shift focus to new objects or tasks was enhanced by minocycline treatment in both young and adult Fmr1 KO mice, but its lasting effects were maintained only in young Fmr1 KO mice.
Figure 4. Minocycline decreased perseverative behavior in young and adult Fmr1 KO mice and demonstrated maintenance of the effect in young Fmr1 KO mice.
(A–D) Quantitative analysis of the number of marbles buried by young or adult mice: after 4 or 8 wks (A, C) of continuous treatment and 4 wks after the treatment was stopped (4+4 wks or 8+4 wks, B, D). Vertical bars indicate SEM (n = 16 WT H2O, 23 Fmr1 KO H2O, 17 WT Mino, 16 Fmr1 KO Mino young mice, n = 15 WT H2O, 19 Fmr1 KO H2O, 14 WT Mino, 14 Fmr1 KO Mino adult mice; *, p<0.05; **, p<0.01; ***, p<0.001).
3.5 Minocycline Moderately Reduces Audiogenic Seizures in Fmr1 KO Mice
At 4 wks of age Fmr1 KO mice are highly susceptible to audiogenic seizures (Fig. 5) that are often fatal (Musumeci et al., 2000; Yan et al., 2004; Yan et al., 2005); whereas, WT mice are resistant to audiogenic seizures (Table 2). Minocycline treatment reduced seizure susceptibility in Fmr1 KO mice as the number of animals that never seized significantly increased after minocycline treatment (p = 0.0491, Fig. 5A), although minocycline treated Fmr1 KO mice displayed WRJ (Table 2) similar to untreated Fmr1 KO mice. Among the mice that did seize, minocycline treated Fmr1 KO mice had significantly fewer seizures per animal (p = 0.0274, Fig. 5B) and a significantly shorter total duration of seizures (p = 0.0215, Fig. 5C), compared to untreated Fmr1 KO mice. This reduction in seizure susceptibility was accompanied by better survival of Fmr1 KO mice treated with minocycline, though the difference was not statistically significant (Fig. 5D).
TABLE 2.
Audiogenic seizure responses based on genotype and treatment.
| Genotype | Treatment | Number of Mice Tested | Number of Mice Exhibit No Response | Number of Mice Exhibit WRJ | Number of Mice Exhibit Tonic Seizure |
|---|---|---|---|---|---|
| WT | H2O | 21 | 21 (100%) | 0 (0%) | 0 (0%) |
| Mino | 15 | 15 (100%) | 0 (0%) | 0 (0%) | |
| KO | H2O | 26 | 0 (0%) | 26 (100%) | 26 (100%) |
| Mino | 23 | 0 (0%) | 23 (100%) | 19 (82.6%) |
3.6 Impact of minocycline treatment on IgG levels and relative activation states of Akt1/2, MAP kinases and GSKα/β
Since a rare, but serious side-effect of minocycline treatment is the occurrence of drug-induced auto-immunity we analyzed IgG levels in young Fmr1 KO mice to determine if minocycline-treated mice might be more susceptible to auto-immunity than untreated mice. IgG levels were measured immediately following 4 weeks of minocycline treatment and 6 weeks after treatment had ceased. There were no significant differences in IgG levels between treated and untreated Fmr1 KO mice (Table 3).
TABLE 3.
Effects of minocycline treatment on IgG levels
| IgG (μg/mL) | ||||
|---|---|---|---|---|
| Genotype | Treatment | Number of Mice Tested | 4 weeks | 4+6 weeks |
| H2O | 6 | 65.99 ± 4.60 | 260.09 ± 28.91 | |
| Mino | 7 | 71.03 ± 22.25 | 278.93 ± 35.30 | |
Minocycline has been previously shown to affect the activation states of several kinases, including Akt-1, Akt-2, ERK1/2, p38 MAPK and GSKα/β, which have also been implicated in the pathophysiology of FXS (Gallagher et al., 2004; Hou and Klann, 2004; Banko et al., 2006; Narayanan et al., 2007; Kim et al., 2008; Peineau et al., 2008; Min et al., 2009; Mines et al., 2010; Sharma et al., 2010). To test whether minocycline treatment also affected activity of these kinases in Fmr1 KO mice we have measured the relative phosphorylation levels of these kinases in the untreated WT, untreated Fmr1 KO and minocycline-treated Fmr1 KO. While we observed significantly higher phosphorylation levels of Akt1, Akt2 and ERK1/2 in the hippocampus of Fmr1 KO mice as compared to WT mice, minocycline treatment did not alter the phosphorylation levels of these kinases as it remained significantly higher in the hippocampus of minocycline-treated Fmr1 KO mice as compared to untreated WT mice (Table 4). Interestingly, phosphorylation levels of GSK3α were significantly lower in Fmr1 KO mice as compared to WT mice, but the difference were less significant after minocycline treatment, suggesting possible effects of minocycline on GSK signaling in Fmr1 KO mice that will be explored in future studies.
TABLE 4.
Impact of minocycline on relative phosphorylation levels of Akt1/2, pERK1/2, p38 MAPK, or GSK3α/β within the hippocampus of Fmr1 KO mice.
| Genotype | Treatment | Number of Mice Tested | Akt-1 | Akt-2 | ERK1/2 | p38 MAPK | GSK3α | GSK3β |
|---|---|---|---|---|---|---|---|---|
| WT | H2O | 3 | 1.000 ± 0.030 | 1.000 ± 0.015 | 1.000 ± 0.028 | 1.000 ± 0.218 | 1.000 ± 0.046 | 1.000 ± 0.036 |
| KO | H2O | 3 | 2.118 ± 0.347 a | 2.835 ± 0.496 a | 2.508 ± 0.207 a | 1.167 ± 0.273 | 0.440 ± 0.158 a | 0.878 ± 0.079 |
| Mino | 3 | 2.612 ± 0.092 | 3.485 ± 0.239 | 2.003 ± 0.775 | 1.361 ± 0.133 | 0.768 ± 0.256 | 1.108 ± 0.101 |
All values were corrected for total protein levels and then quantified as relative values to WT for each specific kinase. The level of WT group was set at 1.
Means statistically significant difference between WT and Fmr1 KO mice (p<0.05).
There were no significant differences between untreated and minocycline-treated Fmr1 KO mice.
4. Discussion
Here we report the effectiveness of minocycline treatment in alleviating FXS-like behaviors in both young and adult Fmr1 KO mice. We have clarified the importance of continuous minocycline administration in maintaining behavioral benefits in both age groups. Behaviors such as anxiety and locomotor activity were measured in the open field test by assessing the tendency of mice to travel to the center of an open field and by scoring the total number of line crosses, respectively (Yan et al., 2005; Spencer et al., 2011). While differences in open field behavior are highly dependent upon genetic background, previous studies have demonstrated increased locomotor activity of Fmr1 KO mice on the FVB background (Spencer et al., 2011). Others have also reported higher locomotor activity in Fmr1 KO mice, which also correlated with increased tendency of these mice to travel through the center of the open field (Yan et al., 2004; Yan et al., 2005). In our studies, minocycline treatment significantly reduced the total number of center entries and line crosses in both young and adult Fmr1 KO mice, demonstrating its effectiveness in reducing locomotor activity in Fmr1 KO mice. Interesting differences emerged in the ways minocycline treatment affected young versus adult Fmr1 KO animals after the treatment was stopped. The decreased tendency of young Fmr1 KO mice to travel through the center of the open field persisted post-treatment, but there was no significant difference in overall locomotor activity between untreated and treated Fmr1 KO mice after minocycline treatment was stopped. On the other hand, adult Fmr1 KO mice required continuous minocycline treatment to maintain reductions in locomotor activity and decreased tendency to travel through the center of an open field, as both effects were lost 4 wks post-treatment. Increased center activity in the open field may be an indication of lower anxiety (Prut and Belzung, 2003; Simon et al. 1994). Notably, drugs that demonstrate anxiolytic properties in humans often have little or even opposing effects in rodents tested in the open field (Prut and Belzung, 2003); although rodent drug responses do correlate with human anxiolytic behavior when assessed in the elevated plus maze (Pellow and File, 1986).
Minocycline effect on anxiolytic behavior was also observed in young Fmr1 KO mice in the elevated-plus maze and was retained 4 wks post-treatment. We and others have found this test to be a reliable indicator of anxiety in young animals, but inconclusive for adult animals (Carobrez and Bertoglio, 2005; Qin and Smith, 2008; Bilousova et al., 2009; Romero-Zerbo et al., 2009; Yuskaitis et al., 2010). Similar to the open field test, we found significant differences in total number of entries and time spent in the open arms within the elevated plus maze between young Fmr1 KO and WT mice, both of which were significantly improved by minocycline. The effects of minocycline on behavior in the elevated plus maze were maintained 4 weeks post-treatment, demonstrating a prolonged effectiveness for treating young Fmr1 KO mice.
We have also shown beneficial effects of minocycline on perseverative behavior in Fmr1 KO mice, as indicated by marble burying behavior. While there may be an anxiety component in this test, marble burying seems to correlate with focus and perseverance, a facet of obsessive-compulsive tendencies (Gyertyan, 1995; Thomas et al., 2009). Thomas and colleagues (2009) discovered that mice on the FVB background inherently bury more than other strains, and that inherent burying and digging behavior significantly decreased if animals were tested in a familiar environment (“home cage”) where they had been housed overnight prior to testing (Thomas et al., 2009). Although single-housing is known to be a stressor to both C57Bl/6 mice and the albino Swiss male mice (used to derive the original FVB line; Garattini et al., 1981; Valzelli, 1973; Voikar et al., 2005), the nature of this perseverance test required that mice be alone in the testing cage. Therefore, habituating the mice to individual cages overnight prior to testing helped to decrease their initial level of stress and inherent digging behavior. This experimental design allowed us to determine how minocycline treatment affected perseverance-like behavior in Fmr1 KO mice. Our results consistently showed more marble burying in Fmr1 KO mice than WT, and we demonstrated a significant reduction of this behavior during minocycline treatment in both young and adult animals. Furthermore, prolonged administration of minocycline was required to maintain this effect in adult, but not young, Fmr1 KO mice.
From 2–8 years of age, humans with FXS are susceptible to seizures when presented with loud sounds (Musumeci et al., 1999), which is replicated in young Fmr1 KO mice, most prominently around 4 wks of age (Yan et al., 2004; Yan et al., 2005). Minocycline treatment partially attenuated seizure susceptibility in young Fmr1 KO mice by decreasing the number and duration of seizures per animal, and by increasing the percentage of animals that never seized, along with a higher survival trend.
Altogether, findings presented here demonstrate that minocycline treatment reverses key behavioral deficits in Fmr1 KO mice and that these beneficial effects are maintained in young Fmr1 KO mice even after treatment was stopped, indicating a developmental window when minocycline treatment is most effective. Although the effects of minocycline treatment were most profound in young animals, benefits of this drug were not age-restricted as adult mice also responded to minocycline treatment. These results indicate that minocycline treatment has promise for treating both young and adult human subjects with FXS, and benefits may be more permanent when administration is started at an early age. However, our findings indicate that continuous minocycline treatment may be required to maintain behavioral improvements in adults. A concern of long-term minocycline treatment in both young and adult subjects, is a higher risk of developing autoimmunity, which has been reported to occur in ~1 in 10,000, especially in younger individuals (Elkayam et al., 1999; Lawson et al., 2001). We analyzed levels of total immunoglobulin G (IgG; Bethyl Laboratories, E99–131), the class of antibodies responsible for most antibody-based autoimmunity (Corley, 2004), but we did not detect any elevations after 4 wks of continuous minocycline treatment or 6 wks after minocycline treatment was stopped in young Fmr1 KO mice, although a frequency of 1 in 10,000 would not have been detectable in these studies.
Aside from its antibacterial properties minocycline has also been shown to impact brain function on several levels. Minocycline inhibits microglial proliferation and neuronal apoptosis through effects on signaling pathways, including changes in mitogen activated protein kinase (MAPK) activity (Yao et al., 2004; Hashimoto and Ishima, 2010) and Akt phosphorylation (Pi et al., 2004; Wilkins et al., 2004; Yao et al., 2004; Hashimoto and Ishima, 2010). FMRP effects on protein synthesis have been suggested to depend on MAPK activation and the phosphoinositide3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway (Gallagher et al., 2004; Hou and Klann, 2004; Banko et al., 2006; Narayanan et al., 2007; Kim et al., 2008; Sharma et al., 2010). Both of these pathways affect the activation states of glycogen synthase kinases (GSKs), which have also been implicated in FXS (Peineau et al., 2008; Min et al., 2009; Mines et al., 2010). However, minocycline treatment did not alter the relative phosphorylation levels of Akt, MAPK or GSK3 in the hippocampus of 4 week old Fmr1 KO mice.
Our previous work indicated that the beneficial effects of minocycline on dendritic spine morphology relate to its inhibitory action on matrix metalloproteinase-9 (MMP-9) expression and activity, and to counteract the increased expression and activity of MMP-9 in the hippocampus of Fmr1 KO mice (Bilousova et al., 2009). Earlier studies have shown that treatment of mice with 30 mg/kg/day of minocycline for 4 wks significantly decreased MMP-9 expression and activity in young and adult brains (Lee et al., 2006; Bilousova et al., 2009). It is possible that reducing excessive MMP-9 activity in young animals, during a critical developmental window, may prevent a delay in synapse development, which would be expected to impact behavior. More recent clinical studies also report elevated MMP-9 levels in blood plasma of the human subjects with FXS and these levels are reduced during minocycline treatment (Dziembowska et al., 2013). Therefore, the behavioral effects of minocycline may be attributable to its effects on MMPs (Siller and Broadie, 2012). Recent studies by Siller and Broadie (2011) support this hypothesis by demonstrating that TIMP overexpression, an endogenous MMP inhibitor, and mmp1 deficiency can both rescue synaptic architecture within the neuromuscular junction of the dfmr1 null mutant, a Drosophila FXS model (Siller and Broadie, 2011). Minocycline treatment also prevented both structural over-elaboration and synaptic developmental defects in a wide range of circuits in dfmr1 null mutants (Siller and Broadie, 2011). Moreover, others have demonstrated a role for MMP-9 in synaptic plasticity, showing the activity-dependent dendritic localization and translation of MMP-9 following the induction and maintenance of long-term potentiation and the elongation and thinning of dendritic spines induced by MMP-9 activity (Nagy et al., 2006; Bozdagi et al., 2007; Okulski et al., 2007; Wang et al., 2008; Michaluk et al., 2011; Dziembowska et al., 2012). MMP-9 levels are elevated in mild cognitive impairment (Bruno et al., 2009) and during experimental epileptogenesis (Wilczynski et al., 2008), but MMP-9 activation is also necessary for inhibitory avoidance learning and long-term memory (Nagy et al., 2007). New treatments that focus on more specific MMP-9 inhibitors may prove beneficial for the treatment of FXS and possibly other cognitive disorders.
TABLE 1.
Baseline differences in open field behavior of adult WT and Fmr1 KO prior to minocycline treatment.
| Genotype | Number of Mice Tested | Number of Center Entries | Total Number of Crosses | Overall Velocity (mm/s) | Thigmotaxis (mm/s) |
|---|---|---|---|---|---|
| WT | 29 | 11.69 ± 0.92 | 159.88 ± 5.02 | 53.19 ± 1.47 | 42.83 ± 1.50 |
| KO | 33 | 20.52 ± 1.38 | 225.96 ± 8.44 | 72.91 ± 2.46 | 61.92 ± 2.96 |
| p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | ||
Effectiveness of minocycline treatment in alleviating FXS-like behaviors in Fmr1 KO mice
Minocycline reduces locomotor activity and anxiety in young and adult Fmr1 KO mice
Continuous minocycline treatment is required to maintain the effects in adult Fmr1 KO mice
Beneficial effects of minocycline on perseverative behavior in Fmr1 KO mice
Minocycline partially attenuates the number and severity of audiogenic seizures in Fmr1 KO mice
Acknowledgments
We thank Dr. Michael Tranfaglia and members of the both Ethell laboratories for helpful discussions. We would also like to thank Adrian Gamez and Sadaf Sherzai for assistance with behavioral testing. The studies were supported by the grant from the FRAXA Research Foundation.
Abbreviations
- dfmr1
Drosophila Fragile X Syndrome model
- FXS
Fragile X Syndrome
- FMR1
Fragile X mental retardation gene
- Fmr1 KO
Fragile X mental retardation gene knock-out
- FMRP
Fragile X mental retardation protein
- GSK
Glycogen synthase kinase
- LSD
Least significant difference
- mTOR
Mammalian target of rapamycin
- MMP
Matrix Metalloproteinase
- MAPK
Mitogen activated protein kinase
- MCB
Multiple comparison with the best
- OCD
Obsessive compulsive disorder
- PI3K
Phosphoinositide-3 kinase
- wks
Weeks
- WRJ
Wild running and jumping
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet. 1999;29:263–271. [Google Scholar]
- Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD, Warren ST. Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem. 1998;273:15521–15527. doi: 10.1074/jbc.273.25.15521. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Mufson EJ, Wuu J, Cuello AC. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68:1309–1318. doi: 10.1097/NEN.0b013e3181c22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR, Kaczmarek L. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32:14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ, Tassone F. High activity levels of MMP-9 in fragile X syndrome are lowered by minocycline. Am J of Med Gen. 2013 doi: 10.1002/ajmg.a.36023. In press. [DOI] [PubMed] [Google Scholar]
- Elkayam O, Yaron M, Caspi D. Minocycline-induced autoimmune syndromes: an overview. Semin Arthritis Rheum. 1999;28:392–397. doi: 10.1016/s0049-0172(99)80004-3. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini S, Valzelli L. Is the isolated animal a possible model for phobia and anxiety? Prog Neuropsychopharmacol. 1981;5:159–165. doi: 10.1016/0364-7722(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. PNAS. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyan I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995;6:24–31. [PubMed] [Google Scholar]
- Hagerman R, Hagerman P. Fragile X Syndrome: diagnosis, treatment, and research. Baltimore: The Johns Hopkins University Press; 2002. The physical and behavioral phenotype; pp. 3–109. [Google Scholar]
- Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ishima T. A novel target of action of minocycline in NGF-induced neurite outgrowth in PC12 cells: translation initiation [corrected] factor eIF4AI. PLoS One. 2010;5:e15430. doi: 10.1371/journal.pone.0015430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Khandjian EW. Biology of the fragile X mental retardation protein, an RNA-binding protein. Biochem Cell Biol. 1999;77:331–342. [PubMed] [Google Scholar]
- Kim SH, Markham JA, Weiler IJ, Greenough WT. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. PNAS. 2008;105:4429–4434. doi: 10.1073/pnas.0800257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson TM, Amos N, Bulgen D, Williams BD. Minocycline-induced lupus: clinical features and response to rechallenge. Rheumatology (Oxford) 2001;40:329–335. doi: 10.1093/rheumatology/40.3.329. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, Lin E, Yang GY, Young WL. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006;26:1157–1164. doi: 10.1038/sj.jcbfm.9600268. [DOI] [PubMed] [Google Scholar]
- Leigh MJ, Nguyen DV, Mu Y, Winarni TI, Schneider A, Checi T, Polussa J, Doucet P, Tassone F, Rivera S, Hessl D, Hagerman RJ. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with Fragile X sydrome. J Dev Behav Ped. 2012;34:147–155. doi: 10.1097/DBP.0b013e318287cd17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wei X, Bales KR, Paul AB, Ma Z, Yan G, Paul SM, Du Y. Minocycline blocks bilirubin neurotoxicity and prevents hyperbilirubinemia-induced cerebellar hypoplasia in the Gunn rat. Eur J Neurosci. 2005;22:21–27. doi: 10.1111/j.1460-9568.2005.04182.x. [DOI] [PubMed] [Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res. 2009;1300:146–158. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci. 2011;124:3369–3380. doi: 10.1242/jcs.090852. [DOI] [PubMed] [Google Scholar]
- Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP. Elevated glycogen synthase kinase-3 activity in Fragile X mice: Key metabolic regulator with evidence for treatment potential. Neuropharmacol. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem. 2007;14:655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sánchez-Capelo A, Mallet J, Kaczmarek L. TIMP-1 Abolishes MMP-9-Dependent Long-lasting Long-term Potentiation in the Prefrontal Cortex. Biol Psychiatry. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Pan D, Sciascia A, 2nd, Vorhees CV, Williams MT. Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, Ethell DW. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153(Suppl 1):S428–437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The State of Synapses in Fragile X Syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC, Li M, Han Y. Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem. 2004;91:1219–1230. doi: 10.1111/j.1471-4159.2004.02796.x. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Qin M, Smith CB. Unaltered hormonal response to stress in a mouse model of fragile X syndrome. Psychoneuroendocrinology. 2008;33:883–889. doi: 10.1016/j.psyneuen.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Zerbo Y, Decara J, el Bekay R, Sanchez-Salido L, Del Arco-Herrera I, de Fonseca FR, de Diego-Otero Y. Protective effects of melatonin against oxidative stress in Fmr1 knockout mice: a therapeutic research model for the fragile X syndrome. J Pineal Res. 2009;46:224–234. doi: 10.1111/j.1600-079X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller SS, Broadie K. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Model Mech. 2011 doi: 10.1242/dmm.008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller SS, Broadie K. Matrix metalloproteinases and minocycline: therapeutic avenues for fragile x syndrome. Neural Plast. 2012;2012:124548. doi: 10.1155/2012/124548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Panissaud C, Costentin J. The stimulant effect of modafinil on wakefulness is not associated with an increase in anxiety in mice. A comparison with dexamphetamine. Psychopharmacology (Berl) 1994;114:597–600. doi: 10.1007/BF02244990. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, Faradz SM, Ethell IM, Nguyen DV. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil. 2010;115:433–443. doi: 10.1352/1944-7558-115.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wang X-b, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. PNAS. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A, Michaluk P, Wawrzyniak M, Malinowska M, Okulski P, Kolodziej LR, Konopka W, Duniec K, Mioduszewska B, Nikolaev E, Walczak A, Owczarek D, Gorecki DC, Zuschratter W, Ottersen OP, Kaczmarek L. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, Duncan I. Minocycline attenuates nitric oxide-mediated neuronal and axonal destruction in vitro. Neuron Glia Biol. 2004;1:297–305. doi: 10.1017/S1740925X05000104. [DOI] [PubMed] [Google Scholar]
- Winarni TI, Schneider A, Borodyanskara M, Hagerman RJ. Early Intervention Combined with Targeted Treatment Promotes Cognitive and Behavioral Improvements in Young Children with Fragile X Syndrome. Case Reports in Genetics. 2012;2012:4. doi: 10.1155/2012/280813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacol. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res. 2004;95:364–371. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Achsel T, Bagni C. mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol. 2006;16:265–269. doi: 10.1016/j.conb.2006.05.010. [DOI] [PubMed] [Google Scholar]