Abstract
Eosinophilic esophagitis (EoE) is a chronic immune-mediated condition believed to have an allergic component, but the timing of the initial allergen triggers that cause the disease is poorly understood. While the clinical presentation of EoE is often of longstanding symptoms, in animal models, acute exposure to an allergen challenge successfully produces EoE. In this report, we present three cases of individuals who developed esophageal eosinophilia and EoE shortly after a clearly identified exposure to aeroallergens. These cases highlight the allergic etiology of EoE, and provide a link from humans to the previously described experimental mechanisms.
Keywords: allergen, eosinophilic esophagitis, etiology
Introduction
Eosinophilic esophagitis (EoE) is a recently recognized disease defined by symptoms of esophageal dysfunction and an eosinophilic infiltrate in the esophageal mucosa.[1] The underlying pathogenesis is thought to involve a Th2 immune response to allergens.[2, 3] It has been hypothesized that food and aeroallergens play a role in the etiology of EoE, and in humans elimination or elemental diets lead to symptom improvement,[4–7] there is seasonal variation in diagnosis,[8–10] and esophageal eosinophilia varies based on climate zone.[11] In murine models, EoE has been produced by intra-nasal exposure to allergens such as Aspergillus fumigatus or ovalbumin after both respiratory and epicutaneous sensitization.[12–14] While this mechanism is presumed to operate in humans, to date there are no direct data to support this hypothesis, and on a clinical basis, the ability to determine a clear allergen exposure responsible for the onset of EoE is rare. Here, we present three instances where patients developed esophageal eosinophilia and EoE after a large and clearly identifiable aeroallergen exposure.
Case presentations
Case 1
The patient is a 22 year-old man with a history of anxiety, depression, obesity, and seasonal allergies who presented with dysphagia and heartburn. Three months prior to presentation, which was during the mid-spring, he was mowing his lawn. The mower jammed, backfired, and expelled a large amount of grass clippings into his face. He believed he may have swallowed some of the debris. Beginning within days of this exposure, he began having progressive solid-food dysphagia with a sense of food hanging in his upper esophagus. He had no difficulty with liquids. He also had associated substernal burning, a hoarse voice, globus sensation, and poor appetite. He had a subsequent 50 lb weight loss (though he remained obese). His initial esophagogastroduodenoscopy (EGD) 2 months after his exposure showed a ringed esophagus with longitudinal furrows (Figure 1A), and mid-esophageal biopsies revealed 45 eosinophils per high powered field (eos/hpf; hpf area = 0.24mm2). He was then started on omeprazole 20 mg twice daily for 8 weeks with an improvement in his symptoms. Follow up EGD showed persistent rings and only subtle furrows (Figure 1B), and biopsies demonstrated 4 eos/hpf in the distal esophagus and 2 eos/hpf in the proximal esophagus. This clinical picture was felt to be consistent with proton-pump inhibitor-responsive esophageal eosinophilia (PPI-REE), and omeprazole was decreased to once daily dosing. He did well for three months of follow-up, was monitored expectantly thereafter, and has not sought further care for recurrent symptoms.
Figure 1.
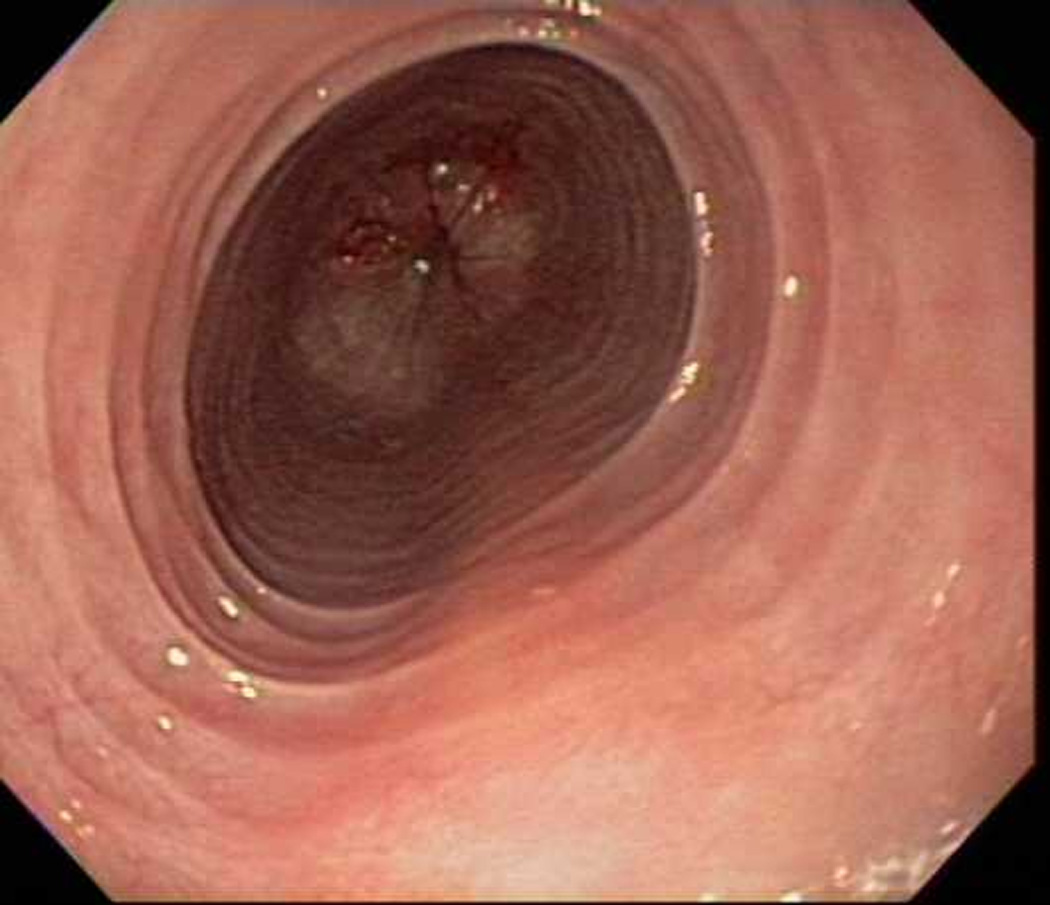
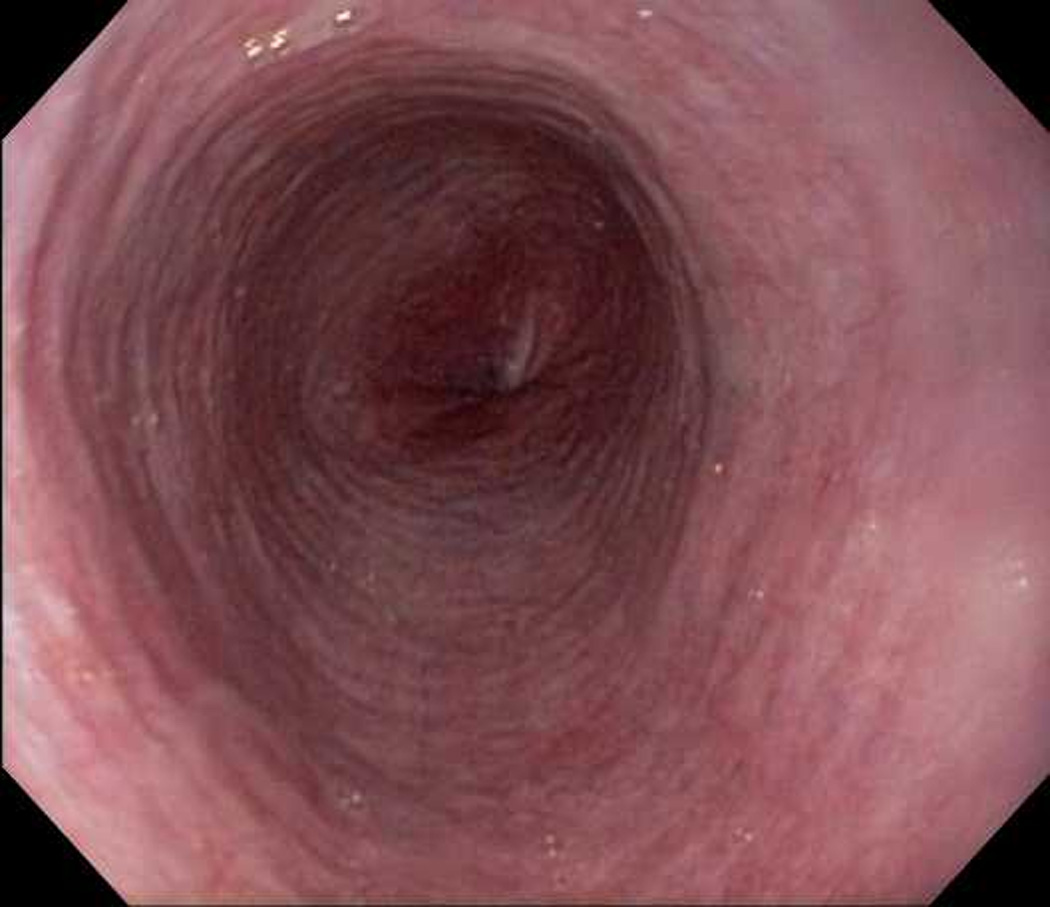
Endoscopic views of the esophagus in case 1 prior to (A) and after (B) PPI therapy. Before PPI, there are prominent fixed esophageal rings and decreased vascularity. After PPI, the rings are nearly resolved and vascularity is normal.
Case 2
The patient is a 20 year-old man with a history of depression and no known atopy who presented with dysphagia and chest pain. Prior to symptom onset, he was tasked at work to clean a small enclosed room that had become moldy. During the cleaning process, he believed that he inhaled and swallowed mold and dust. Subsequently, he began having a sore throat and increased difficulty swallowing. Within 10 days of his exposure he presented to an outside emergency department with a frank food impaction. EGD there revealed a feline esophagus with furrows and plaques, and random esophageal biopsies showed 30 eos/hpf. His symptoms did not resolve after treatment with swallowed topical fluticasone and budesonide, oral prednisone, and intravenous methylprednisolone. On presentation to our facility, he had ongoing dysphagia to solids with food sticking in the upper esophagus that would pass with large amounts of fluid intake, chest discomfort, and a 25–30 lb weight loss which he attributed primarily to decreased oral intake. A repeat EGD about 6 months after his exposure and after 8 weeks of treatment with esomeprazole 40 mg twice daily showed esophageal rings, linear furrows, and decreased vascularity (Figure 2). Biopsies showed 40 eos/hpf in both the distal and proximal esophagus consistent with EoE. The plan was to institute high-dose topical steroid therapy but the patient moved out of state and did not follow-up. Allergy testing was not performed on this patient, so sensitization remains unknown.
Figure 2.
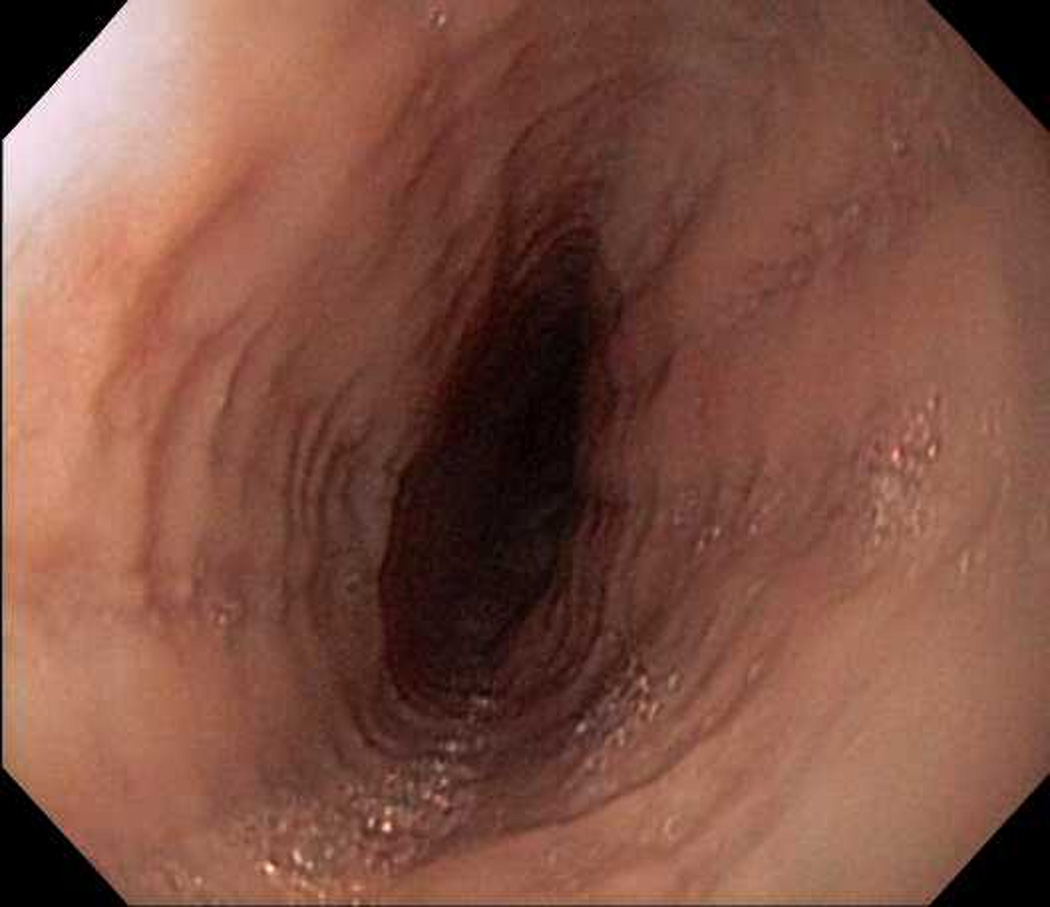
Endoscopic view of case 2 with esophageal rings, linear furrows, and decreased vascularity.
Case 3
This patient is a 39 year-old man with a history of asthma who presented with dysphagia which developed after renovating a home he purchased as a foreclosure property. He noted exposure to saw dust, mold, and dust during this work, particularly when he was removing rotted drywall and wood. About six months after his exposure, he began having solid food dysphagia and a sense of transient impaction of food. He was initially able to control symptoms by shifting to a soft food diet with nutritional supplements but could not meet his caloric requirements and lost 20 lbs. Allergy testing with skin prick showed no food allergies but did reveal IgE-sensitization to mites, house dust, grasses, and cats. Upper endoscopy one year after his exposure showed esophageal rings, furrows, and decreased vascularity (Figure 3), with distal esophageal scarring associated with pseudodiverticula and erythema. Pathology revealed >60 eos/hpf distally and 25 eos/hpf proximally with eosinophilic microabscesses. Lansoprazole 30 mg twice daily was started, and on repeat EGD there were persistent esophageal rings, scarring, and pseudodiverticuli, but the furrowing and erythema had resolved. Biopsies showed persistent esophageal eosinophilia with 40 eos/hpf in both the distal and proximal esophagus, confirming the diagnosis of EoE. He declined repeat allergy evaluation, and started an oral viscous budesonide slurry (1 mg twice daily mixed with 5g sucralose). Symptoms persisted and repeat EGD showed continuing endoscopic changes with ongoing esophageal eosinophilia on biopsy.
Figure 3.
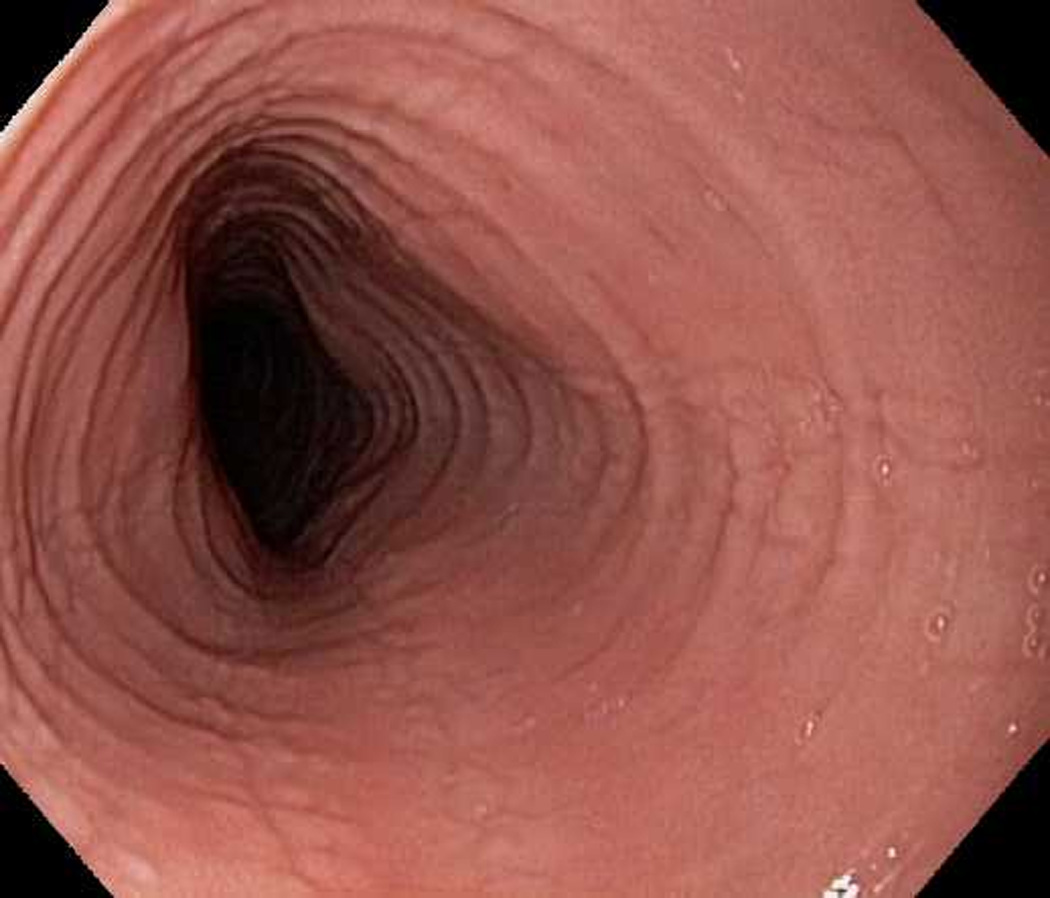
Endoscopic view of case 3 with esophageal rings, linear furrows, and decreased vascularity.
Discussion
Eosinophilic esophagitis is an immune-mediated disorder that has rapidly become an important cause of upper GI morbidity.[1] The evidence base for EoE as immune-mediated comes from a wide range of publications. First, a number of studies have noted a strong association between EoE and other atopic disorders such as asthma, allergic rhinitis and sinusitis, atopic dermatitis, and food allergies.[9, 15–19] Second, dietary therapy of EoE with elimination or elemental diets leads to symptom improvement and resolution of esophageal eosinophilia.[4–7] Third, seasonal variation in the diagnosis of EoE has been reported at a number of centers,[8–10] and the prevalence of esophageal eosinophilia varies based on climate zone in the United States.[11] Fourth, there has been a report of a few cases of EoE possibly induced by oral immunotherapy (OIT), i.e., as a result of allergen exposure.[20] Finally, a wealth of basic science research has shown that EoE is a Th2 mediated process where proinflammatory cytokines such as IL-4, IL-5, and IL-13 and the chemokine eotaxin-3 promote eosinophilic recruitment and infiltration of the esophageal mucosa, and that this process is reversible after eliminating offending allergens.[1–3, 21, 22] While the allergic basis of EoE is becoming more well characterized, it is difficult to identify the initial allergen insult. Even a food elimination and reintroduction protocol, while effective for many patients in identifying certain triggers,[7, 23] does not necessarily pinpoint the inciting event that causes EoE.
In this report, we present three cases where development of symptoms of esophageal dysfunction, esophageal eosinophilia, and EoE occurred shortly after a large and identifiable allergen exposure. In the first, the patient had known seasonal allergies and developed PPI-REE after inhalation and possible swallowing of grass clippings. In the second and third, while neither patient had known allergies before participating in cleaning and renovations that led to mold and dust exposures and development of frank EoE, allergic sensitization was demonstrated after the fact for the third patient. Both of these cases were also refractory to initial attempts at topical steroid therapy. To our knowledge, these are the first reports of esophageal eosinophilia and EoE that developed after large allergen exposures in humans. Despite only three case descriptions, we feel there are several important issues raised by our observations.
First, in these cases, the human development of EoE appears to mimic the mechanism seen in animal models. In murine models first established by Mishra and colleagues and subsequently used to understand the pathogenesis of EoE,[12, 14, 24, 25] the core mechanism for producing EoE is allergen exposure. Specifically, mice with respiratory, oral, or epicutaneous sensitization that are rechallenged with intra-nasal exposure to allergens develop esophageal eosinophilic infiltrates which mimics EoE.[12, 14] Longer follow-up of experimental EoE models also show esophageal remodeling and strictures.[26] For our cases, it is interesting that patients 1 and 3 either had a history of seasonal allergies or eventually tested positive for environmental allergens, potentially indicating past sensitization. All three had a large antigen challenge, likely by the respiratory and possibly by a swallowed route. For cases 2 and 3, they were exposed to mold, which echoes the Aspergillus challenge in mice.[13] While it has been long supposed that the murine model recapitulated the clinical scenario, until now human cases have been lacking to support that exact mechanism. Total serum IgE levels and peripheral eosinophil counts were not available for these patients, however.
Second, these cases suggest the importance of aeroallergen exposure in EoE. This is consistent with findings of seasonality and geographic distribution of EoE cases.[3, 8–11, 27–29] However, previous studies have explored associations between atopic disease and EoE, or month of diagnosis of EoE, but none show the initial allergen exposure that caused EoE.
Third, it is interesting to note the two patients with EoE in this report were refractory to initial treatments with topical steroids. This is consistent with some data showing that patients with atopic disease or ongoing allergen exposure may be more difficult to treat with topical corticosteroids.[30]
Finally, case 1 may be the first link of the PPI-REE phenotype to an allergic mechanism. PPI-REE has been only recently described,[31–34] and there are few details available to characterize these patients. It is not currently known if PPI-REE is a manifestation of GERD, a sub-type of EoE, or a different disease process,[1] but this case raises the question of whether PPI-REE might be immune-mediated.
These observations must be tempered by that acknowledgement that this is a case series of three patients. Nevertheless, it is interesting that these patients developed esophageal eosinophilia and EoE via a mechanism that is similar to the murine model for EoE. The cases also generate hypotheses that can be investigated in larger studies, and provide examples that, in some cases, the initial instigating trigger for EoE can be identified.
Acknowledgments
Grant support: This research was supported, in part, by NIH awards K23DK090073 (ESD)
Footnotes
Disclosures: There are no potential conflicts of interest for any of the authors pertaining to this study.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128 doi: 10.1016/j.jaci.2011.02.040. 3-20.e6. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology. 2009;137:1238–1249. doi: 10.1053/j.gastro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 4.Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–1512. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 6.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves N, Yang GY, Doerfler B, et al. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology. 2012:142. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–833. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107:698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108:891–894. doi: 10.1067/mai.2001.120095. [DOI] [PubMed] [Google Scholar]
- 14.Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–994. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 16.Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2010;10:231–237. doi: 10.1097/ACI.0b013e328338cbab. [DOI] [PubMed] [Google Scholar]
- 17.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The Role of Allergy Evaluation in Adults With Eosinophilic Esophagitis. J Clin Gastroenterol. 2010;44:22–27. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 18.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic Characteristics of Adult Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–535. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol. 2012;129:1155–1157. doi: 10.1016/j.jaci.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard C, Wang N, Rothenberg ME. Eosinophilic esophagitis: pathogenesis, genetics, and therapy. J Allergy Clin Immunol. 2006;118:1054–1059. doi: 10.1016/j.jaci.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–149. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 24.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1347–G1355. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31:509–515. doi: 10.1111/j.1365-2036.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41:451–453. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 29.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796–797. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]
- 30.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus--peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666–1670. doi: 10.1111/j.1572-0241.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 32.Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100. doi: 10.1016/j.jpeds.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment With High-dose Proton Pump Inhibitors Helps Distinguish Eosinophilic Esophagitis From Noneosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2009;49:393–399. doi: 10.1097/MPG.0b013e31819c4b3e. [DOI] [PubMed] [Google Scholar]
- 34.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal Eosinophilic Infiltration Responds to Proton Pump Inhibition in Most Adults. Clin Gastroenterol Hepatol. 2011;9:110–117. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]


