Abstract
Euphorbia wallichii a perennial herb growing mainly in Himalayas has been widely used in folk medicines for its medicinal properties. In the present study, the crude methanolic root extract (CME) and its fractions; n-Hexane Fraction (NHF), n-Butanol Fraction (NBF), Chloroform Fraction (CHF), Ethyl acetate Fraction (EAF) and Aqueous Fraction (AQF) of this plant specie were investigated for antioxidant and cytotoxic activities and phytochemical analysis. Antioxidant activity was determined by using 2,2-diphenyl-1-picryl-hydrazyl free radical (DPPH) and DNA protection assay performed on pBR322 plasmid DNA. In both these assays, promising results were obtained for CME as well as other fractions. The IC50 values for DPPH assay were in a range of 7.89 to 63.35 μg/ml in which EAF showed the best anti-oxidant potential and almost all the tested samples showed certain level of DNA protection. The cytotoxic activity was assessed by using Sulforhodamine B (SRB) assay on human cell lines; H157 (Lung Carcinoma) and HT144 (Malignant Melanoma). The IC50 values of the tested samples ranged from 0.18 to 1.4 mg/mL against H157 cell line whereas against HT144 cell line the IC50 values ranged from 0.46 to 17.88 mg/mL with NBF fraction showing maximum potential for both. Furthermore, the phytochemical analysis of CME and its fractions showed the presences of flavonoids, saponins, tannins, terpenoides and cardiac glycosides with varying concentrations.
Key Words: Antioxidant, Cytotoxicity, DNA protection, E. wallichii, Phytochemical analysis
Introduction
The Euphorbiaceae Family is one of the largest families of higher plants, about 300 genera and 7,500 species have been reported (1, 2). There are about 2,160 species in genus Euphorbia and it is regarded as the 6th largest genus among flowering plants. Many plants of this genus have been used in folk medicine for centuries against various diseases including infections, skin diseases as well as cancer (1-5).
E. wallichii is a perennial herb growing mainly in the Himalayas, including Qinghai-Tibetan Plateau area of China, India, Nepal, and Kashmir. The roots of this plant have been conventionally used in folk medicine for treatment of edema, skin disease, cutaneous anthrax and exanthema (6, 7). A chemical literature survey of E. wallichii, showed the presence of abietane (8), diterpenoids (6, 7) and triterpenoid of the multiflorane class (1). Recently Ali et al. (2) reported significant results for in vitro phytotoxicity, cytotoxicity and antibacterial activity of E. wallichii root extract and fractions. Plants of the genus Euphorbia are known to possess considerable anti-cancer (7), anti-tumor and antioxidant (7, 9) potential. However antioxidant and cytotoxic activity (towards cancer cells) of E. wallichii in comparison to its phytochemicals have not been reported previously. This provoked us to investigate E. wallichii for its cytotoxic and antioxidant activity along with phytochemical studies.
Experimental
Plant material
The roots of E. wallichii were collected from Mushkpuri tract, Nathia Gali, N.W.F.P. Pakistan in July 2008. The plant was identified by taxonomist Dr Rizwana Aleem Qureshi, Associate Professor, Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan with reference to “Flora of Pakistan” and comparing with already identified herbarium sheets preserved in the herbarium. A voucher specimen (Specimen No. 125755) was deposited in ISL Herbarium, Quaid-i-Azam University, Islamabad Pakistan.
Extraction and fractionation
Fresh roots of the E. wallichii were washed, sliced and dried under shade and ground. The root extract was prepared in analytical grade methanol (5 kg in 12 L) for 72 h, then methanol was removed and residue was immersed in methanol for further five days. Thereafter, the methanol was decanted and filtered with filter paper. The filtrate was subsequently concentrated under reduced pressure at 45°C in rotary evaporator (Rotavapor R-200 Buchi, Switzerland) and dried to a constant weight (700 gram) in vacuum oven at 45°C (Vacucell, Einrichtungen GmbH). This was called crude methanolic root extract (CME).
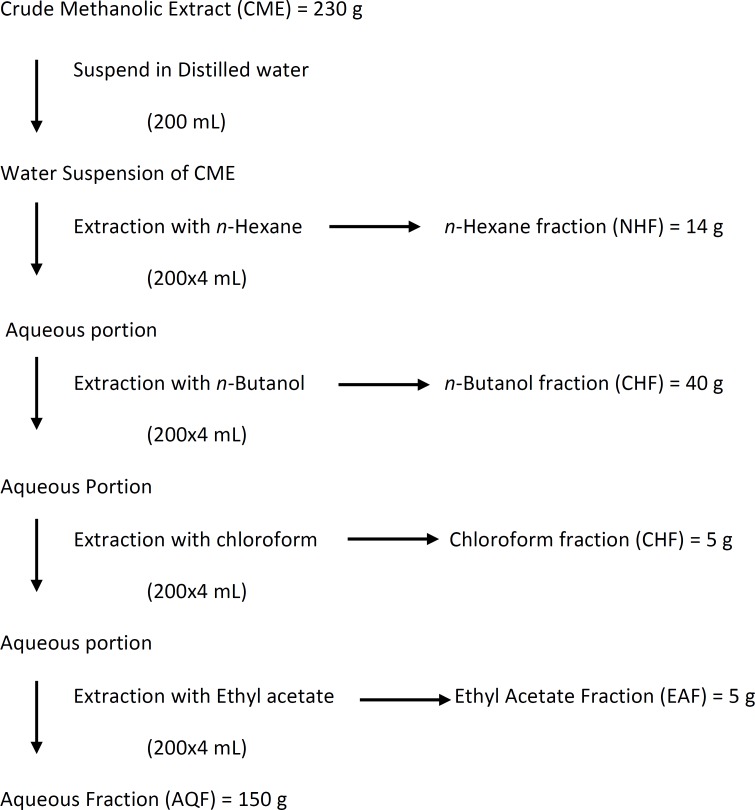
The CME was then subjected to fractionation, where 230 g of CME was suspended in 200 mL of distilled water. This aqueous suspension was further subjected to solvent-solvent extraction for five fractions, namely; n-Hexane Fraction (NHF), n-Butanol Fraction (NBF), Chloroform Fraction (CHF), Ethyl acetate Fraction (EAF) and aqueous Fraction (AQF). The overall fractionation procedure is given in flowchart diagram (Figure 4).
Figure 4.
Schematic diagram for fractionation of crude methanolic root extract (CME).
Biological activities
Determination of antioxidant activity
The free radical scavenging activity was measured by using 2,2-diphenyl-1-picryl-hydrazyl free radical (DPPH) assay. DPPH assay was performed according to the procedure described by Kulisic et al. (10) modified by Obeid et al. (11). DPPH solution was prepared by dissolving 3.2 mg DPPH in 100 mL of 82% methanol. 2800 μL of DPPH solution was added to glass vials followed by the addition of 200 μL of CME solution in Methanol; leading to the final concentration of 100 μg/mL, 50 μg/mL, 25 μg/mL, 10 μg/mL, 5 μg/mL, 2 μg/mL and 1 μg/mL. Mixtures were shaken well and kept in dark at controlled room temperature (25°C-28°C) for one hour. Absorbance was measured at 517 nm by using spectrophotometer (DAD 8453, Agilent). Methanol (82%) was used as blank while mixture of 200 μL of methanol and 2800 μL of DPPH solutions were taken as negative control. Ascorbic acid was used as positive control. Each test was performed in triplicates and percentage inhibition was measured according to formula given below and IC50 values were calculated by graphical method.
Scavenging effect (%) = [(Ac-As)/Ac] x100
Where “Ac” means Absorbance of negative control and “As” means Absorbance of test sample. In order to determine the antioxidant activity of different fractions the same procedure was then repeated with all of the fractions i.e. NBF, NHF, EAF and AQF.
DNA protection assay
To study the effects of CME and its fractions on plasmid DNA the procedure of Tian and Hua (12), modified by Nawaz et al. (13) was adopted. The reaction was conducted in an Eppendorf tube at a total volume of 15 μL containing following components; 0.5 μg pBR322 DNA suspended in 3 μL of 50mM phosphate buffer (pH 7.4), 3 μL of 2 mM FeSO4, 5 μL of tested samples (CME and its fractions) and 4 μL of 30% H2O2. Resulting mixture was incubated at 37°C for 1 h and was subjected to 1% agarose gel electrophoresis for 1 h at 100 volts. DNA bands (supercoiled, linear, and open circular) were stained with ethidium bromide and were qualitatively analyzed by scanning with Doc-IT computer program (VWR). Evaluations of antioxidant or prooxidant effects on DNA were based on the increase or loss percentage of supercoiled monomer, compared with the control value. To avoid the effects of photoexcitation of samples, experiments were done in the dark and untreated supercoiled DNA, supercoiled DNA treated with 2 mM FeSO4, supercoiled DNA treated with 30% H2O2 and supercoiled DNA treated with 2 mM FeSO4 + 30% H2O2 were used as control along with the test samples.
Cytotoxic activity by sulforhodamine B (SRB) assay
The human cancer cell lines H157 (lung carcinoma) and HT144 (malignant melanoma) were cultured in RPMI1640 media (Gibco BRL, Life Technologies, Inc) supplemented with 10% heat inactivated fetal bovine serum in a humidified incubator at 37°C with 5% CO2. The cells were subcultured approximately once every four days by 98% trypsin EDTA solution (pH 7.2). Growth inhibition of H157 and HT144 cells was determined by using the modified SRB assay as described by Skehan et al. (14). Briefly, cells were seeded at a density of 5×103 cells/well in 96-well plates. After 24 h, serial dilutions of samples (CME and fractions) and standard drug (Methotrexate) solutions were added for each concentration. The cells were exposed to test samples and drugs for continuous 72 h. For cell fixation, the culture medium was removed and trichloroacetic acid (50%, 100 μL) was added in each plate. Then the plates were air-dried and 0.4% SRB (sigma) in 1% acetic acid was added for 30 min and unbound dye was washed out with 1% acetic acid. After air-drying, SRB dye within cells were dissolved with 100 μL solution of tris-base 10mM (pH 10.5). The optical density of the extracted SRB dye was measured with a microplate reader (Platos R 496) at 490nm. The 50% inhibitory concentration (IC50) of the test drugs was calculated using a Probit analysis program. Chemosensitivity of H157 and HT144 cells transfected with control vector was determined by SRB assay as described above.
Phytochemical analysis
The crude methanol extract and its fractions were screened phytochemically for the presence of tannins, alkaloids, saponins, flavonoids, steriods phlobatannins, terpenoids and cardiac glycosides by standard methods of phytochemical analysis (15-19). For total flavonoid determination ammonium chloride chlorimetric method was used (20). The total phenolic contents were determined according to Velioglu et al. (21) method and Folin-Ciocalteu reagent was used.
Results and Discussion
Biological activities
Antioxidant activity
DPPH free radical scavenging assay was used to evaluate antioxidant potential of our samples. The DPPH scavenging assay is a reliable and easy method for antioxidant property evaluation. This assay has been used for investigating antioxidant properties of wheat, vegetables, herbs, edible seed oils, conjugated linoleic acids, and flours in several different organic solvent systems including ethanol, aqueous acetone, methanol, aqueous alcohol, and benzene (9, 22-25).
CME as well as fractions showed effective free radical scavenging activity as determined by DPPH assay. The results of free radical scavenging assay are given in Table 1.
Table 1.
Percentage scavenging and IC50 of antioxidant assay for CME and Fractions of E. wallichii root
| S. No. | Extract |
Scavenging effect (%) at different concentrations ± STDEV *
|
IC
50
μg/mL |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 25 μg/mL | 10 μg/mL | 5 μg/mL | 2 μg/mL | 1 μg/mL | |||
| 1. | CME | 92.6 ± 0.23 | 93.0 ± 0.23 | 93.0 ± 0.32 | 53.0 ± 0.21 | 44.0 ± 0.00 | 18.0 ± 0.32 | 6.0 ± 0.56 | 8.410 |
| 2. | NHF | 12.2 ± 0.13 | - | - | - | - | - | - | >100 |
| 3. | NBF | 93.1 ± 0.60 | 93.2 ± 0.72 | 93.0 ± 0.52 | 37.0 ± 0.23 | 34.2 ± 0.34 | 3.1 ± 0.02 | - | 13.55 |
| 4. | CHF | 90.2 ± 0.72 | 31.1 ± 0.42 | 9.1 ± 0.35 | - | - | - | - | 63.35 |
| 5. | EAF | 92.7 ± 0.56 | 93.7 ± 0.45 | 91.3 ± 0.35 | 54.1 ± 0.34 | 48.0 ± 0.25 | 29.1 ± 0.21 | 5.0 ± 0.13 | 7.89 |
| 6. | AQF | 91.0 ± 0.32 | 90.2 ± 0.26 | 90.1 ± 0.76 | 29.0 ± 0.59 | 5.8 ± 0.32 | - | - | 15.23 |
| 7. | A.A. | 95.0 ± 0.53 | 94.8 ± 0.61 | 90.0 ± 0.48 | 66.4 ± 1.2 | 44.7 ± 0.23 | 30.0 ± 0.41 | 11.7 ± 0.71 | 5.63 |
*Data represents mean values of three replicates, A.A.: Ascorbic acid, - : shows no activity
Crude extract showed promising antioxidant activity with IC50 value of 8.41 μg/mL while EAF has maximum antioxidant activity with IC50 value of 7.89 μg/mL. Other fractions; NBF, AQF and CHF have IC50 values of 13.55, 15.23 and 63.35 μg/mL respectively, while NHF showed lowest free radical scavenging activity and has IC50 >100 μg/mL. EAF has excellent free radical scavenging with IC50 7.89 μg/mL which is comparable to ascorbic acid, a very important antioxidant compound (26). Phytochemical assay of the EAF shows that it has high concentrations of tannins which are known to be potent antioxidants (27). The presence of tannins in EAF may be responsible for its antioxidant activity. Our phytochemical analyses (Table 3) also support this hypothesis as antioxidant activity varies with the change in tannins concentration.
Table 3.
Phytochemical analysis of CME and Fractions of E. wallichii
| S/No | Costituents/ test |
Extracts / Fractions
|
|||||
|---|---|---|---|---|---|---|---|
| CME | NHF | NBF | CHF | EAF | AQF | ||
| 1 | Alkaloids | ||||||
| Dragendoff's | + | - | - | - | - | - | |
| Mayer's | + | - | - | - | - | - | |
| Wagners | + | - | - | - | - | - | |
| 2 | Flavonoids | ||||||
| Harborne | ++ | + | ++ | + | ++ | + | |
| 3 | Steriods | ||||||
| Liebermann-Burchard reaction | - | - | - | - | - | - | |
| 4 | Saponins | ||||||
| Frothing Test | + | - | ++ | + | ++ | +++ | |
| 5 | Tannins | ||||||
| FeCl3 Test | +++ | - | +++ | - | +++ | +++ | |
| 6 | Phlobatannins | - | - | - | - | - | - |
| 7 | Terpenoids | +++ | +++ | - | ++ | - | - |
| 8 | Cardiac Glycosides | ||||||
| Keller-Kiliani Test | +++ | +++ | - | ++ | - | - | |
+ : weekly present, ++: moderately present, +++: highly present , - : not present
Transition metal iron, in human, may react with H2O2 to produce ●OH trough a Fenton like reaction. The ●OH is the most influential oxidizing agent among other reactive oxygen radicals, and it can oxidize biomolecules` including DNA, protein, lipid, and carbohydrate (27,28,29). Oxidative DNA damage from reactive oxygen species (ROS) can play a serious role in many biological processes including mutagenesis, aging and carcinogenesis (31-33).
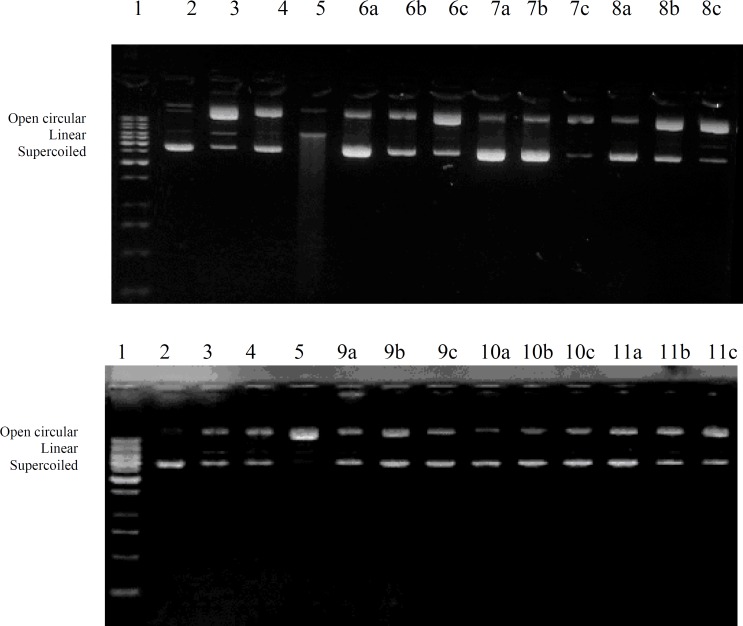
Results of DNA protection assay demonstrated that CME has concentration dependent DNA protection properties (Figure 1). Among other fractions NHF has good DNA protection activity
Figure 1.
DNA protection affect of crude extract and different fractions of E. wallichii roots.1 = 1Kb DNA Ladder, 2 = Plasmid DNA (pBR 322), 3 = Plasmid DNA treated with FeSO4, 4 = Plasmid DNA treated with H2O2, 5 = Plasmid DNA treated with FeSO4 and H2O2, a= 1000 μg/mL, b = 100 μg/mL, c = 10 μg/mL, 6 = CME, 7 = NHF, 8 = NBF, 9 = CHF, 10 = EAF, 11 = AQF in which a,b,c shows three replicates for each test sample
at 1000 μL/mL and 100 μL/mL while weak protection activity at 10 μL/mL. NBF also has good protection activity at 1000 μL/mL while moderate to weak protection activity at 100 μL/mL and 10 μL/mL respectively. CHF and EAF have good protection activity at all tested concentrations, while AQF has concentration dependent DNA protection activity as shown in the Figure 1.
Cytotoxic activity
Infectious diseases and cancer have a primary position in use of medicinal plants for drug discovery and 60-75% anticancer and antiinfectious drugs have origin from natural products (34). The connotation in discovery of natural drugs for cancer chemoprevention is rapidly increasing (35-37). Several plant species rich in phenolics and flavonoids are reported for anticancer therapeutic properties. For cytotoxic activity, the SRB assay was conducted on CME of E. wallichii and its fractions by using human cell lines H157 (Lung Carcinoma) and HT144 (Malignant Melanoma). NBF shows the highest cytotoxicity on H157 cell line and has IC50 value 0.18 mg/mL followed by EAF at 0.22 mg/mL. The IC50 value of CME was 0.29 mg/mL against this cell line whereas standard drug Methotrexate has shown IC50 value 5.9E-5 mg/mL. Against Malignant Melanoma (HT144) cell line similar results were obtained. In this test the cytotoxic effect of NBF was highest with IC50 value of 0.46 mg/mL followed by EAF fraction having IC50 of 0.54 mg/mL and CME having IC50 value of 0.87 mg/mL. Standard drug Methotrexate has shown IC50 value 0.02 mg/mL for this cell line as shown in Table 2.
Table 2.
Cytotoxic activity of CME and Fractions of E. wallichii root *.
| Extract Name |
Treatment (mg/mL) against H157 cell line
|
IC 50 mg/mL |
Treatment (mg/mL) against HT144 cell line
|
IC 50 mg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 1 | 2.5 | 5 | 0.1 | 0.5 | 1 | 2.5 | 5 | |||
| CME | 38 ± 0.9 | 56 ± 1.0 | 64 ± 0.6 | 73 ± 0.7 | 75 ± 0.8 | 0.29 | 20 ± 0.8 | 45 ± 1.1 | 58 ± 1.1 | 63 ± 1.2 | 67 ± 1.1 | 0.87 |
| NHF | 38 ± 0.7 | 51 ± 1.0 | 54 ± 1.1 | 54 ± 0.6 | 58 ± 0.9 | 0.74 | 11 ± 0.9 | 15 ± 0.8 | 30 ± 0.9 | 38 ± 0.9 | 38 ± 0.9 | 17.88 |
| NBF | 41 ± 0.9 | 61 ± 2.0 | 73 ± 0.7 | 75 ± 0.9 | 76 ± 0.9 | 0.18 | 28 ± 1.0 | 54 ± 0.9 | 66 ± 1.1 | 68 ± 1.3 | 74 ± 0.8 | 0.46 |
| CHF | 29 ± 0.7 | 33 ± 2.1 | 49 ± 1.1 | 55 ± 1.1 | 65 ± 1.1 | 1.36 | 7 ± 1.1 | 28 ± 1.1 | 48 ± 1.3 | 60 ± 1.1 | 61 ± 1.1 | 1.69 |
| EAF | 42 ± 1.1 | 57 ± 1.0 | 67 ± 1.2 | 76 ± 1.1 | 78 ± 1.1 | 0.22 | 37 ± 1.1 | 43 ± 1.1 | 57 ± 1.2 | 64 ± 1.1 | 75 ± 1.1 | 0.54 |
| AQF | 22 ± 0.7 | 34 ± 0.8 | 44 ± 1.1 | 57 ± 0.7 | 66 ± 0.9 | 1.4 | 7 ± 0.9 | 17 ± 0.9 | 27 ± 0.9 | 33 ± 0.9 | 45 ± 0.9 | 11.7 |
| Methotrexate | 81 ± 0.9 | 92 ± 1.1 | 97 ± 1.1 | 98 ± 0.3 | 98 ± 0.7 | 5.9E-5 | 61 ± 0.9 | 67 ± 1.1 | 69 ± 0.9 | 74 ± 1.3 | 85 ± 1.2 | 0.02 |
*a) Activity given as % inhibition of cancer cells. b) H157 (lung carcinoma cells) and HT144 (malignant melanoma cells). c) IC50 was calculated by graphical method using MS-Excel 2007.
Cytotoxic activity recorded in the present study seems in accordance with our finding of phytochemicals in this plant species (Table 3). Since the phytochemical evaluation indicated the presence of flavonoids in CME and its fractions particularly in EAF, NBF and AQF which also shows promising cytotoxic potential. Among these active fractions NBF and EAF have highest cytotoxic and antioxidant potential. As mentioned earlier, these fractions have higher phenolic contents as well (Table 3).
The presence of tannins at high or moderate level in NBF, EAF and AQF and in CME may also be responsible for their promising antioxidant/DNA protection and cytotoxic activity. Khan et al (38) reported that, “tannins exhibit a stronger anti-oxidant effect by their ability to quench hydroxyl radical and singlet oxygen mediated DNA cleavage”. Therefore, the present work on antioxidant/DNA protection or cytotoxic activity and phytochemical analysis of E. wallichii can be a good start for isolation of valuable secondary metabolites from root extract of this plant.
Phytochemical analysis
Phytochemical analysis showed the presence of different classes and concentrations of compounds in CME and fractions (Table 3). Analysis shows the presence of flavonoids, saponins, tannins, terpenoids and cardiac glycosides whereas alkaloids were completely absent. On fractionation these compounds distributed in solvents of different polarities on the basis of solubility as shown in Table 3. Moderate to high concentrations of saponins, tannins and cardiac glycosides were present in CME and its fractions.
The flavonoid contents
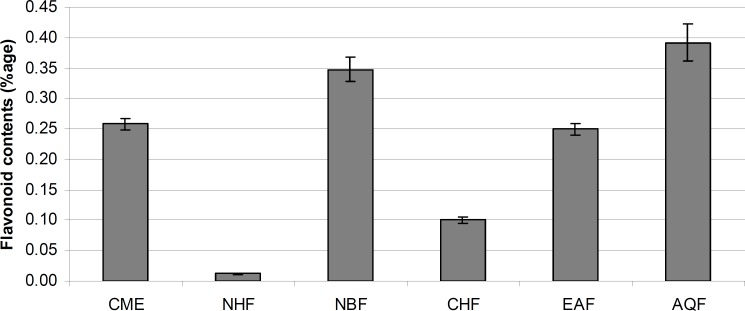
The total flavonoid contents of the CME and its fractions were determined in terms of flavonoids percentage as shown in Figure 2. In CME the flavonoid contents were 0.26%. Among fractions, the highest flavonoid content were found in AQF (0.39%) followed by NBF (0.35%), EAF (0.25%), CHF (0.10%) and NHF (0.01 %). It is well documented that the flavonoids show antioxidant activity and have considerable effects on human health and nutrition (39, 40).
Figure 2.
Flavonoid contents (% w/w dry extract/fraction) of E. wallichii CME and its fractions with standard deviations
The phenolic contents
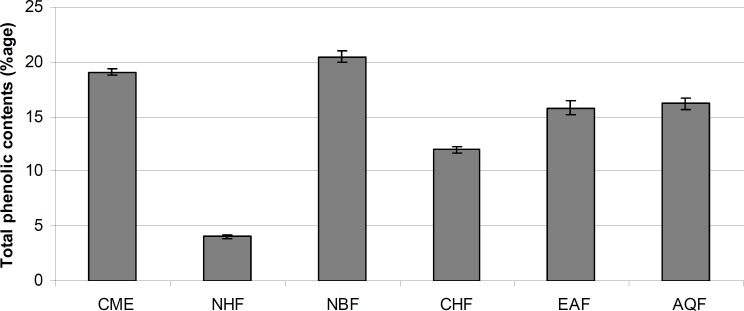
The total phenolic contents of the CME and its fractions are shown in Figure 3. Among CME and its fractions, NBF fraction has highest phenolic content (20.49%), followed by CME fraction (19.08%), AQF (16.18%), EAF (15.81%), CHF (12.01%) and NHF (4.03%). The relationships between phenolic content of medicinal plants and antioxidant activity is well documented (21, 41).
Figure 3.
Total phenolic contents (% w/w of dry extract/fraction) of E. wallichii CME and its fractions with standard deviations
Acknowledgment
The authors are thankful to the Higher Education Commission of Pakistan for financial support. We thank Dr. Rizwana Aleem Qureshi, Quaid-i-Azam University Islamabad for identifying the plant.
References
- 1.Ali MS, Ahmed S, Saleem M. Spirowallichiione: A rearranged multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae). Molecules. 2008;13:405–411. doi: 10.3390/molecules13020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali I, Naz R, Khan WN, Gul R. Biological screening of different root extracts of Euphorbia wallichii. Pak. J. Bot. 2009;41:1737–1741. [Google Scholar]
- 3.Smith-Kielland I, Dornish JM, Malteruel KE, Hvistendahl G, Romming C, Bockman OC, Kolsaker P, Stenstrom Y, Norelal A. Cytotoxic triterpenoids from the leaves of Euphorbia pulcherrima. Planta Med. 1966;62:322–325. doi: 10.1055/s-2006-957893. [DOI] [PubMed] [Google Scholar]
- 4.Javidnia K, Miri R, Amirghofran Z, Jafari A, Amoozega Z. Cytotoxicity and antimicrobial assessment of Euphorbia hebecarpa. Iranian J. Pharm. Res. 2004;Suppl. 2:75–75. [Google Scholar]
- 5.Singla AK, Pathak K. Phytoconstituents of Euphorbia species. Fitoterapia. 1990;61:483–516. [Google Scholar]
- 6.Pan L, Yu KB, Peng SL, Zhou Y, Ding LS. Ent-3b,16-dihydroxyisopimar-7-ene-2,15- dione: a new tricyclic diterpenoid from the roots of Euphorbia wallichii. Acta Crystallogr. E. 2006;62:01643–01644. [Google Scholar]
- 7.Pan L, Zhou P, Zhang XF, Peng S, Ding LS, Qiu SX. Skeleton-rearranged pentacyclic diterpenoids possessing a cyclobutane ring from Euphorbia wallichii. Org. Lett. 2006;8:2775–2778. doi: 10.1021/ol0608552. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Zhang X, Cai X, Ma Y, Luo X. Three new diterpenoids from Euphorbia wallichii. Chinese J. Chem. 2004;22:199–202. [Google Scholar]
- 9.Parry J, Su L, Luther M, Zhou KQ, Yurawecz MP, Whittaker P, Yu LL. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005;53:566–573. doi: 10.1021/jf048615t. [DOI] [PubMed] [Google Scholar]
- 10.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. [Google Scholar]
- 11.Obeid HK, Allen MS, Bedgood DR, Prenzied PD, Robards K, Stockmann R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005;53:823–837. doi: 10.1021/jf048569x. [DOI] [PubMed] [Google Scholar]
- 12.Tian B, Hua Y. Concentration dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91:413–418. [Google Scholar]
- 13.Nawaz H, Akhter Z, Yameen S, Siddiqi HM, Mirza B, Rifat A. Synthesis and biological evaluation of some Schiff-bases esters of ferrocenyl aniline and simple aniline. J. Organomet. Chem. 2009;694:2198–2203. [Google Scholar]
- 14.Skehan P, Storeng R, Scudiero D, Monks A, McMahan J, Vistica D, Warren JT, Bokesch H, Kenny S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 15.Harborne JB. Phytochemical Methods. London : Academic Press; 1993. pp. 89–131. [Google Scholar]
- 16.Sofowara A. Medicinal Plants and Traditional Medicine in Africa. Ibadan: Spectrum Books Ltd.; 1993. p. 289. [Google Scholar]
- 17.Evans WC. Trease and Evans’ Pharmacognosy. 15th ed. London: Saunders Publishers; 2002. pp. 42–393. [Google Scholar]
- 18.Edeoga OH, Okwu ED, Mbaebie OB. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;7:685–688. [Google Scholar]
- 19.Parekh J, Chanda V, Sume In-vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007;31:53–58. [Google Scholar]
- 20.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 21.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998;46:4113–4117. [Google Scholar]
- 22.Yu LL. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001;49:3452–3456. doi: 10.1021/jf010172v. [DOI] [PubMed] [Google Scholar]
- 23.Huang DJ, Ou BX, Prior RL. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 24.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 25.Zhou KQ, Yin JJ, Yu LL. Phenolic acid, tocopherol and carotenoid compositions, and antioxidant functions of hard red winter wheat bran. J. Agr. Food Chem. 2005;53:3916–3922. doi: 10.1021/jf050117c. [DOI] [PubMed] [Google Scholar]
- 26.Arrigoni O, Tullio MCD. Ascorbic acid: much more than just an antioxidant. Biochim. Biophys. Acta. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Buelga C, Scalbert A. Proantocyanidins and tannin like compounds-nature, occurrence dietary intake and effects on nutrition and health. J. Agric. Food Chem. 2000;80:1094–1117. [Google Scholar]
- 28.Frank H, Thiel D, MacLoad J. Mass spectrometric detection of cross-linked fatty acids formed during radical induced lesion of lipid membranes. Biochem. J. 1989;260:873–878. doi: 10.1042/bj2600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breen AP, Murphy JA. Reactions of oxyl radicals with DNA Free Radicals. Free Radical Bio. Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 30.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 32.Cerruti PA. Oxy-radicals and cancer. Lancet. 1984;344:862–863. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 33.Von-Sonntag C. The Chemical Basis of Radiation Biology. New York: Taylor & Francis; [Google Scholar]
- 34.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 35.Kucuk O. New opportunities in chemoprevention research. Cancer Invest. 2002;20:237–245. doi: 10.1081/cnv-120001151. [DOI] [PubMed] [Google Scholar]
- 36.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J. Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 38.Khan NS, Ahmad A, Hadi SM. Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem-Biol. Interac. 2000;125:177–189. doi: 10.1016/s0009-2797(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 39.Younes M. Inhibitory action of some flavonoids on enhanced spontaneous lipid peroxidation following glutathione depletion. Planta Med. 1981;43:240–245. doi: 10.1055/s-2007-971503. [DOI] [PubMed] [Google Scholar]
- 40.Das NP, Pereira TA. Effects of flavonoids on thermal autooxidation of Palm oil: structure- activity relationship. J. Am. Oil Chem. Soc. 1990;67:255–258. [Google Scholar]
- 41.Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]