Abstract
Considerable interest has been focused on the triazole structure, which has been known to possess a broad spectrum of biological activities such as antitumor, anti-inflammatory, antimicrobial, antiviral, and anticonvulsant activities. Before this, several heterocyclic compounds containing triazole were synthesized that had shown considerable anticonvulsant activity. As part of our continuous research in this area, we have synthesized several new 7-substituted-5-phenyl-[1,2,4] triazolo[1,5-a] pyrimidines (compounds 3a-3i, 5a-5j) through incorporating triazole moiety into the pyrimidine ring, which are expected to have the synergistic effect in dealing with the epilepsy. Their anticonvulsant activities were measured through the Maximal electroshock (MES) test. Carbamazepine and valproate were considered as positive control drugs with anticonvulsant effects [ED50 = 11.8 and 272 mg/Kg]. Amongst the compounds tested, compound 3f, 7-(heptyloxy)-5-phenyl-[1,2,4] triazolo[1,5-a] pyrimidine, showed potent anticonvulsant activity with ED50 84.9 mg/Kg, which was weaker than carbamazepine, but better than valproate.
Key Words: Synthesis, Triazole, Pyrimidine, Anticonvulsant, Maximal electroshock
Introduction
Epilepsy, one of the most frequent neurological afflictions in men characterized via excessive temporary neuronal discharges resulting in uncontrolled convulsion, inflicts more than 60 million people worldwide (1, 2). Despite the development of several new anticonvulsants, the treatment of epilepsy remains still inadequate. It is roughly estimated that up to 28-30% of patients are poorly treated with the available antiepileptic drugs (AEDs) (3, 4). Moreover, many AEDs have serious side effects (5-10) and lifelong medication may be required. Therefore, there is a continuing demand for new anticonvulsant agents with more selectivity and lower toxicity.
In the effort to get those agents, we have reported (11-17) several heterocyclic compounds containing triazole, which have shown considerable anticonvulsant activities. From the currently used AEDs, the major characteristics important in newly synthesized compounds are the inclusion of a hydrophobic site and H-bond donors/acceptors. With respect to the compounds we reported previously, the hydrophobic site is obviously the phenyl group and the substituents on it, and the H-bond acceptor is the triazole.
As a part of our continuous research in this area, we have designed and synthesized several new 7-substituted-5-phenyl-[1,2,4] triazolo[1,5-a] pyrimidines (compound 3a-3i, 5a-5j) through incorporating triazole moiety into the pyrimidine ring, which are expected to have the synergistic effect in dealing with the epilepsy. In this series of compounds, the 5th position phenyl group and the 7th position substitutes is the hydrophobic site, which could contribute to the traverse blood-brain barrier and also the H-bond acceptor is still the triazole.
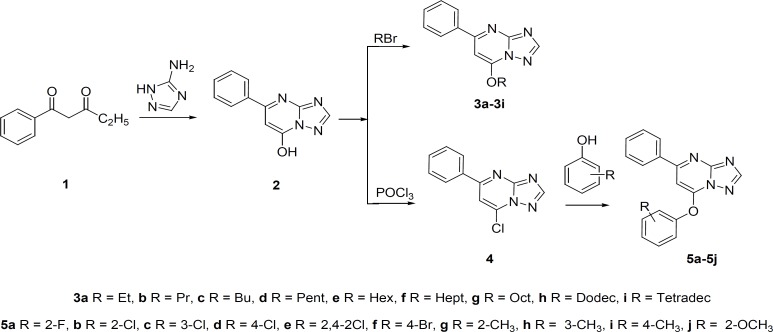
New compounds were synthesized according to Figure 1.
Figure 1.
The synthesis route of target compounds
The reaction of 1-phenylpentane-1,3-dione (1) with 2H-1,2,4-triazol-3-amine under 160oC afforded 5-phenyl-[1,2,4] triazolo[1,5-a]pyrimidin-7-ol (2). The alkylation of compound 2 with appropriate alkyl bromide in Dimethylformamide (DMF) in the presence of NaOH and KI at 80oC afforded 7-alkoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine derivatives (compound 3a-3i). Compound 4 was obtained by means of boiling compound 3 with excessive POCl3. The refluxing of compound 4 with appropriate substituted phenol in acetonitrile in the presence of sodium hydroxide gave substituted 7-phenoxy-5-phenyl-[1,2,4] triazolo[1,5-a] pyrimidine derivatives (compound 5a-5j) (Figure 1).
The anticonvulsant activity of synthesized compounds was determined through the maximal electroshock seizure (MES), which is one of the animal seizure models most widely used in the search for new AEDs (18).
Experimental
Preparation of compounds
5-Phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-ol (compound 2)
The 1-phenylpentane-1,3-dione (compound 1) (3.00 g, 15.6 mmol) and 2H-1,2,4-triazol-3-amine (2.00 g, 23.8 mmol) were reacted at 160°C for 2 h with no solvent. After cooling, the mixture was filtered and washed with dichloromethane to afford compound 2 in 92% yield. M.p. 201-203°C, IR (KBr) cm-1: 1616 (C=N), 1537 (C=C), 1194 (N–N). MS m/z 213 (M+1). 1H-NMR (DMSO-d6, 300 MHz) δ 6.35 (s, 1H, H-6), 7.48 (s, 1H, -OH), 7.53-7.55 (m, 3H, Ph-H), 7.89-7.93 (m, 2H, Ph-H), 8.38 (s, 1H, H-2). Anal.Calcd. for C11H8N4O: C, 62.26; H, 3.80; N, 26.40. Found: C, 62.12; H, 3.65; N, 26.56.
7-Chloro-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 4)
Compound 2 (1.00 g, 4.72 mmol) were placed into a 100 mL round-bottomed flask containing 30 mL of POCl3 equipped with a reflux condenser connected with a drying tube. The mixture was stirred and heated at 100°C for 3 h. Then, most of the solvent was removed under reduced pressure and the mixture was poured into ice-water. The precipitate was filtered and washed with water and recrystallized from CH3CO2C2H5 to afford compound 4 in 85% yield. M.p. 146-148°C, IR (KBr) cm-1: 1636 (C=N), 1557 (C=C), 1213 (N–N). MS m/z 231 (M+1). 1H-NMR (CDCl3, 300 MHz) δ7.72 (s, 1H, H-6), 7.56-7.66 (m, 3H, Ph-H), 8.19-8.22 (m, 2H, Ph-H), 8.59 (s, 1H, H-2). Anal.Calcd. for C11H7ClN4: C, 57.28; H, 3.06; N, 24.29. Found: C, 57.13; H, 3.22; N, 24.44.
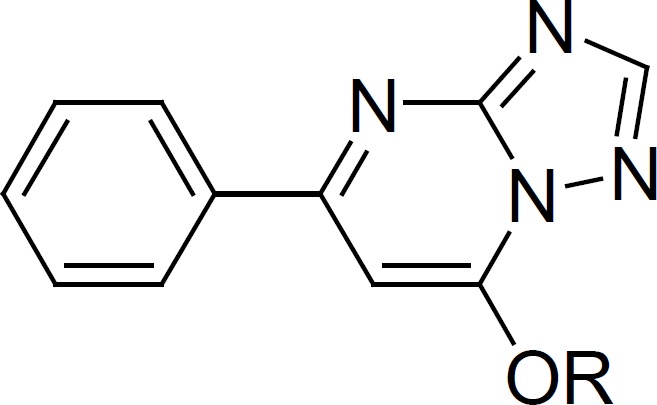
7-Alkoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine derivatives (compounds 3a-3i)
Compound 2 (0.30 g, 1.42 mmol) and NaOH (0.06 g, 1.50 mmol) were placed into a 100 mL round-bottomed flask containing 30 mL of DMF. After the mixture was stirred and heated at 80°C for 3 h, various kinds of substituted alkyl bromide (1.68 mmol) and KI (1.68 mmol) were added. After stirring for about 16 h, the solvent was removed under reduced pressure. The mixture was extracted twice with dichloromethane. The dichloromethane layer was dried over anhydrous MgSO4. The evaporation of the solvents got a crude product, which was purified through silica gel column chromatography with CH2Cl2 and CH3OH (30:1) to obtain compounds 3a-3i. The yield, melting point and spectral data of each compound were given below.
Substituted 7-phenoxy -5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine derivatives (compounds5a-5j)
Various kinds of Substituted phenol (1.50 mmol) and NaOH (0.06 g, 1.50 mmol) were placed into a 100 mL round-bottomed flask containing 30 mL of CH3CN. After the mixture was stirred and heated at 80°C for 1 h, Compound 4 (0.3 g, 1.30 mmol) was added. Following the stirring for about 5 h, the solvent was removed under reduced pressure. The mixture was extracted twice with dichloromethane. The dichloromethane layer was dried over anhydrous MgSO4. The evaporation of solvents get a crude product, which was purified by silica gel column chromatography with CH2Cl2 and CH3OH (25:1) to obtain compounds 5a-5j. The yield, melting point and spectral data of each compound were given below.
7-Ethoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3a)
M.p. > 270°C; yield 27.5%; 1H-NMR (CDCl3, 300 MHz) δ 1.63 (t, 3H, J = 7.3 Hz, -CH3), 4.28 (q, 2H, J = 7.3 Hz, -OCH2-), 6.72 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.15 (s, 1H, H-2); IR (KBr) cm-1: 1680 (C=N), 1537 (C=C), 1146 (N–N); MS m/z 241 (M+1); Anal.Calcd. for C13H12N4O: C, 64.99; H, 5.03; N, 23.32. Found: C, 64.86; H, 5.12; N, 23.50.
7-Propoxy-5-phenyl- [1,2,4]triazolo[1,5-a]pyrimidine (compound 3b)
M.p. 228-230°C; yield 39.7%. 1H-NMR (CDCl3, 300 MHz) δ 1.07 (t, 3H, J = 7.4 Hz, -CH3), 1.96-2.08 (m, 2H, -CH2-), 4.18 (t, 2H, J = 7.2 Hz, -OCH2-), 6.72 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.11 (s, 1H, H-2); IR (KBr) cm-1: 1681 (C=N), 1538 (C=C), 1146 (N–N); MS m/z 255 (M+1); Anal.Calcd. for C14H14N4O: C, 66.13; H, 5.55; N, 22.03. Found: C, 66.32; H, 5.66; N, 22.11.
7-Butoxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3c)
M.p. 209-210°C; yield 42.2%. 1H-NMR (CDCl3, 300 MHz) δ 1.03 (t, 3H, J = 7.3 Hz, -CH3), 1.40-1.52 (m, 2H, -CH2-), 1.90-2.01 (m, 2H, -CH2-), 4.21 (t, 2H, J = 7.2 Hz, -OCH2-), 6.72 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.13 (s, 1H, H-2); IR (KBr) cm-1: 1681 (C=N), 1540 (C=C), 1150 (N–N); MS m/z 269 (M+1); Anal.Calcd. for C15H16N4O: C, 67.15; H, 6.01; N, 20.88. Found: C, 67.28; H, 6.13; N, 20.99.
7-Pentyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3d)
M.p. 168-171°C; yield 39.6%. 1H-NMR (CDCl3, 300 MHz) δ 0.94 (t, 3H, J = 6.5 Hz, -CH3), 1.41-1.44 (m, 4H, -(CH2)2-), 1.95-2.00 (m, 2H, -CH2-), 4.20 (t, 2H, J = 7.3 Hz, -OCH2-), 6.73 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.11 (s, 1H, H-2); IR (KBr) cm-1: 1683 (C=N), 1543 (C=C), 1153 (N–N); MS m/z 283 (M+1); Anal.Calcd. for C16H18N4O: C, 68.06; H, 6.43; N, 19.84. Found: C, 68.23; H, 6.61; N, 19.62.
7-Hexyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3e)
M.p. 157-160°C; yield 29.1%. 1H-NMR (CDCl3, 300 MHz) δ 0.90 (t, 3H, J = 6.9 Hz, -CH3), 1.35-1.39 (m, 6H, -(CH2)3-), 1.95-1.99 (m, 2H, -CH2-), 4.20 (t, 2H, J = 7.3 Hz, -OCH2-), 6.72 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.12 (s, 1H, H-2); IR (KBr) cm-1: 1683 (C=N), 1543 (C=C), 1152 (N–N); MS m/z 297 (M+1); Anal.Calcd. for C17H20N4O: C, 68.89; H, 6.80; N, 18.90. Found: C, 68.71; H, 6.93; N, 18.75.
7-Heptyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3f)
M.p. 139-141°C; yield 33.7%. 1H-NMR (CDCl3, 300 MHz) δ 0.89 (t, 3H, J = 6.9 Hz, -CH3), 1.30-1.41 (m, 8H, -CH2-CH2-CH2-CH2-), 1.95-1.99 (m, 2H, -CH2-), 4.20 (t, 2H, J = 7.2 Hz, -OCH2-), 6.73 (s, 1H, H-6), 7.48-7.50 (m, 3H, Ph-H), 8.00-8.02 (m, 2H, Ph-H), 8.13 (s, 1H, H-2); IR (KBr) cm-1: 1684 (C=N), 1546 (C=C), 1158 (N–N); MS m/z 311 (M+1); Anal.Calcd. for C18H22N4O: C, 69.65; H, 7.14; N, 18.05. Found: C, 69.42; H, 7.02; N, 18.21.
7-Octyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3g)
M.p. 125-128oC; yield 32.8%. 1H-NMR (CDCl3, 300 MHz) δ 0.87 (t, 3H, J = 6.9 Hz, -CH3), 1.27-1.39 (m, 10H, -(CH2)5-), 1.94-1.97 (m, 2H, -CH2-), 4.20 (t, 2H, J = 7.2 Hz, -OCH2-), 6.73 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.02 (m, 2H, Ph-H), 8.11 (s, 1H, H-2); IR (KBr) cm-1: 1684 (C=N), 1545 (C=C), 1156 (N–N); MS m/z 325 (M+1); Anal.Calcd. for C19H24N4O: C, 70.34; H, 7.46; N, 17.27. Found: C, 70.52; H, 7.33; N, 17.45.
7-Dodecyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3h)
M.p. 117-119oC; yield 32.1%. 1H-NMR (CDCl3, 300 MHz) δ 0.88 (t, 3H, J = 6.6 Hz, -CH3), 1.25-1.40 (m, 18H, -(CH2)9-), 1.95-1.99 (m, 2H, -CH2-), 4.19 (t, 2H, J = 7.2 Hz, -OCH2-), 6.73 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.01-8.02 (m, 2H, Ph-H), 8.09 (s, 1H, H-2); IR (KBr) cm-1: 1688 (C=N), 1551 (C=C), 1160 (N–N); MS m/z 381 (M+1); Anal.Calcd. for C23H32N4O: C, 72.60; H, 8.48; N, 14.72. Found: C, 72.82; H, 8.66; N, 14.50.
7-Tetradecyloxy-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 3i)
M.p. 106-109°C; yield 41.6%. 1H-NMR (CDCl3, 300 MHz) δ 0.88 (t, 3H, J = 6.6 Hz, -CH3), 1.25-1.40 (m, 22H, -(CH2)11-), 1.94-1.97 (m, 2H, -CH2-), 4.20 (t, 2H, J = 7.2 Hz, -OCH2-), 6.72 (s, 1H, H-6), 7.47-7.49 (m, 3H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.11 (s, 1H, H-2); IR (KBr) cm-1: 1689 (C=N), 1551 (C=C), 1159 (N–N); MS m/z 409 (M+1); Anal.Calcd. for C25H36N4O: C, 73.49; H, 8.88; N, 13.71. Found: C, 73.71; H, 8.79; N, 13.93.
7-(4-Fluorophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5a)
M.p. 160-162°C; yield 61.1%. 1H-NMR (CDCl3, 300 MHz) δ 6.59 (s, 1H, H-6), 7.25-7.30 (m, 2H, Ph-H), 7.36 (d, 2H, J = 7.9 Hz, Ph-H), 7.46-7.54 (m, 3H, Ph-H), 8.01 (d, 2H, J = 7.9 Hz, Ph-H), 8.55 (s, 1H, H-2); IR (KBr) cm-1: 1628 (C=N), 1543 (C=C), 1196 (N–N); MS m/z 307 (M+1); Anal.Calcd. for C17H11FN4O: C, 66.66; H, 3.62; N, 18.29. Found: C, 66.82; H,3.46; N, 18.34.
7-(2-Chlorophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5b)
M.p. 158-161°C; yield 63.5%. 1H-NMR (CDCl3, 300 MHz) δ 6.49 (s, 1H, H-6), 7.42-7.49 (m, 6H, Ph-H), 7.63-7.66 (m, 1H, Ph-H), 7.99-8.02 (m, 2H, Ph-H), 8.57 (s, 1H, H-2); IR (KBr) cm-1: 1627 (C=N), 1542 (C=C), 1208 (N–N); MS m/z 323 (M+1); Anal.Calcd. for C17H11ClN4O: C, 63.26; H, 3.44; N, 17.36. Found: C, 63.02; H, 3.29; N, 17.45.
7-(3-Chlorophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5c)
M.p. 144-147°C; yield 28.6%. 1H-NMR (CDCl3, 300 MHz) δ 6.70 (s, 1H, H-6), 7.71-7.54 (m, 7H, Ph-H), 8.03-8.05 (m, 2H, Ph-H), 8.60 (s, 1H, H-2); IR (KBr) cm-1: 1625 (C=N), 1544 (C=C), 1207 (N–N); MS m/z 323 (M+1); Anal.Calcd. for C17H11ClN4O: C, 63.26; H, 3.44; N, 17.36. Found: C, 63.12; H, 3.31; N, 17.52.
7-(4-Chlorophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5d)
M.p. 146-148°C; yield 62.5%. 1H-NMR (CDCl3, 300 MHz) δ 6.62 (s, 1H, H-6), 7.33 (d, 2H, J = 8.0 Hz, Ph-H), 7.46-7.57 (m, 5H, Ph-H), 8.02 (d, 2H, J = 8.0 Hz, Ph-H), 8.54 (s, 1H, H-2); IR (KBr) cm-1: 1625 (C=N), 1547 (C=C), 1210 (N–N); MS m/z 323 (M+1); Anal.Calcd. for C17H11ClN4O: C, 63.26; H, 3.44; N, 17.36. Found: C, 63.19; H, 3.38; N, 17.49.
7-(2,4-Dichlorophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5e)
M.p. 211-214°C; yield 41.9%. 1H-NMR (CDCl3, 300 MHz) δ 6.50 (s, 1H, H-6), 7.32-7.51 (m, 5H, Ph-H), 7.65-7.66 (m, 1H, Ph-H), 8.01-8.03 (m, 2H, Ph-H), 8.57 (s, 1H, H-2); IR (KBr) cm-1: 1626 (C=N), 1545 (C=C), 1204 (N–N); MS m/z 357 (M+1); Anal.Calcd. for C17H10Cl2N4O: C, 57.16; H, 2.82; N, 15.69. Found: C, 57.32; H, 2.70; N, 15.84.
7-(4-Bromophenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5f)
M.p. 143-145°C; yield 55.0%. 1H-NMR (CDCl3, 300 MHz) δ 6.63 (s, 1H, H-6), 7.27 (d, 2H, J = 7.4 Hz, Ph-H), 7.47-7.53 (m, 3H, Ph-H), 7.72 (d, 2H, J = 7.4 Hz, Ph-H), 8.01-8.04 (m, 2H, Ph-H), 8.55 (s, 1H, H-2); IR (KBr) cm-1: 1622 (C=N), 1539 (C=C), 1211 (N–N); MS m/z 367 (M+1); Anal.Calcd. for C17H11BrN4O: C, 55.61; H, 3.02; N, 15.26. Found: C, 55.75; H, 3.13; N, 15.39.
7-(2-Methylphenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5g)
M.p. 118-120°C; yield 61.5%. 1H-NMR (CDCl3, 300 MHz) δ 2.29 (s, 3H, -CH3), 6.49 (s, 1H, H-6), 7.28-7.29 (m, 1H, Ph-H), 7.36-7.48 (m, 6H, Ph-H), 7.96-8.00 (m, 2H, Ph-H), 8.55 (s, 1H, H-2); IR (KBr) cm-1: 1612 (C=N), 1544(C=C), 1165 (N–N); MS m/z 303 (M+1); Anal.Calcd. for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.66; H, 4.59; N, 18.69.
7-(3-Methylphenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5h)
M.p. 123-126°C; yield 61.6%. 1H-NMR (CDCl3, 300 MHz) δ 2.46 (s, 1H, CH3), 6.64 (s, 1H, H-6), 7.15-7.49 (m, 7H, Ph-H), 8.00-8.03 (m, 2H, Ph-H), 8.56 (s, 1H, H-2); IR (KBr) cm-1: 1611 (C=N), 1545 (C=C), 1163 (N–N);MS m/z 303 (M+1); Anal.Calcd. for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.65; H, 4.79; N, 18.66.
7-(4-Methylphenoxy)-5-phenyl- [1,2,4]triazolo[1,5-a]pyrimidine (compound 5i)
M.p. 167-169°C; yield 50.9%. 1H-NMR (CDCl3, 300 MHz) δ 2.46 (s, 3H, -CH3), 6.61 (s, 1H, H-6), 7.25 (d, 2H, J = 8.0 Hz, Ph-H), 7.34-7.49 (m, 5H, , Ph-H), 7.99-8.02 (m, 3H, Ph-H), 8.01 (d, 2H, J = 8.0 Hz, Ph-H), 8.53 (s, 1H, H-2); IR (KBr) cm-1: 1614 (C=N), 1547 (C=C), 1165 (N–N); MS m/z 303 (M+1); Anal.Calcd. for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.36; H, 4.82; N, 18.66.
7-(2-Methoxyphenoxy)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine (compound 5j)
M.p. 166-168°C; yield 43.5%. 1H-NMR (CDCl3, 300 MHz) δ 3.80 (s, 3H, -OCH3), 6.52 (s, 1H, H-6), 7.12-7.15 (m, 2H, Ph-H), 7.36-7.48 (m, 5H, Ph-H), 7.99-8.02 (m, 2H, Ph-H), 8.55 (s, 1H, H-2); IR (KBr) cm-1: 1610 (C=N), 1542(C=C), 1163(N–N); MS m/z 307 (M+1); Anal.Calcd. for C18H14N4O2: C, 67.91; H, 4.43; N, 17.60. Found: C, 67.76; H, 4.37; N, 17.74.
Pharmacology
Kunming mice (supplied from the Laboratory of Animal Research, Yanbian University, China) weighting 18-22 g were used for pharmacological study. Animals were allowed free access to food and water except during the experiment and housed at controlled room temperature with 12 h light/dark schedule. All compounds were dissolved in Dimethyl sulfoxide DMSO with the injection volume of 0.05 mL per 20 g, which had no effect on the test system.
Anticonvulsant activity in the maximal electroshock seizure (MES) test
Anticonvulsant activity of the synthesized compounds was determined through the evaluation of the compounds ability to protect mice against MES-induced seizures. The MES test was carried out by the methods described in the ADD of the National Institutes of Health (USA) (18, 19). Seizures were elicited with a 60 Hz alternating current of 50 mA intensity in mice. The current was applied via corneal electrodes for 0.2 s. Protection against the spread of MES-induced seizures was defined as the abolition of tonic maximal extension of the hind leg. At 30 min after the administration of compounds, the activities were evaluated in MES test. In phase-I screening, each compound was administered at the dose levels of 100 mg/Kg for evaluating the preliminary anticonvulsant activity. For the determination of median effective dose (ED50) and the median toxic dose (TD50), the phase-II screening was prepared. Groups of 10 mice were given a range of intraperitoneal doses of the tested compound until at least three points were established in the range of 10-90% seizure protection or minimal observed neurotoxicity. From the plot of this data, the respective ED50 and TD50 values, 95% confidence intervals, slope of the regression line, and the standard error of the slope were calculated with the statistical software SPSS 13.0.
Neurotoxicity screening (NT)
The neurotoxicity of the compounds was measured in mice through the rotarod test (19, 20). The mice were trained to stay on a rotarod with a diameter of 3.2 cm that rotates at 10 rpm. Trained animals were given IP-injection of the test compounds. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least 1 min in each of the trials.
Results and Discussion
The maximal electroshock (MES) model was carried out to preliminary evaluate (phase I) the prepared compounds (compounds 3a-3i, 5a-5j) for the anticonvulsant activity. As shown in Table 1, some of the compounds were active in the MES test in dose of 100 mg/Kg, the indicative of their ability to prevent seizure spread. Among alkoxy group substituted derivatives (compounds 3a-3i), compounds 3c-3h showed protection against MES-induced seizure in varying degrees at the dose of 100 mg/Kg. Compound 3f was the best one as its complete protection.
Table 1.
The phase I data of compounds 3a-3i, 5a-5j in the MES in mice (IP).
| Compds. | R | MESa (100 mg/Kg) |
|---|---|---|
| 3a | -C2H5 | 0/6 |
| 3b | -C3H7 | 0/6 |
| 3c | -C4H9 | 1/6 |
| 3d | -C5H11 | 2/6 |
| 3e | -C6H13 | 2/6 |
| 3f | -C7H15 | 6/6 |
| 3g | -C8H17 | 4/6 |
| 3h | -C12H25 | 3/6 |
| 3i | -C14H29 | 0/6 |
| 5a | -C6H4(p-F) | 0/6 |
| 5b | -C6H4(o-Cl) | 0/6 |
| 5c | -C6H4(m-Cl) | 0/6 |
| 5d | -C6H4(p-Cl) | 0/6 |
| 5e | -C6H3(2,4-Cl2) | 0/6 |
| 5f | -C6H4(p-Br) | 0/6 |
| 5g | -C6H4(o-CH3) | 0/6 |
| 5h | -C6H4(m-CH3) | 0/6 |
| 5i | -C6H4(p-CH3) | 0/6 |
| 5j | -C6H4(o-OCH3) | 0/6 |
aMaximal electroshock test (number of animals protected/number of animals tested) (the number of mice is six).
Among phenoxy group substituted derivatives (compounds 5a-5j), none showed protection against MES-induced seizure at dose of 100 mg/Kg. The weak activity of 5a-5j may be due to the big size of their phenoxy group in 7th position, which may reduce the affinity between the triazole and receptor. For the alkoxy substituted derivatives (compounds 3a-3i), the length of the alkoxyl chain appeared to have impact on the anticonvulsant activity of them. From 3c to 3f, as the alkoxyl chain length increased, the anticonvulsant activity was gradually increased with the compound 3f (with the n-heptyloxy group in 7th position) being the most active compound. The trend reversed, however, when the alkyl chain had more than seven carbon atoms (compounds 3f-3h). Obviously, the activity curve of the alkyl chain substituted derivatives is bell-shaped with a maximum activity peak. Compound 3f, with the maximum activity in this series of compounds, reflected the optimal partition coefficient associated with the easiest crossing of the biological membranes and the optimal stereo configuration.
As a result of preliminary screening, compound 3f was subjected to phase II trials for the quantification of its anticonvulsant activity (indicated with ED50) and neurotoxicity (indicated with TD50) in mice. Results of the quantitative test for 3f, along with the data on the standard drugs valproate and carbamazepine, are reported in Table 2.
Table 2.
Phase II quantitative anticonvulsant data in mice (IP).
| Compds. | R | ED 50 (mg·Kg -1 ) | TD 50 (mg·Kg -1 ) | PI(TD 50 /ED 50 ) |
|---|---|---|---|---|
| 3f | -C7H15 | 84.9 (74.3-97.0) | 509.2 (476.3-544.4) | 6.0 |
| Valproate | - | 272.0 (247.1-338.8) | 426.1 (369.4-450.3) | 1.6 |
| Carbamazepine | - | 11.8 (8.5-16.4) | 76.1 (55.8-103.7) | 6.4 |
Compound 3f, which gave an ED50 value of 84.9 mg/Kg, displayed a weaker anticonvulsant activity compared to carbamazepine (ED50 = 11.8 mg/Kg), but a higher activity compared to valproate (ED50 = 272 mg/Kg). Moreover, 3f showed a higher TD50-value (TD50 = 509.2) in comparison to carbamazepine (TD50 = 76.1) and valproate (TD50 = 426), which make its PI value close to carbamazepine and higher than valproate.
For further exploring the anticonvulsant activity of these compounds, PTZ-induced seizure model was made to 3f. As shown in Table 3, no protection was observed at the 100 mg/Kg and 200 mg/Kg doses, which suggested that compound 3f cannot be against the seizure induced by PTZ. PTZ has been reported to produce seizures by inhibiting γ-aminobutyric acid (GABA) neurotransmission. GABA is the main inhibitory neurotransmitter in the brain, and is widely implicated in epilepsy. From the data of Table 3, it is speculated that the mechanism of the novel compounds’ action may not involve in the GABAergic neurotransmission.
Table 3.
PTZ-induced seizure test data of 3f in mice (IP)
| Compds. | Dose (mg/mg) | Number of animals | Number of seizures |
|---|---|---|---|
| Control | - | 10 | 10 |
| 3f | 100 | 10 | 10 |
| 3f | 200 | 10 | 10 |
In conclusion, most of these compounds possessed the weak anticonvulsant effect under dose of 100 mg/Kg, which did not achieve the previously designed expectation, and showed lower activity compared to the compounds with similar chemical structures previously synthesized in our laboratory.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 30860340).
References
- 1.Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46:1133–9. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 2.McNamara OJ, Brunton LL, Lazo JS, Parker KL, editors. (eds. New York: McGraw-Hill; 2006. pp. 501–526. [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 4.Spear BB. Pharmacogenetics and antiepileptic drugs. Epilepsia. 2001;42:31–34. doi: 10.1046/j.1528-1157.2001.0420s5031.x. [DOI] [PubMed] [Google Scholar]
- 5.Rémi J, Hüttenbrenner A, Feddersen B, Noachtar S. Carbamazepine but not pregabalin impairs eye control: a study on acute objective CNS side effects in healthy volunteers. Epilepsy Res. 2010;88:145–50. doi: 10.1016/j.eplepsyres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Meador KJ. Newer anticonvulsants: dosing strategies and cognition in treating patients with mood disorders and epilepsy. J. Clin. Psychiatry. 2003;64(Suppl. 8):30–34. [PubMed] [Google Scholar]
- 7.Belcastro V, Striano P, Gorgone G, Costa C, Ciampa C, Caccamo D, Pisani LR, Oteri G, Marciani MG, Aguglia U, Striano S, Ientile R, Calabresi P, Pisani F. Hyperhomocysteinemia in epileptic patients on new antiepileptic drugs. Epilepsia. 2010;51:274–279. doi: 10.1111/j.1528-1167.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- 8.Bootsma HP, Ricker L, Hekster YA, Hulsman J, Lambrechts D, Majoie M, Schellekens A, Krom M, Aldenkamp AP. The impact of side effects on long-term retention in three new antiepileptic drugs. Seizure. 2009;18:327–331. doi: 10.1016/j.seizure.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22:739–760. doi: 10.2165/00023210-200822090-00003. [DOI] [PubMed] [Google Scholar]
- 10.Penovich PE, Willmore LJ. Use of a new antiepileptic drug or an old one as first drug for treatment of absence epilepsy. Epilepsia. 2009;50:37–41. doi: 10.1111/j.1528-1167.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 11.Xie ZF, Chai KY, Piao HR, Kwak KC, Quan ZS. Synthesis and anticonvulsant activity of 7-alkoxyl- 4,5-dihydro-[1,2,4]triazolo[4,3-a] quinolines. Bioorg. Med. Chem. Lett. 2005;15:4803–4805. doi: 10.1016/j.bmcl.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Mahdavi M, Akbarzadeh T, Sheibani V, Abbasi M, Firoozpour L, Tabatabai SA, Shafiee A, Foroumadi A. Synthesis of two novel 3-Amino-5-[4-chloro-2-phenoxyphenyl]-4H-1,2,4-triazoles with anticonvulsant activity. Iranian J. Pharm. Res. 2010;9:265–269. [PMC free article] [PubMed] [Google Scholar]
- 13.Guo LJ, Wei CX, Jia JH, Zhao LM, Quan ZS. Synthesis of 5-alkoxy-[1,2,4]triazolo[4,3-a]quinoline derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2009;44:954–958. doi: 10.1016/j.ejmech.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Guan LP, Sun XY, Wei CX, Chai KY, Quan ZS. Synthesis and Anticonvulsant activity Evaluation of 6-Alkoxy-[1,2,4] triazolo[3,4-a] phthalazines. Chem. Biol. Drug Des. 2009;73:313–319. doi: 10.1111/j.1747-0285.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 15.Deng XQ, Wei CX, Li FN, Sun ZG, Quan ZS. Design and synthesis of 10-alkoxy-5, 6-dihydro-triazolo[4,3-d]benzo[f][1,4]oxazepine derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2010;45:3080–3086. doi: 10.1016/j.ejmech.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Zhang LQ, Guan LP, Wei CX, Deng XQ, Quan ZS. Synthesis and anticonvulsant activity of some 7-alkoxy-2H-1,4-benzothiazin-3(4H)-ones and 7-alkoxy-4H-[1,2,4]triazolo[4, 3-d]benzo[b][1,4]thiazines. Chem. Pharm. Bull. 2010;58:326–331. doi: 10.1248/cpb.58.326. [DOI] [PubMed] [Google Scholar]
- 17.Deng XQ, Song MX, Wei CX, Li FN, Quan ZS. Synthesis and anticonvulsant activity of 7-alkoxy-triazolo-[3,4-b]benzo[d]thiazoles. Med. Chem. 2010;45:3080–3086. doi: 10.2174/157340610793358855. [DOI] [PubMed] [Google Scholar]
- 18.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44 (Suppl. 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 19.Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia. 1978;19:409–28. doi: 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 20.Porter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, Scoville B, White BG. Antiepileptic Drug Development Program. Cleve Clin. Q. 1984;51:293–305. doi: 10.3949/ccjm.51.2.293. [DOI] [PubMed] [Google Scholar]