Abstract
3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins), are effective serum cholesterol-lowering agents which also have anti-inflammatory properties. The objective of this study was to evaluate the effect of atorvastatin on bronchial hyperresponsiveness.
Adult patients (age 14 to 65 years) with bronchial hyperresponsiveness (BHR) diagnosis based on the spirometry with methacholine challenge test were entered into the study. The study was conducted in the National Research Institute of Tuberculosis and Lung Disease. Patients were randomized to receive either atorvastatin 20 mg/day or placebo for 4 weeks. Spirometric parameters were determined at baseline and at completion of the study. Twenty two patients with the age of 32.95±10.30 years completed the trial.
Changes in airway responsiveness categories (moderate to severe, mild, borderline, normal) after the intervention were not significant in atorvastatin group as in placebo group (p-value= 0.131 for atorvastatin group and p-value = 0.305 for placebo group). Also, changes in methacholine solution number (different concentrations of methacholine) which caused at least 20% decrease in FEV1 were not significant between groups (p-value = 0.089). Although we could not find a significant difference, the patients’ fall in FEV1 in atorvastatin group was observed in higher concentrations of methacholine. Median before treatment versus after treatment in atorvastatin group was 1 versus 4 mg/mL, while those were 2 versus 1 mg/mL in placebo group.
This study showed a better but not significant hyperresponsiveness control in the treatment group. The result might be presented more pronounced, if we could increase the sample size.
Key Words: Bronchial hyperresponsiveness, Atorvastatin, Methacholine, Clinical Trial, Lung function, Statin
Introduction
Statins or 3-hydroxy-3-methylgluteryl coenzyme A (HMG-CoA) reductase inhibitors have pleiotropic anti-inflammatory effects that may be beneficial in the treatment of chronic inflammatory diseases in addition to reducing cholesterol biosynthesis (1, 2). Studies have demonstrated that statins reduce cardiovascular morbidity and mortality in patients with or without coronary artery disease and/or elevated cholesterol levels (3, 4). Administration of atorvastatin can prevent nitrate tolerance in diabetic as well as normal rats (5). Preclinical in-vitro and in-vivo studies, including experimental models of allergic lung inflammation, have shown that statins decrease components of airway inflammation probably relevant to the pathogenesis of asthma (6, 7). Statins have been shown to reduce the production of interleukin (IL)-6 (8, 9, 10). Statins inhibit T cell activation by decreasing the expression of MHC II on monocytes induced by IFN-γ (11). They may also reduce tumor necrosis factor-α (TNF-α) (12).
It has been shown that airway hyper- responsiveness is a feature of bronchial asthma, and it has been stressed that airway inflammation has an important role in bronchial hyper- responsiveness in humans (13). Statins have the potential to modify T lymphocyte driven diseases (14-15).
Th2 lymphocytes are thought to play a key role in the initiation and perpetuation of this airway inflammation, mediated by the functions of their signal cytokines such as IL-3, IL-4, IL-5, and IL-6 (16). There is now evidence that Th1 cells may also contribute to bronchial hyperresponsiveness, and IFN-γ secretion may exacerbate airway inflammation (17-18-19). The potential benefits of statin therapy on inflammatory airway disease were demonstrated in an experimental animal model of allergic airways disease. Simvastatin reduced inflammatory cell infiltrate and eosinophilia in bronchoalveolar lavage (BAL) fluid and decreased IFN-γ, IL-4 and IL-5 in-vitro (6). The same anti-inflammatory effects of pravastatin have been reported in a similar animal model of allergic airway inflammation (20). Another animal study demonstrated that simvastatin used in emphysema, reduced mRNA expression of IFN-γ, TNF-α and MMP-12 in the whole lung and reduced the number of neutrophils and lymphocytes and the concentration of TNF-α in BAL fluid, thus reduced inflammation and remodeling (21). The intergroup differences in the anti-inflammatory potency of statins should be kept in mind. It has been suggested that less hydrophil statins such as simvastatin and atorvastatin could have more capacity to suppress inflammation and TNF-γ production (22, 23). Though atorvastatin is a stronger inhibitor of the inflammatory response compared to simvastatin, as indicated by NF-kappaB block activation (24). Atorvastatin has been associated with marked down-regulation of HLA-DR and the CD38 activation on peripheral T cells. On the contrary, super antigen-mediated T cell activation was restrained by simvastatin (25). The anti-inflammatory properties of statins are numerous, complex and, although incompletely understood, they so they might prove to be of clinical benefit in the treatment of a range of inflammatory lung diseases.
Methacholine challenge testing (MCT) is considered when asthma is high possibility and spirometry could not confirm the diagnosis. Methacholine chloride is widely accepted as one of the drugs of choice for bronchial challenge testing to assess airway hyperresponsiveness in both clinical and research settings (26).
The objective of this study was to evaluate the effect of atorvastatin on FEV1 and bronchial hyperresponsiveness using MCT.
Experimental
Study design
This study was a randomized, double-blinded, placebo-controlled clinical trial with 2 treatment groups comparing the effect of oral atorvastatin 20 mg (Sobhan Pharmaceuticals, Iran) daily for 1 month with that of a matched placebo on the lung function. The study protocol was registered, reviewed and approved by Australian New Zealand Clinical Trial Registry and the registry number is ACTRN12609000704291.
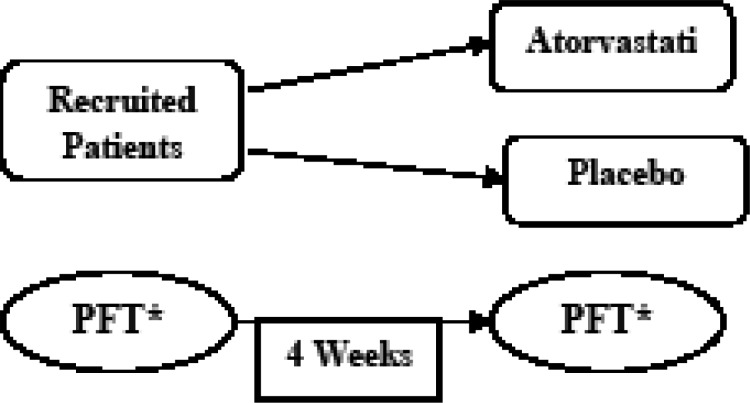
The study was conducted in the pulmonary clinic of the National Research Institute of Tuberculosis and Lung Disease (NRITLD). Consent form was obtained from all the patients entered the study. Patients were randomly allocated to receive either medicine or placebo in a double-blind fashionl (Figure 1).
Figure 1.
Illustration of the study design *PFT: Pulmonary Function Test
All investigators (data collecting investigator, outcome assessor, clinical trial consultant and study statistician) were masked to the treatment allocation of the patients.
Methacholine preparation
Methacholine powder (Sigma-Aldrich, Fluka, US) has been used and the following 8 doubling concentrations of methacholine in sterile vials were prepared.
Solution number:
1 2 3 4 5 6 7 8
Concentration (mg/mL):
0.125 0.25 0.5 1 2 4 8 16
Patients
Adult patients (age 14 to 65 years) with Bronchial Hyperresponsiveness (BHR) diagnosis based on the spirometry with methacholine challenge test and clinical symptoms were entered into the study. The following exclusion criteria were applied: cardiovascular disease, hepatic disease, acute upper and lower airway infections, use of any lipid lowering drugs during the past six months, use of concurrent drugs affecting atorvastatin metabolism, pregnancy or lactation, clinically significant rise in creatinine phosphokinase (CPK), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, appearance of severe statin side effects e.g. myopathy), secondary diseases that cause worsening of BHR symptoms, using cholinesterase inhibitor agents, uncontrolled hypertension and aortic aneurysm during the study.
All the laboratory tests were confirmed by the pathologist. After confirmation of normal lipid profile, participants were randomized to receive either placebo or atorvastatin orally 20 mg once daily, for 4 weeks. Allocation concealed by sealed opaque envelopes. The pharmacist dispensed either active drug or placebo according to randomization table from a statistic book.
Spirometry and MCT
Study outcomes were spirometric parameters i.e. peak expiratory flow rate (PEFR) and forced expiratory volume in the 1st second (FEV1). After baseline spirometry the concentration was sequentially increased one concentration step at a time, until a decrease in FEV1 greater than 20 percent was seen. The lowest concentration of methacholine which caused ≥ 20% (PC20) fall in FEV1 was considered as BHR, while the lack of a 20% fall in FEV1 at the highest concentration (16 mg/mL) ruled out BHR (27). These parameters were measured at baseline and 4 weeks post drug or placebo administration.
Airway responsiveness was categorized as: PC20 <1 mg/mL = moderate to severe BHR, 1- 4 mg/mL = mild BHR, 4 – 16 mg/mL = borderline, >16 mg/mL = normal based on ATS guideline (28).
Data analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS 16.0, SPSS Inc., Chicago, IL, USA). Independent and paired sample t-test, Mann-whitney and Wilcoxon signed ranks tests were used for comparisons. The results are expressed as mean ± SD, median, ranges. p < 0.05 was regarded as significance level.
Results
Twenty two patients with BHR diagnosis (12 females and 10 males) with mean±SD age of 32.95 ± 10.30 years could complete the entire 4 weeks fully blinded study protocol.
The number of patients who received atorvastatin 20 mg/day versus those received placebo was 9 versus 13. The minimum duration of BHR symptoms in patients before the start of the study was one month with mean ± SD duration of 16.60 ± 84.53 months. The chief complaints of these patients were cough (22.7%), dyspnea (18.2%), dry cough (4.5%), and palpitation (4.5%). 36.4% of them had a history of allergic rhinitis. Among all, 27.3% had a family history of asthma and 18.2% had a family history of allergic rhinitis. Drug sensitivity in 9.1% and food allergy in 27.3% of the patients were reported.
The measured parameters, before and after atorvastatin and placebo therapy are shown in Table 1.
Table 1.
Spirometric parameters of subjects before and after treatment
| Patient | Before treatment |
|
After treatment |
group | change in methacholine solution* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 (%) | PC20 (mg/ml) |
AR category | FEV1 (%) | PC20 (mg/ml) |
AR category | |||||
| 1 | 98 | 4 | borderline | 93 | 1 | mild | Placebo | -2 | ||
| 2 | 90 | 8 | borderline | 90 | 16 | normal | Placebo | 1 | ||
| 3 | 92 | 0.25 | moderate to severe | 88 | 0.125 | moderate to severe | Drug | -1 | ||
| 4 | 112 | 8 | borderline | 109 | 2 | mild | Drug | -2 | ||
| 5 | 103 | 4 | borderline | 104 | 4 | borderline | Placebo | 0 | ||
| 6 | 76 | 1 | mild | 77 | 1 | mild | Drug | 0 | ||
| 7 | 103 | 0.5 | moderate to severe | 98 | 16 | normal | Drug | 5 | ||
| 8 | 84 | 4 | borderline | 80 | 0.5 | moderate to severe | Placebo | -3 | ||
| 9 | 78 | 4 | borderline | 78 | 2 | mild | Placebo | -1 | ||
| 10 | 110 | 4 | borderline | 110 | 4 | mild | Placebo | 0 | ||
| 11 | 88 | 2 | mild | 89 | 0.25 | moderate to severe | Placebo | -3 | ||
| 12 | 109 | 1 | mild | 88 | 2 | mild | Placebo | 1 | ||
| 13 | 90 | 2 | mild | 91 | 4 | borderline | Drug | 1 | ||
| 14 | 93 | 1 | mild | 94 | 1 | mild | Placebo | 0 | ||
| 15 | 116 | 1 | mild | 119 | 1 | mild | Placebo | 0 | ||
| 16 | 78 | 0.25 | moderate to severe | 86 | 1 | mild | Drug | 2 | ||
| 17 | 81 | 1 | mild | 93 | 16 | normal | Drug | 4 | ||
| 18 | 102 | 4 | borderline | 100 | 8 | borderline | Drug | 2 | ||
| 19 | 109 | 8 | borderline | 103 | 8 | borderline | Drug | 0 | ||
| 20 | 117 | 0.5 | moderate to severe | 110 | 0.5 | moderate to severe | Placebo | 0 | ||
| 21 | 90 | 0.5 | moderate to severe | 90 | 8 | borderline | Placebo | 4 | ||
| 22 | 82 | 2 | mild | 90 | 0.25 | moderate to severe | Placebo | -3 | ||
AR: Airway responsiveness* Increase or decrease in solution concentration which caused more than 20% decrease in FEV1
Data analysis revealed no significant differences between baseline FEV1% in the two groups (t= 0.539, p-value= 0.596). Changes in airway responsiveness categories (e.g. from mild to borderline airway responsiveness) were not significant in atorvastatin group and placebo group (p-value= 0.131 for atorvastatin group and p value = 0.305 for placebo group). Changes in methacholine solution number which caused greater than 20% decrease in FEV1 were not significant between groups, either (p-value = 0.089). Descriptive parameters of spirometric findings in placebo and atorvastatin group are shown in Table 2.
Table 2.
Descriptive parameters of spirometeric findings in placebo and atorvastatin group
| Median | Minimum | Maximum | Percentile 25 | Percentile 75 | ||
|---|---|---|---|---|---|---|
| Placebo | PC20 before (mg/mL) | 2 | 0.5 | 8 | 1 | 4 |
| PC20 after (mg/mL) | 1 | 0.25 | 16 | 0.5 | 4 | |
| Airway responsiveness category (before treatment) | 2 | 1 | 3 | 2 | 3 | |
| Airway responsiveness category (after treatment) | 2 | 1 | 4 | 1 | 2 | |
| change in methacholine solution* (solution number) | 0 | -3 | 4 | -2 | 0 | |
| Drug | PC20 before (mg/mL) | 1 | 0.25 | 8 | 0.5 | 4 |
| PC20 after (mg/mL) | 4 | 0.125 | 16 | 1 | 8 | |
| Airway responsiveness category (before treatment) | 2 | 1 | 3 | 1 | 3 | |
| Airway responsiveness category (after treatment) | 3 | 1 | 4 | 2 | 3 | |
| change in methacholine solution* (solution number) | 1 | -2 | 5 | 0 | 2 | |
* Increase or decrease in solution number which caused more than 20% decrease in FEV1
There were no significant difference between the alterations of PEFR, FEV1 and FEV1% in neither placebo nor atorvastatin group.
Discussion
According to the results of this study, using atorvastatin 20 mg/day for a month had no significant effect on spirometric indices of BHR normolipid patients. Although we could not find a significant difference, there was a trend in treatment group i.e. changes in methacholine solution number, which shows that the patients in that group had drop in FEV1 in higher concentrations of methacholine.
Median of PC20 in atorvastatin group before treatment was less than after treatment (1 versus 4 mg/mL), while those were 2 versus 1 mg/mL in placebo group. This could be considered a better hyperresponsiveness control in the treatment group. This is the first study on BHR patients with statins. The result may be presented more pronounced, if we could increase the sample size. Although many studies have been done in this area but due to complexity and the nature of inflammatory disease, there are controversies about the anti-inflammatory effects of statins and their effect on the inflammatory disease. The result of this study is in contravention with some of the findings of previous studies that have been successful to report anti-inflammatory effects of statins (2, 6, 29, 30, 31).
Of course there are contradictions in the statins effects reported in the previous studies (22, 32, 33, 34).The effects of statins have mainly been studied on cellular markers and few clinical trials on human have been done (14, 20, 32-34).To our knowledge the effects of atorvastatin on human asthma and BHR are unexplored clearly. However, in 2004 Mckay and colleagues examined the anti-inflammatory activity of simvastatin in a murine model of allergic asthma. The result has shown a reduction in IL-4, IL-5, IL-6, and IFN-γ secretion in thoracic lymph node cultures from simvastatin-treated mice. This study was conducted on allergic asthma, while we studied on patients with Bronchial Hyperresponsiveness (BHR). In animal trial study, there were two ways of administration: Intra peritoneal (IP) and oral administration. One study has shown IP that route obtained better results (6).This is probably due to first-pass hepatic metabolism of the drug after absorption from the gastrointestinal tract. In 2006 Verhoeven evaluated atherosclerotic plaques in 378 patients that more than half of them were taking different doses of statins. Although IL-6 levels in the group who were taking statins had a decrease, but IL-8 levels in the two groups did not differ and CD68 as a marker of macrophages in the atorvastatin group had increased. While that of IL-4, IL-5, IL-6 as inflammatory mediators involved in BHR (35). In 2006, thirty young healthy male participants who received an injection of the bacterial cell wall endotoxin product to induce systemic inflammation were administered simvastatin 20 mg daily for 14 days. Plasma cytokines (TNF-alpha, IL-6 and IL-1) as well as total leukocyte counts increased in all participants upon endotoxin challenge but were not affected by simvastatin treatment unlike study done by Erikstrup et al. (36).
Evidence of anti-inflammatory effects of statins in autoimmune disease is also obtained. Lawman and colleagues in 2004 showed reduced progression of lupus in mice by atorvastatin (37). It should be noted that lupus is a complex inflammatory disease with a systemic inflammatory while asthma is a localized inflammation and TH2 dominant disease. Further studies were carried on to evaluate the effect of statins in autoimmune diseases with TH1 dominant such as rheumatoid arthritis. Also, there is no evidence about the effect of statins on inflammation induced by Th2-interference (30).
In a similar study in 2008, 44 patients with allergic asthma were treated with atorvastatin 40 mg and placebo for 8 weeks. After 6 weeks of washout, a second 8-week treatment period was carried on. Spirometric parameters, inflammatory markers such as IL6, IL5, IL8, TNFα, B4 leukotriene, CRP, NO levels in exhaled breath and blood samples at the beginning, middle and end of each period was measured. The results showed no improvement and no decrease in spirometric indices and inflammatory parameters in these patients (38). The study duration was longer than our study (4-6 weeks).
CRP levels decreased in previous studies after taking atorvastatin over 4 weeks and in another study after only 2 days (39, 40). Also the anti-inflammatory effects of simvastatin in animal models with allergic asthma have been observed after 28 days (6). In contrast, in another study that was conducted in 2007 by Menzies, there was no anti-inflammatory effect after taking simvastatin for 1 month in asthmatic patients (22). Based on these findings, it is clear that duration of using statins does not seem to determine anti-inflammatory effects of them and 4 weeks should be long enough for study the anti-inflammatory effects of statins. In the study which was conducted in the University of Glasgow, all patients have been used inhaled corticosteroids with atorvastatin simultaneously. Although, this did not change spirometric indices significantly, it is possible that reduction in amount of macrophages and leukotrienes B4 in the sputum of patients was due to synergistic effect of inhaled corticosteroids with atorvastatin (41). We did not determine macrophages and other inflammatory markers in sputum of patients.
Besides, we used atorvastatin 20 mg/day which failed to make a difference in the outcomes.
Kiener and colleagues also showed that lipophylic statins such as simvastatin has more anti-inflammatory effect in human and mouse models than pravastatin (a hydrophilic statin) (23). In contrast Joukhadar and colleagues by comparing atorvastatin, simvastatin and pravastatin have shown no difference between their effect on inflammatory parameters (5). There is a possibility that using other statins may increases anti-inflammatory effects in comparison with atorvastatin, which according to some studies this possibility is not far-fetched (6, 20).
In the study conducted by Menzies and colleagues before starting treatment, using inhaled corticosteroids were prohibited and patients only could use inhaled long-acting beta-agonists (22). According to the reports of other study anti-inflammatory effects of other drug classes can cover the inflammatory effects of statins (42).
In a new study, 40 asthma patients were studied for one year. Twenty patients received statin and the rest were not taking statins. Patients taking statins showed more decrease in lung function compared to controls (43).
In conclusion, administration of oral atorvastatin 20 mg/day for 4 weeks did not illustrate a significant impact on spirometric parameters and airway responsiveness in normolipidemic BHR patients.
Since we had only assessed the effects on clinical symptoms and spirometric parameters, we could not conclude that statins lack anti-inflammatory properties at tissue, endothelial or cellular levels. Although the response to different concentrations of methacholine did not show a significant difference between the treatment and placebo group of patients, a trend was observed. Interestingly, patients in atorvastatin group were provoked in higher concentrations while this was reversed in placebo group.
Methacholine challenge test might be a better predictor of the probable effect of statins in BHR patients. Though, for more accurate conclusion on the anti-inflammatory effect of HMG CoA reductase inhibitors, study of the systemic inflammatory biomarkers and inflammatory changes at tissue, endothelial or cellular levels of the respiratory system with a larger sample size is recommended.
Acknowledgment
The authors would like to acknowledge Sobhan Pharmaceuticals, Iran for the preparation of the placebo. We would like to thank Dr. Hamid Reza Moghimi and Dr. Jamshid Salamzadeh for their kind support, and Golnar Radmand for her assistance in data analysis. Also, the collaboration of Dr. Solmaz Sohrabi in the preparation of the proposal of this project and Dr. Sepideh Taghikhani are highly appreciated.
The study was supported financially by the National Research Institute of Tuberculosis and Lung Disease.
The study is the result of a pulmonology fellowship project.
References
- 1.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease, from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;6:358–70. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–34. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briel M, Studer M, Glass TR, Bucher HC. Effects of statins on stroke prevention in patients with and without coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2004;117:596–606. doi: 10.1016/j.amjmed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Joukhadar C, Klein N, Prinz M, Schrolnberger C, Vukovich T, Wolzt M, Schmetterer L, Dorner GT. Similar effects of Atorvastatin, Simvastatin and Pravastatin on thrombogenic and inflammatory parameters in patients with hypercholesterolemia. Thromb. Haemost. 2001;85:47–51. [PubMed] [Google Scholar]
- 5.Imenshahidi M, Karimi G, Kazemzadeh E. Effects of Atorvastatin on nitrate tolerance in diabetic rats. Iranian J. Pharm. Res. 2010;9:55–59. [PMC free article] [PubMed] [Google Scholar]
- 6.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A Novel Anti- Inflammatory Role of Simvastatin in a Murine Model of Allergic Asthma. J. Immunol. 2004;17:2903–08. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 7.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin Inhibits Airway Hyper reactivity: Implications for the Mevalonate Pathway and beyond. Am. J. Respir. Crit. Care Med. 2009;180:731–40. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda U, Shimada K. Statins and monocytes. Lancet. 1999;353 doi: 10.1016/S0140-6736(05)77885-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferro D, Parrotto S, Basili S, Alessandri C, Violi F. Simvastatin inhibits the monocyte expression of proinflammatory cytokinesin patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2000;36:427–31. doi: 10.1016/s0735-1097(00)00771-3. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immune modulator. Nat. Med. 2000;6:1399–403. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–4. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 13.Nadel JA. Inflammation and asthma. J. Allergy Clin. Immunol. 1984;73:651–53. doi: 10.1016/0091-6749(84)90299-9. [DOI] [PubMed] [Google Scholar]
- 14.Weitz-Schmit G, Welzenbach K, Brinkmannn V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding a novel regulatory integrin site. Nat. Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 15.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as newly recognized type of immune modulator. Nat. Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma. Pharmacol. Rev. 1998;50:515–96. [PubMed] [Google Scholar]
- 17.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyper reactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J. Immunol. 1999;162:2375–83. [PubMed] [Google Scholar]
- 19.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferonx regulate allergic airway inflammation and mucus production. J. Exp. Med. 1999;190:1309–17. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J. Biomed. Sci. 2004;11:599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi S, Nakamura H, Furuuchi M, Minematsu N, Nakajima T, Tsujimura S, Seki M, Ishizaka A. Simvastatin suppresses the development of elastase-induced emphysema in mice. Proc. Am. Thor. Soc. 2005;2:A135. [Google Scholar]
- 22.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J. Allergy Clin. Immunol. 2007;119:328–35. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Kiener PA, Davis PM, Murray JL, Youssef S, Rankin BM, Kowala M. Stimulation of inflammatory responses in vitro and in vivo by lipophilic HMG-CoA reeducates inhibitors. Int. Immuno pharmacol. 2001;1:105–18. doi: 10.1016/s0162-3109(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 24.Hilgendorff A, Muth H, Parviz B, Staubitz A, Haberbosch W, Tillmanns H, Hölschermann H. Statins differ in their ability to block NF-γB activation in human blood monocytes. Int. J. Clin. Pharmacol. Ther. 2003;41:397–401. doi: 10.5414/cpp41397. [DOI] [PubMed] [Google Scholar]
- 25.Fehr T, Kahlert C, Fierz W, Joller Jemelka HI, Riesen WF, Rickli H, Wüthrich RP, Ammann P. Statin-induced immunomodulatory effects on human T cells in-vivo. Atherosclerosis. 2004;175:83–90. doi: 10.1016/j.atherosclerosis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Laflamme-Procyshen S. Methacholine challenge testing- An evidence-based review, business briefing. North American Pharmacotherapy. 2004;2:1–3. [Google Scholar]
- 27.Birnbaum S, Barreiro TJ. Methacholine challenge testing, identifying its diagnostic role, testing, coding, and reimbursement. Chest. 2007;131:1932–5. doi: 10.1378/chest.06-1385. [DOI] [PubMed] [Google Scholar]
- 28.The American Thoracic Society Guidelines for methacholine and exercise challenge testing. Am. J. Respir. Crit. Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 29.Samson KT, Minoguchi K, Tanaka A. Inhibitory effects of fluvastatin on cytokine and chemokine production by peripheral blood mononuclear cells in patients with allergic asthma. Clin. Exp. Allergy. 2006;36:475–82. doi: 10.1111/j.1365-2222.2006.02470.x. [DOI] [PubMed] [Google Scholar]
- 30.Asberg A, Hartmann A, Fjeldså E, Holdaas H. Atorvastatin improves endothelial function in renal-transplant recipients. Nephrol. Dial. Transplant. 2001;16:1920–24. doi: 10.1093/ndt/16.9.1920. [DOI] [PubMed] [Google Scholar]
- 31.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, Chen JW, Leu HB. Statin use in patients with asthma – a nationwide population-based study. Eur. J. Clin. Invest. 2011;41:507–12. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur. J. Pharmacol. 2007;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Fahimi F, Salamzadeh J, Jamaati HR, Sohrabi S. Do statins improve lung function in asthmatic patients? A randomized and double-blind trial. IJPS. 2009;5:13–20. [Google Scholar]
- 34.Erikstrup C, Ullum H, Pedersen BK. Short-term Simvastatin treatment has no effect on plasma cytokine response in a human in-vivo model of low-grade inflammation. Clin. Exp. Immunol. 2006;144:94–100. doi: 10.1111/j.1365-2249.2006.03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corsini A, Arnaboldi L, Raiteri M, Quarato P, Faggiotto A, Paoletti R. Effect of the new HMG-CoA reductase inhibitor cerivastatin (BAY W 6228) on migration, proliferation and cholesterol synthesis in arterial monocytes. Pharmacol. Res. 1996;33:55–61. doi: 10.1006/phrs.1996.0009. [DOI] [PubMed] [Google Scholar]
- 36.Bellosta S, Via D, Canavesi M, Pfister P, Fumagalli R, Paoletti R, Bernini F. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler. Thromb. Vasc. Biol. 1998;18:1671–8. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 37.Choi M, Rolle S, Rane M, Haller H, Luft FC, Kettritz R. Extracellular signalregulated kinase inhibition by statins inhibits neutrophil activation by ANCA. Kidney International. 2003;63:96–106. doi: 10.1046/j.1523-1755.2003.00718.x. [DOI] [PubMed] [Google Scholar]
- 38.Verhoeven BA, Moll FL, Koekkoek JA, Koekkoek JAF, Van der Wal AC, De Kleijn DPV, De Vries JPPM H, Verheijen JH, Velema E, Busser E, Schoneveld A, Virmani R, Pasterkamp G. Statin treatment is not associated with consistent alterations in inflammatory status of carotid atherosclerotic plaque. Stroke. 2006;37:2054–60. doi: 10.1161/01.STR.0000231685.82795.e5. [DOI] [PubMed] [Google Scholar]
- 39.Erikstrup C, Ullum H, Pedersen BK. Short-term Simvastatin treatment has no effect on plasma cytokine response in a human in vivo model of low-grade inflammation. Clin. Exp. Immunol. 2006;144:94–100. doi: 10.1111/j.1365-2249.2006.03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein MR. Atorvastatin inhibits auto reactive B cell activation and delays lupus development in Newzeland black/white F1 mice. J. immunol. 2004;173:7641–6. doi: 10.4049/jimmunol.173.12.7641. [DOI] [PubMed] [Google Scholar]
- 41.Hothersall , Eleanor Jane. Effect of Atorvastatin on asthma control and airway inflammation. [dissertation] University of Glasgow; 2008. [Google Scholar]
- 42.Dobreanu M, Gălăţeanu C, Simionescu A, Deac R. Effects of Atorvastatin on some inflammatory parameters in severe primary hypercholesterolemia. Rom. J. Intern. Med. 2002;40:61–73. [PubMed] [Google Scholar]
- 43.Laufs U, Wassmann S, Hilgers S, Ribaudo N, Böhm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of Atorvastatin in healthy, Normocholesterolemic men. Am. J. Cardiol. 2001;88:1306–7. doi: 10.1016/s0002-9149(01)02095-1. [DOI] [PubMed] [Google Scholar]
- 44.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, Lafferty J, Lloyd SM, Spears M, Mair F, Thomson NC. Effects of short-term treatment with Atorvastatin in smokers with asthma. BMC Pulm. Med. 2011;11:16. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, Bailey M, Wilson JW, Haydn Walters E. An anti-inflammatory effect of salmetrol, a long-acting beta 2 agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. Am. J. Respir. Crit. Care Med. 1999;160:1493–9. doi: 10.1164/ajrccm.160.5.9811052. [DOI] [PubMed] [Google Scholar]
- 46.American College of Allergy. Asthma and Immunology Annual Meeting. Boston: The Institute; 2011. Nov. pp. 3–8. [Google Scholar]