Abstract
In the present study, protective effect of Teucrium polium L. (Labiatae) extract on acetaminophen-induced hepatotoxicity was investigated in mice. Animals were divided into six groups, each group consist of 8 mice. Group one as the negative control group received normal saline, while group two received only crude extract of T. polium L. (500 mg/Kg) for five days and group three as the positive group received acetaminophen (500 mg/Kg). Groups four, five and six received crude extract in doses of 125, 250 and 500 mg/Kg, respectively, and on the fifth day, one hour after the last administration, acetaminophen was given orally (500 mg/Kg). Then on the 6th day, animals were sacrificed, their blood was collected to determine serum enzyme activities of ALT, AST and ALP to measure the serum levels of directed and total bilirubin. The livers were removed for histological examination. The results of this study showed the protective effect in all doses but the most significant protection was observed in doses of 250 and 500 mg/Kg (p < 0.05). Also these findings were supported and confirmed by histological examination.
Key Words: Hepatotoxicity, Acetaminophen, Teucrium polium L, Protective
Introduction
It is well known that medicinal plants play an important role in health care system and can be called as a main source of new chemical substances with potential therapeutic effects (1). In recent years, there has been a shift towards therapeutic evaluation of herbal products in liver diseases (2). The genus Teucrium L. (Labiatae) is very diverse and contains more than 300 species mainly found in the Mediterranean region (3). Teucrium polium L. is a wild-growing flowering plant, which belongs to Labiatae family (4). This plant has been used in folk medicine for various purposes such as anti-inflammatory, anti-bacterial, anti-pyretic, anti-spasmodic, anti-hypertensive and anti-hyperlipedemic (5, 6). Furthermore, the plant possesses hypoglycemic, insulinotropic, diuretic, diaphoretic, tonic, cholagogic and antioxidant properties (8-11). Acetaminophen is a widely used nonprescription analgesic and antipyretic drug that has a very low rate of liver toxicity at normal therapeutic doses, however, it causes hepatic and renal injuries in humans and experimental animals when administered in high doses (12-14 ). Liver, as a major vital organ, metabolizes acetaminophen in the form of glucuronidated and sulfated product and the subsequent metabolite is excreted by urine (15), but small fraction metabolized by cytochrome P450 to a highly reactive free radical, n-acetyl-p-benzoquinone imine (NAPQI) (16). This metabolite is a strong electrophile oxidizing agent normally detoxified by reduced glutathione (GSH) in the liver (17). However, after acetaminophen overdose, the glucuronidation and sulfation pathways become saturated, and more acetaminophen becomes available for activation by the cytochrome P450, which produces a large amount of NAPQI leading to rapid depletion of hepatic GSH levels. Subsequently, NAPQI metabolite binds covalently to cell macromolecules that results in cell damage or cell death (18, 19). The aim of this study was to evaluate the hepatoprotective activities of hydro alcoholic extract of T. polium on acetaminophen-induced hepatotoxicity.
Experimental
Animals
Studies were carried out using male ICR mice (6-8 weeks old, 25-30 g), obtained from Animal house of Ahvaz Jundishapur University of Medical Science, Iran. Mice were kept in polycarbonate cages under standard condition (temperature 25 ± 2°C) with 12 h light/dark cycle. They were provided with standard pellet diet and free access to drinking water ad libitum. The animals were acclimatized to the environment for a week before the commencement of experiment. The investigation was performed according to the Local Animal Ethics Committee guidelines for the use of experimental animals.
Chemicals
All the chemicals were of analytical grade. Solvents were purchased from Merck (Darmstadt, Germany). The acetaminophen powder was purchased from Darou pakhsh Company (Iran). Preparation of plant extract
Plant was collected from Larestan region, Iran in April 2008 and shade-dried. The plants were identified at the Herbarium of Department of Pharmacognosy, School of Pharmacy, Ahvaz, Iran, where the voucher specimens were preserved (number voucher: T-0157). The whole plant was crushed into small pieces and soaked in an 80% aqueous-ethanol solution in a large container for 3 days with occasional shaking. The extract was filtered through a clean cotton cloth and the filtrate was dried by using a rotary evaporator at 40°C. The extract yield was 16% w/w (19).
Study design
Plant extract was dissolved in normal saline before the administration to mice. Acetaminophen was first dissolved in normal saline at 70°C, and then cooled to 37°C for administration; it was administered orally in a single dose of 500 mg/Kg. Mice were divided randomly into six groups, each of which consisted of eight animals. All mice were treated orally for five consecutive days. Group one as the negative control group received normal saline (10 mL/Kg), while group two received only crude extract of T. polium L. (500 mg/Kg) for five days and group three as the positive group received acetaminophen (500 mg/Kg) on the fifth day. Groups four, five and six received crude extract during five days in doses of 125, 250 and 500 mg/Kg, respectively and on the fifth day, acetaminophen was administered (500 mg/Kg) one hour after the last administration of the crude extract. Then, on the 6th day, animals were sacrificed and their blood was collected to estimate ALT, AST and ALP, direct and total bilirubin. Liver was removed and kept in 10% formalin solution for histopathological examination.
Biochemical assays
The blood samples were allowed to clot for 40 min at room temperature. Serum was separated by centrifugation at 2500 rpm at 30°C for 15 min. Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum were determined employing the method of Reitman and Frankel. In this procedure, transaminase reacts with 2, 4-dinitrophenyl-hydrazones reagent and produces color complex that is proportional to the AST and ALT concentrations (21). Alkaline phosphatase (ALP) was estimated according to king (22), while serum levels of direct and total bilirubin were determined according to the method of Watson and Rogers. In this method, the conventional diazo reagent was added to plasma, and then the color densities were determined (23).
Histopathological assessments
The liver specimens were fixed in 10% neutral buffered-formalin and then, Liver tissues were dehydrated with a sequence of ethanol solutions, embedded in paraffin, cut into 5 μm sections, stained with hematoxylin and eosin dye (H and E stain) and observed under a photomicroscope (24). The observed morphological changes included cell necrosis, fatty change and the infiltration of lymphocytes and Kupffer cells.
Statistical analysis
Statistical analysis was performed using the statistical package SPSS 16.0 for Windows. The results were expressed as mean ± SD. One-way ANOVA followed by Tukey post-test were applied for statistical analysis with the level of significance set at p < 0.05.
Results
It is clear that an increase in serum levels of AST, ALT, ALP and bilirubin concentrations in blood indirectly reflects the failure of liver function due to the acetaminophen-induced hepatotoxicity. The effects of pre-treatment with different doses of T. polium extract (125, 250 and 500 mg/Kg) on serum levels of liver enzymes and bilirubin in acetaminophen-induced hepatotoxicity are shown in Tables 1 and 2.
Table 1.
Effects of the pretreatment with Teucrium polium L. extract on the serum activities of AST, ALT and ALP in acetaminophen-induced hepatotoxicity
| Groups | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|
| 1. normal saline | 73.87 ± 20.08 b | 63.25 ± 8.92 b | 88.62 ± 10.79b |
| 2. T. polium (500 mg/Kg) | 81.75 ± 21.75b | 61.62 ± 8.65b | 95.62 ± 13.98 b |
| 3. Acetaminophen (500 mg/Kg) | 848.50 ± 38.26a | 683.50 ± 42.63a, | 171.62 ± 16.87 a |
| 4. T. polium 125 mg/Kg + Acetaminophen | 702.12 ± 24.58a,b | 547.00 ± 41.70 a,b | 128.50 ± 15.08 a b |
| 5. T. polium250 mg/Kg + Acetaminophen | 406.00 ± 20.52a,b | 338.75 ± 45.24 a,b | 101.00 ± 16.97 b |
| 6. T. polium500 mg/Kg + Acetaminophen | 441.50 ± 112.68a,b | 354.00 ± 43.98 a,b | 96.50 ± 12.85 b |
Table 2.
Effects of the pretreatment with Teucrium polium L. extract on the serum levels of direct and total bilirubin in acetaminophen-induced hepatotoxicity
| Groups | Direct bilirubin | Total bilirubin |
|---|---|---|
| 1. normal saline | .3100 ± 06437 | .4925 ± .12361 |
| 2. T. polium (500 mg/Kg) | .3438 ± .08450 | .5112 ±.14025 |
| 3. Acetaminophen (500 mg/Kg) | .4225 ± .08940 | .6075 ±.18211 |
| 4. T. polium 125 mg/Kg + Acetaminophen | .4488 ± .09658 | .5762 ±.13511 |
| 5. T. polium 250 mg/Kg + Acetaminophen | .3900 ± .10981 | .5925 ±.15360 |
| 6. T. polium 500 mg/Kg + Acetaminophen | .3712 ± .11319 | .5412 ±.15797 |
Animals in group 1 received normal saline solution, whereas animals in group 2 received T. polium L. extract (500 mg/Kg) for 5 days. Group 3 received acetaminophen on the fifth day. The mice in groups 4, 5 and 6 were pretreated with T. polium L. extract (125, 250 and 500 mg/Kg, po, respectively) once daily for five consecutive days. One hour after the final treatment, the mice were treated with acetaminophen (500 mg/Kg, po). Each value represents the mean ± SD for eight mice. a: Significantly different from the control (p < 0.05). b: Significantly different from acetaminophen group ( p < 0.05).
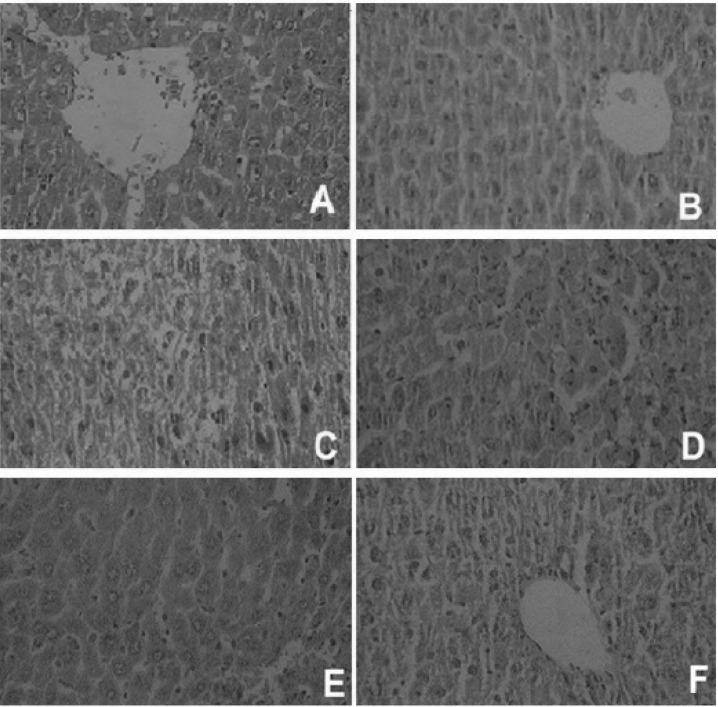
Data showed that 24 h after the acetaminophen administration mice developed significant hepatocellular damage as it was evident from a significant increase in the serum activities of ALT, AST and ALP (p < 0.05) when compared with the negative control group (Table 1). The positive control group showed slightly (not significant) increase in serum direct and total bilirubin concentration, as shown in Table 2. Pretreatment of mice with 125, 250 and 500 mg/Kg of hydroalcoholic extracts of T. polium exhibited a significant (p < 0.05) reduction in the levels of AST, ALT and ALP as compared with the positive control group (Table 1). Direct and total bilirubin concentration, elevated by acetaminophen, exhibited a slight decrease in response to the pre-treatment with the plant extract (Table 2). The histopathological study of liver in the negative control group and the second group with only crude extract showed a normal hepatic architecture with distinct hepatic cells, sinusoidal spaces and a central vein (Figures 1A and 1B).
Figure 1.
Histopathological observations (liver sections stained with Hematoxylin and Eosin, magnification x 100) showing the effects of Teucrium polium extract on acetaminophen-induced histopathological changes in mouse liver. (A) Normal, (B) T. polium L. extract (500 mg/Kg) shows normal hepatic architecture with distinct hepatic cells, sinusoidal spaces and a central vein; (C) acetaminophen-treated group shows severe centrilobular hepatic necrosis, fatty changes, ballooning degeneration, and infiltrating lymphocytes; (D), (E) and (F) are acetaminophen groups pre-treated with 125, 250 and 500 mg/Kg of T. polium L extract, respectively. D shows milder degree of hepatocyte necrosis, fatty changes, ballooning degeneration, and infiltrating lymphocytes. In pictures E and F, only mild inflammation and lymphocyte infiltration are observed
Acetaminophen-intoxicated group exhibited severe histopathological changes, such as centrilobular hepatic necrosis, fatty changes, ballooning degeneration, and infiltrating lymphocytes (Figure 1C). The group that received 125 mg/Kg of T. polium extract showed partial protection of hepatocytes (Figure 1D), while pretreatment with doses of 250 and 500 mg/Kg of hydro alcoholic extract showed best results and prevented these histopathological changes associated with the hepatotoxicity induced by acetaminophen. Results of groups that received 250 mg/Kg and 500 mg/Kg of the extract of T. polium are shown in Figure 1E and Figure 1F.
Discussion
Liver is one of the largest organs in human body and the major site for metabolism and excretion (25). It has a wide range of functions, including detoxification, protein synthesis, and production of biochemical’s necessary for digestion (26). Liver diseases have become one of the major causes of morbidity and mortality in human all over the world and hepatotoxicity due to drugs appears to be the most common contributing factor (27). Protection against acetaminophen induced toxicity has been used as a test for a potential hepatoprotective agent by several investigators (28-31).
Acetaminophen, as an analgesic and antipyretic drug, is readily available without prescription. In therapeutic doses, acetaminophen is well tolerated; side effects and interaction with other drugs are usually not observed. However, overdose of acetaminophen causes acute centrilobular hepatic necrosis in humans and experimental animals (32, 33). The rise in serum levels of transaminases (AST and ALT) and ALP have been attributed to the damaged structural integrity of the liver, because these are normally located in the cytoplasm and are released into the circulation after cellular damage (34). The elevation in the levels of bilirubin has been reported in acetaminophen-induced hepatotoxicity. Bilirubin, an endogenous organic anion binds reversibly to albumin and it is transported to the liver, and then conjugated with glucuronic acid and excreted in the bile. In hepatobiliary disease, bilirubin concentration exceeds the upper limits of normal (35, 36). Since hepatic damage induced by acetaminophen is mediated by its free radical metabolites (16-18), antioxidant activity or inhibition of the generation of free radicals is important in the protection against acetaminophen-induced liver injury (37). It has been reported that T. polium possess profound anti-inflammatory and antioxidant activity (10, 11, 38). Furthermore, T. polium extract enhances intracellular glutathione levels by promoting the glutathione biosynthetic pathway (39). These properties motivate us to study its hepatoprotectivity in acetaminophen-induced liver toxicity.
Panovska et al. investigated hepatoprotective activity of the ethyl acetate extract of Teucrium polium L. against CCl4-induced liver damage. They reported that intraperitoneal injection of T. polium extract for 7 days resulted in restoration of liver damage to the normal state (40).
Our results provided strong evidence that T. polium extract significantly inhibited the acute liver toxicity induced by high doses of acetaminophen in mice, as shown by a decrease in serum liver enzyme activities (AST, ALT and ALP) and bilirubin concentrations (Tables 1 and 2). Moreover, the liver morphology and histopathology findings confirm the protective activity of this extract against the acetaminophen-induced liver damage as it is evident by the reversal of centrilobular necrosis, fatty changes (steatosis) and scattered lymphocytes infiltrate in hepatic parenchyma by T. polium administration. Thus, as shown in Figures 1E and 1F, only mild inflammation and lymphocyte infiltration were observed. Although this protective effect was dose-dependent, there was no significant difference between doses of 250 and 500 mg/Kg of T. polium extract (Table 1). Despite the fact that T. polium extract significantly reduced ALT and AST levels in groups 4, 5 and 6, it can’t completely restore these biochemical parameters to the normal values. Moreover, group two that received only crude extract of T. polium L (500 mg/Kg, once daily, for 5 days) did not have any significant differences with negative control group based on biochemical parameters (AST, ALT , ALP and bilirubin) and histopathological findings and did not cause liver damage. Similarly, in the study of Iriadam et al, oral administration of T. polium L (82 mg/Kg) did not cause any adverse effect on liver (39).
In conclusion, this herbal extract had potential protective effect in all doses but the most significant protection was observed in doses of 250 and 500 mg/Kg (p < 0.05). In addition, these findings were supported and confirmed by histological examination. Further studies are required to isolate and purify the bioconstituents involved in hepatoprotection of this plant as well as to confirm the mechanisms responsible for hepatoprotective activity.
Acknowledgment
This work was a part of M.Sc thesis of Hossein Foruozandeh which was supported by the grant number of TRC-8901 provided by Deputy of Research Affairs of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
References
- 1.Blumenthal M. The complete German Commission E monographs: therapeutic guide to herbal medicines. Thieme Medical Pub; 1998. [Google Scholar]
- 2.Susunaga GS, Siani AC, Pizzolati MG, Yunes RA, Delle Monache F. Triterpenes from the resin of Protium heptaphyllum. Fitoterapia. 2001;72:709–711. doi: 10.1016/s0367-326x(01)00289-1. [DOI] [PubMed] [Google Scholar]
- 3.Boulila A, Bejaoui A, Messaoud C, Boussaid M. Variation of Volatiles in Tunisian Populations of Teucrium polium L.(Lamiaceae) Chemistry and Biodiversity. 2008;5:1389–1400. doi: 10.1002/cbdv.200890127. [DOI] [PubMed] [Google Scholar]
- 4.Khoshnood-Mansoorkhani MJ, Moein MR, Oveisi N. Anticonvulsant Activity of Teucrium polium Against Seizure Induced by PTZ and MES in Mice. Iranian J. Pharm. Res. 2010;9:395–401. [PMC free article] [PubMed] [Google Scholar]
- 5.Mousavi SE, Shahriari A, Ahangarpour A, Vatanpour H, Jolodar A. Effects of Teucrium polium Ethylacetate Extract on Serum, Liver and Muscle Triglyceride Content of Sucrose-induced Insulin Resistance in Rat. Iranian J. Pharm. Res. 2012;11:347–355. [PMC free article] [PubMed] [Google Scholar]
- 6.Ardestani A, Yazdanparast R. Inhibitory effects of ethyl acetate extract of Teucrium polium on in-vitro protein glycoxidation. Food and Chemical Toxicology. 2007;45:2402–2411. doi: 10.1016/j.fct.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Rasekh H, Khoshnood-Mansourkhani M, Kamalinejad M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia. 2001;72:937–939. doi: 10.1016/s0367-326x(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 8.Mousavi SE, Shahriari A, Ahangarpour A Vatanpour H, Jolodar A. Effects of Teucrium polium Ethylacetate Extract on Serum, Liver and Muscle Triglyceride Content of Sucrose-induced Insulin Resistance in Rat. Iranian J. Pharm. Res. 2012;11:347–355. [PMC free article] [PubMed] [Google Scholar]
- 9.Esmaeili MA, Yazdanparast R. Hypoglycaemic effect of Teucrium polium: studies with rat pancreatic islets. J. ethnopharmacology. 2004;95:27–30. doi: 10.1016/j.jep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in-vitro. Evidence Based Complementary and Alternative Medicine. 2006;3:329–338. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couladis M, Tzakou O, Verykokidou E, Harvala C. Screening of some Greek aromatic plants for antioxidant activity. Phytotherapy Research. 2003;17:194–195. doi: 10.1002/ptr.1261. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell M, Schenker S, Avant G, Speeg Jr K. Cimetidine protects against acetaminophen hepatotoxicity in rats. Gastroenterology. 1981;81 [PubMed] [Google Scholar]
- 13.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: Lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 14.Richie JP, Lang CA, Chen T S. Acetaminophen-Induced Depletion Of Glutathione And Cysteine In The Aging Mouse Kidney. Biochemical Pharmacology. 1992;44:129–135. doi: 10.1016/0006-2952(92)90046-l. [DOI] [PubMed] [Google Scholar]
- 15.McClain CJ, Price S, Barve S, Devalarja R, Shedlofsky S. Acetaminophen Hepatotoxicity:An Update. Current Gastroenterology Reports. 1999;1:42–49. doi: 10.1007/s11894-999-0086-3. [DOI] [PubMed] [Google Scholar]
- 16.Zaher H, Buters JTM, Ward JM, Bruno MK. Protection against Acetaminophen Toxicity in CYP1A2 and CYP2E1 Double-Null Mice. Toxicology and Applied Pharmacology. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 17.Sener G, Sehirli AO, Ayanoglu-Dulger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J. Pineal Res. 2003;35:61–68. doi: 10.1034/j.1600-079x.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 18.Yoon MY, Kim SJ, Lee BH, Chung JH, Kim YC. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biological and Pharmaceutical Bulletin. 2006;29:1618–1624. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, You HJ, Park SJ, Kim YS. Hepatoprotective effects of Platycodon grandiflorum on acetaminophen-induced liver damage in mice. Cancer Letters. 2001;174:73–81. doi: 10.1016/s0304-3835(01)00678-4. [DOI] [PubMed] [Google Scholar]
- 20.Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction Technologies For Medicinal And Aromatic Plants International Centre For Science And High Technology. Trieste. 2008:74–80. [Google Scholar]
- 21.Reitman S, Frankel S. A colorimetric method for the determination of serum levels of glutamic oxaloacetic acid and pyruvic acid transaminases. Am. J. Clin. Pathol. 1957;10:394–399. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.King J. The phosphohydrolases and alkaline phosphatases: Practical clinical enzymology. London : Van Nostrand Company limited ; 1965. [Google Scholar]
- 23.Watson D, Rogers JA. A study of six representative methods of plasma bilirubin analysis. J. Clin. Pathol. 1961;14:271–278. doi: 10.1136/jcp.14.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancroft JD and Stevens A. Theory and practice of histological techniques. 4th ed. New York: Churchill Livingstone; 1996. [Google Scholar]
- 25.Ahsan R, Islam KM, Musaddik A, Haque E. Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride induced hepatotoxicity in albino rats. Global J. Pharmacology. 2009;3:116–122. [Google Scholar]
- 26.Angelico M, Gridelli B, Strazzabosco M. A.I.S.F. Commission on liver transplantation. Practice of adult liver transplantation in Italy: Recommendations of the Italian association for the study of the liver (A.I.S.F.) Digest. Liver Dis. 2005;37:461–467. doi: 10.1016/j.dld.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Bhawna S, Kumar SU. Hepatoprotective activity of some indigenous plants. International J. Pharm. Tech. Res. 2009;1:1330–1334. [Google Scholar]
- 28.Dwivedi Y, Rastogi R, Garg N, Dhawan B. Prevention of paracetamol-induced hepatic damage in rats by picroliv, the standardized active fraction from Picrorhiza kurroa. Phytotherapy Research. 1991;5:115–119. [Google Scholar]
- 29.Visen PKS, Shukla B, Patnaik GK, Dhawan BN. Andrographalide protects rat hepatocytes against paracetamol induced damage. J. Ethnopharmacol. 1993;40:131–136. doi: 10.1016/0378-8741(93)90058-d. [DOI] [PubMed] [Google Scholar]
- 30.Singh A, Handa SS. Hepatoprotective activity of Apium graeolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. J. Ethnopharmacol. 1995;49:119–126. doi: 10.1016/0378-8741(95)01291-5. [DOI] [PubMed] [Google Scholar]
- 31.Kalantari H, Khorsandi LS, Taherimobarekeh M. The protective effect of the Curcuma longa extract on acetaminophen induced hepatotoxicity in mice. Jundishapur Journal of Natural pharmaceutical products. 2007;2:7–12. [Google Scholar]
- 32.Ita S, Akpanyung E, Umoh B, Ben E, Ukafia S. Acetaminophen induced hepatic toxicity: Protective role of Ageratum conyzoides. Pakistan J. Nutrition. 2009;8:928–932. [Google Scholar]
- 33.Jollow D, et al. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. Journal of Pharmacology and Experimental Therapeutics. 1973;187 [PubMed] [Google Scholar]
- 34.Chenoweth MB, Hake CL. The smaller halogenated aliphatic hydrocarbons. Annual Review of Pharmacology. 1962;2:363–398. [Google Scholar]
- 35.Ellenhorn MJ, Ellenhorn S. Medical toxicology and treatment of human poisoning. Philadelphia : Williams and Willkins Company; 1997. [Google Scholar]
- 36.Yamaguchi T, Terakado M, Hario F. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem. Biophys Res. Commun. 1996;223:129–135. doi: 10.1006/bbrc.1996.0857. [DOI] [PubMed] [Google Scholar]
- 37.Bhoopata L, Srichairatanakoob S, Kanjanapothic D. Hepatoprotective effects of lychee (Litchi chinensis Sonn.): A combination of antioxidant and anti-apoptotic activities. Journal of Ethnopharmacology. 2011;136:55–66. doi: 10.1016/j.jep.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 38.Tariq M, Ageel AM, Al-Said MS. Antiinflammatory activity of T. polium. Int. J. Tissue React. 1989;11:185–188. [PubMed] [Google Scholar]
- 39.Shtukmaster S, Ljubuncic P, Bomzon A. The effect of an aqueous extract of Teucrium polium on glutathione homeostasis in-vitro: A possible mechanism of its hepatoprotectant action. Hindawi Publishing Corporation. 2010:1–7. doi: 10.1155/2010/938324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panovska TK, Kulevanova S, Gjorgoski I, BogdanovA M, Petrushevska G. Hepatoprotective effect of the ethyl acetate extract of Teucrium polium L against carbontetrachloride-induced hepatic injury in rats. Acta. Pharm. 2007;57:241–8. doi: 10.2478/v10007-007-0020-x. [DOI] [PubMed] [Google Scholar]
- 41.Iriadam M, Musa D, Gumushan H, Baba F. Effects of two Turkish medicinal plants Artemisia herba-alba and Teucrium polium on blood glucose levels and other biochemical parameters in rabbits. J. Cell and Molecular Biology. 2006;5:19–24. [Google Scholar]