Abstract
A series of curcumin derivatives containing heterocyclic moiety have been synthesized and evaluated for their antibacterial activities. The chemical structures of the synthesized compounds were verified on the basis of spectral data and elemental analyses. Investigation of antimicrobial activity of the derivatives demonstrated the ability to inhibit Gram-positive microorganisms with zone of inhibition ranging from 14-18 mm, MIC ranging between 0.0625 and 0.25 mg/mL. Among all tested derivatives, diazepine 4 exhibited remarkable potency against Gram-positive bacteria S. aureus. An extensive study is underway to optimize the effectiveness of diazepine type of compounds and to determine their mode of action.
Key Words: Antimicrobial activity, pyrazoles, isoxazoles, diazepine, disc diffusion
Introduction
Bacterial resistance to many available antibacterial agents is a growing problem. Accordingly, the development of new antibacterial agents that could overcome the resistance problem has become the subject of an ongoing research (1-6). In the present work, we have employed curcumin as a naturally occurring skeleton for the construction of heterocyclic systems such as pyrazole, isoxazole, and diazepine which might exhibit promising antibacterial activity.
Curcumin, (E,E)-l,7-bis(4-hydroxy-3- methoxyphenyl)-l,6-heptadiene-3,5- dione, also known as turmeric yellow, is a natural yellow pigment derived from the roots of curcuma plants, e.g. C. tinctoria, C. xanthorrhiza and C. domestica, and is known since several hundred years. It is used as a food coloring agent and in traditional Indian medicine for treatment of various diseases that include biliary disorders, anorexia, cough, diabetic wounds, hepatic disorder, rheumatism, blood purification and rheumatoid arthritis (7-10).
Several studies have shown that curcumin has various pharmacological activities including potent antioxidant, anti-inflammatory and antiviral activities (11-16), as well as anticancer activities against different forms of cancer, e.g., cervical cancer caused by HPV (17-19). In addition, other studies have shown that curcumin represents a hopeful approach for delaying or preventing the progression of Alzheimer›s disease (20-24), and has been identified as an inhibitor of HIV-1 LTR directed gene expression and viral replication, besides its ability to block HIV replication by inhibiting HIV-integrase and protease (25-30). Heterocyclic compounds, in general, are very important class of organic compounds with various bioactivities ranging from antibacterial to anticancer (31-47). For example, diazepines and benzodiazepines have various therapeutic applications. Many members of the diazepine family are widely used as anticonvulsants, anti-anxiolytics, analgesics, sedatives, antidepressants and hypnotic agents (48-51). These literature findings have led us to synthesize the proposed group of curcumin-based heterocycles and screen them against representative panel of Gram-positive and Gram-negative bacteria.
Results and Discussion
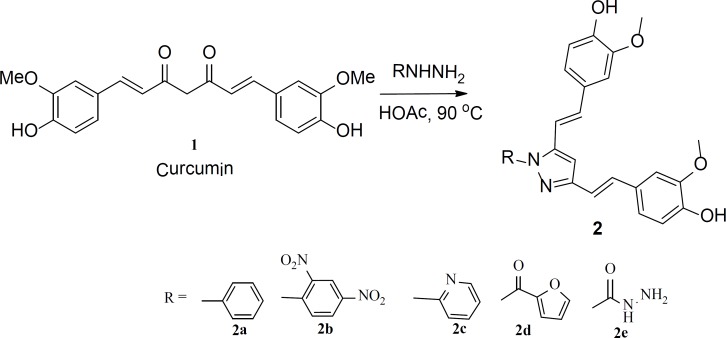
Compounds used in this study were prepared following a literature procedure with minor modification (52-54). In this procedure, curcumin (compound 1) was refluxed with various hydrazines in acidic media, which performs dual function as catalyst and solvent. Acid used for this purpose was either glacial acetic acid or polyphosphoric acid. The progress of the reaction was monitored by TLC. Some reactions required more reflux time than the others. Followed procedure produced only the expected product; some starting materials were also observed in some reactions, as in the case of synthesis of 1-(2,4-dinitrophenyl)-3,5-bis-2-(4-hydroxy-3- methoxystyryl)-1H-pyrazole (2b). Similar yields were obtained using either acetic acid or PPA as a solvent. Purification of the products was performed using flash chromatography. Purified products were analyzed by various analytical and spectroscopic techniques, such as: melting point, LC/MS, 1H and 13C NMR, and elemental analysis. In all cases, results are consistent with the expected structures. All compounds were obtained in acceptable yield (65 % to 90%).
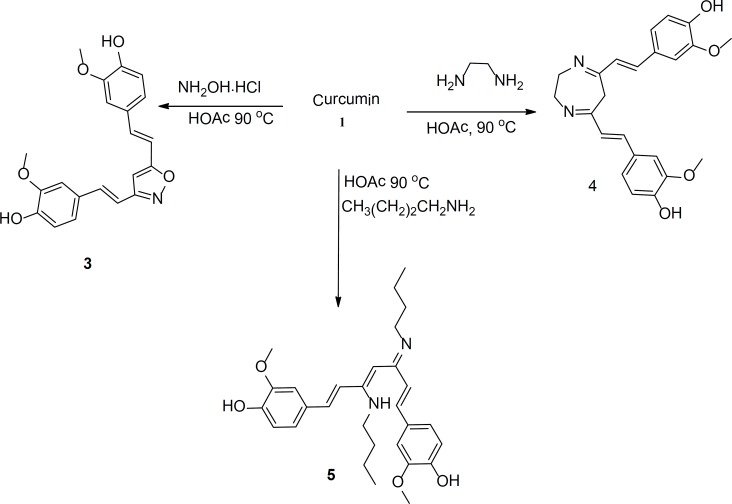
The structures and the characterization data for the prepared compounds are summarized in Figure 1 and the experimental part, respectively. Another set of curcumin-based heterocycles was prepared by reacting curcumin with various amines such as ethylenediamine and hydroxylamine. Compound 5 was prepared from reacting curcumin with n-butylamine; it was added to the set of evaluated derivatives for a comparison purpose. The structures and the characterization data for these compounds are also summarized in Figure 1 and the experimental part, respectively.
Figure 1.
Curcumin based pyrazoles prepared form reacting curcumin with various hydrazines
Antibacterial activity
The in-vitro antimicrobial activity was performed on four types of bacteria strains: S. aureus, E. coli, Proteus mirabilis and Pseudomonas aeruginosa using a disk diffusion assay reported in the literature by Perez et al. (55). All strains were isolated from patients suffering from bacterial infections with the relevant bacteria. Ampicillin, Methicillin, and Carbenicillin were used as references of antibacterial compounds in antimicrobial assay.
Screening results
The results of the prepared compounds (2a-e, 3, 4, 5) and the references for preliminary antibacterial testing are shown in Table 1. Results revealed that the majority of the synthesized compounds showed varying degree of inhibition against the tested microorganisms. In general, the potency against Gram-positive organisms is greater than against Gram-negative ones. As shown in Table 1, curcumin and all other derivatives were inactive (zones of inhibition were zero) against Proteus mirabilis and Pseudomonas aeruginosa. However, some compounds showed potency against E. coli, and some others like curcumin, compounds 4, and compound 5 were totally inactive against E. coli. Compound 2b showed the highest activity against E. coli (zone of inhibition is about 13 mm).
Table 1.
Zone of Inhibition of Curcumin and Synthesized Curcumin-Based Heterocycles
| Compound | Gram-positive Bacteria S. aureus |
Gram-negative Bacteria |
||
|---|---|---|---|---|
| P. mirabilis | E. coli | P . aeruginosa | ||
| Curcumin (1) | 11 | 0 | 0 | 0 |
| Ampicillin | 25 | 21 | 18 | NTa |
| Methicillin | 16 | NTa | NTa | NTa |
| Carbenicillin | 15 | 22 | 23 | 20 |
| 2a | 11 | 0 | 11 | 0 |
| 2b | 14 | 0w | 13 | 0 |
| 2c | 16 | 0 | 10 | 0 |
| 2d | 15 | 0 | 12 | 0 |
| 2e | 18 | 0 | 12 | 0 |
| 3 | 16 | 0 | 12 | 0 |
| 4 | 27 | 0 | 0 | 0 |
| 5 | 11 | 0 | 0 | 0 |
All prepared curcumin-based heterocycles have shown to be susceptible to excellent potency against Gram-positive bacteria S. aureus with zone of inhibition ranges from 11 mm to 27 mm. The results were comparable to those of the reference antibiotics Methicillin, and Carbenicillin. Compound 4, showed remarkable activity against S. aureus with zone of inhibition of about 27 mm and its effect is about two-fold more than that of some other derivatives. Compound 4 showed even higher potency against S. aureus than Ampicillin (Table 1). Curcumin-based heterocycles then subjected to minimum inhibition concentration (MIC) testing. Results on S. aureus bacteria are summarized in Tables 2 and 3. As shown in Table 2, MIC of curcumin was 1 mg/mL, which is at least four-fold higher than the MIC of the other curcumin derivatives. Compounds 2a, 2b and 3 showed MIC of about 0.25 mg\mL. Derivatives 2c, 2d, 2e, and 5 showed lower MIC (about 0.0625 mg/mL). These results indicate that compounds 2c, 2d, 2e, and 5 have higher activities than compounds 1, 3, and 5. However, the highest activity was shown by derivative 4 as it showed a MIC of 1.9 μg mL-1.
Table 2.
MIC of curcumin and synthesized Curcumin-Based Heterocycles on S. aureus Bacteria
| Conc.(μg/mL) | Curcumin | 2a | 2b | 2c | 2d | 2e | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|---|---|
| 4 x 10 3 | - | - | - | - | - | - | - | - | - |
| 2 x 10 3 | - | - | - | - | - | - | - | - | - |
| 1 x 10 3 | - | - | - | - | - | - | - | - | - |
| 500 | + | - | - | - | - | - | - | - | - |
| 250 | + | - | - | - | - | - | - | - | - |
| 125 | + | + | + | - | - | - | + | - | - |
| 62.5 | + | + | + | - | - | - | + | - | - |
| 31.25 | + | + | + | + | + | + | + | - | + |
| 15.625 | + | + | + | + | + | + | + | - | + |
| 7.8125 | + | + | + | + | + | + | + | - | + |
| 3.9062 | + | + | + | + | + | + | + | - | + |
| 1.953125 | + | + | + | + | + | + | + | - | + |
| 0.976563 | + | + | + | + | + | + | + | + | + |
| Positive control | + | + | + | + | + | + | + | + | + |
| Sterility control | - | - | - | - | - | - | - | - | - |
Table 3.
A Summary of MIC And MBC Results of Curcumin and Synthesized Curcumin-based Heterocycles on S. aureus Bacteria.
| Compound | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| curcumin | 1 | 1 |
| 2a | 0.25 | 1 |
| 2b | 0.25 | 1 |
| 2c | 0.0625 | 1 |
| 2d | 0.0625 | 1 |
| 2e | 0.0625 | 1 |
| 3 | 0.25 | 1 |
| 4 | 0.0019 | 0.0075 |
| 5 | 0.0625 |
Figure 2.
Curcumin based isoxazoles, diazepine, and amine prepared form reacting curcumin with various amines.
The minimum concentration of each curcumin derivatives that results in a total inhibition of bacterial growth (MBC) was also determined. Compound 4 showed the highest activity. MBC for compound 4 was 0.0075 mg\mL. These results indicate that compound 4 has the highest potency against S. aureus, which may be attributed to the presence of 1,4-diazepine ring. However, compound 5 that lacks heterocyclic system showed the least potency against S. aureus and no activity against E-Coli, which is similar to that of curcumin. These results indicate that the improved potency of the prepared compounds could be attributed to the heterocyclic part of the curcumin-based heterocycles.
Conclusion
We have synthesized series of curcumin-based heterocycles and evaluated their antibacterial activities against Gram-positive and Gram-negative bacteria. The molecules 2b-e and 3 effectively inhibit S. aureus, with zone of inhibition ranging from 14 to 18 mm, MIC ranging between 0.0625 and 0.25 mg/ mL. Among the prepared derivatives, diazepine (4) showed the highest potency against Gram-positive bacteria S. aureus. In conclusion, more extensive study is needed to optimize the effectiveness of diazepine type of compounds and to determine their mode of action. This could be accomplished by preparing a variety of curcumin-based diazepine hybrids and screen their antibacterial activities.
Experimental
General experimental
All chemicals were purchased from Aldrich Chemical Company and used without any further purification unless otherwise specified. All prepared compounds were characterized by 1H-NMR, 13C-NMR, IR spectroscopy, elemental analysis, and melting point. Nuclear Magnetic Resonance spectra were recorded on Varian Gemini 2000, 300 MHz instrument and on Bruker DPX-300 MHz instruments. Infrared spectra were recorded in KBr on a Shimadzu 820 PC FT-IR spectrometer. All 1H-NMR experiments were reported in δ units, parts per million (ppm) downfield from tetramethylsilane (TMS). All 13C-NMR spectra were reported in ppm relative to deuterochloroform (77.0 ppm). All melting points were determined in an open capillary tube and are uncorrected. At least two measurements were carried out for each compound. Elemental analyses were carried out with Elementar Vario EL III elemental analyzer. TLC analysis was performed on silica gel plates pre-coated with Merck Kieselgel 60 F254 and visualization was done using UV lamp. Sample purifications were performed using flash chromatography with silica gel (100-200) mesh.
General Procedure
Preparation of curcumin-based heterocyclic compounds
The general experimental procedure for the preparation of compounds shown in Figure 1 was as follows: In a round bottom flask equipped with magnetic stirring bar and a condenser, curcumin (1.5 mmole, 0.5 g) was dissolved in acetic acid (or polyphosphoric acid (PPA)) (8 mL). The desired hydrazine (1.5 mmole) or amine was added to the solution of curcumin in acid. The produced solution was refluxed for 6-12 h. The progress of the reaction was monitored by TLC. After complete conversion, the mixture was cooled to room temperature and concentrated in-vacuo and redissolved in ethyl acetate (150 mL). The ethyl acetate layer was washed with saturated solution of NaHCO3 and saturated solution of NaCl, dried over Na2SO4 and concentrated in-vacuo. The produced solid was collected by suction filtration and purified by flash chromatography (hexane : EtOAC (6 : 4)). In case where PPA was used, reaction mixture was diluted with water, extracted with ethyl acetate and then the product was purified as shown before.
1-phenyl-3,5-bis-2-(4-hydroxy-3-methoxystyryl)-1H-pyrazole (2a)
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL) and then, phenyl hydrazine hydrochloride (1.5 mmole, 0.25 g) was added. Produced solution was refluxed for about 6 h. Yield 71.6% (0.48 g), mp 127-129°C, IR (KBr ): vmax cm-1 3600 (-C-OH), 3350 (–C–NH), 3100, 1620 (-C=N), 1600, and 1080 (C-O ether) of (–O-CH3). 1H-NMR (300 MHz, CDCl3) δ ppm: 3.90 (s, 6H, OCH3), 6.05 (s, 2H, OH), 6.80 (s, 1H, C4-H), 7.02 (d, 2H, J = 14.8 Hz, C2-H and, C6-H), 7.10 (d, 2H, J = 14.8 Hz, C1-H and C7-H), 7.15–7.32 (m, 8H, Ar-H), 7.45 (m, 2H, Ar-H), 7.72 (m, 1H, Ar-H). 13C NMR (300 MHz, CDCl3) δ ppm: (300 MHz, CDCl3): 56.05, 56.09, 101.22, 110.02, 110.98, 112.57, 115.97, 116.14, 117.64, 120.67, 125.24, 128.17, 128.29, 128.84, 129.02, 129.06, 129.08, 131.22, 133.34, 139.72, 142.82, 147.20, 147.74, 148.2, 148.33, 151.54, 161.00. Anal. Calcd for C27H24N2O4: C 73.62, H 5.49, N 6.36. Found: C 73.43, H 5.42, N 6.48.
1-(2,4-dinitrophenyl)-3,5-bis-2-(4-hydroxy-3-methoxystyryl)-1H-pyrazole (2b)
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL), and to it was added 2,4-dinitrophenyl hydrazine hydrochloride (1.5 mmole, 0.355 g). Produced solution was refluxed for about 12 h (Yield 55 (0.43 g), mp 192-195°C).
IR: vmax cm-1 3610 (-C-OH), 3050, 1635, (-C=N), 1590, 1080 (C-O ether), 1520, 1350.
1H-NMR (300 MHz, CDCl3) δ ppm: 3.78 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 6.16 (s, 2H, OH),
6.80 (s, 1H, C-4H), 6.83 (m, 4H, C1-H, C2-H, C6-H, and, C7-H), 6.90 (m, 1H, Ar-H), 7.10 (d, 2H, Ar-H), 7.20 (d, 2H, Ar-H), 7.40 (d, 1H, Ar-H), 8.00 (d, 1 H, J = 9.43 Hz, Ar-H containing NO2), 8.43 (dd, 1H, J = 2.64 Hz, 9.43 Hz, Ar-H containing NO2), 8.90 (d, 1H, J = 2.64 Hz, Ar-H containing NO2). 13C NMR (300 MHz, CDCl3) δ ppm: 56.15, 56.23, 102.07, 110.00, 110.94, 111.28, 111.72, 116.11, 116.89, 121.59, 121.86, 123.63, 126, 83, 127.91, 128.43, 128.92, 130.23, 133.07, 135.18, 135.69, 147.16, 144.89, 145.48, 146.23, 148.23, 148.42, 154.02. Anal. Calcd for C27H22N4O8: C 61.13, H 4.18, N 10.56. Found: C 61.59, H 4.32, N 10.66.
1-(2-pyridyl)-3,5-bis-2-(4-hydroxy-3-methoxystyryl)- 1 H-pyrazole (2c)
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL), to it was added 2-hydrazinopyridin (1.5 mmole, 0.164 g). Produced solution was refluxed for 12 h. Yield 89.5 (0.6 g), mp 122-125°C IR: vmax cm-1 3550 (-C-OH), 3050, 1640, (-C=N), 1590, 1080 (C-O ether), 1520, 1350. 1H NMR (400 MHz, DMSO-d6) δ: 3.78 (s, 6H, 2OCH3), 6.13 (2H, OH), 6.65 (s, 1H, C4-H), 6.78 (m, 4H), 6.95 (m, 4H), 7.13 (m, 2H), 7.35 (d, 1H), 7.63 (d, 1H), 7.72 (t, 1H), 8.21 (d, 1H). 13C NMR (400 MHz, DMSO-d6) δ: 56.17,101.33, 102.45, 110.135, 110.87, 111.79, 115.56, 116.21, 117.50, 120.62, 122.37, 123.68, 126.69, 127.51, 128.85, 132.38, 132.79, 141.17, 144.02,, 147.81, 148.41, 150.08, 152.20, 163.37, 183.64. Anal. Calcd for C26H23N3O4: C 70.73, H 5.25, N 9.50, Found: C 70.62, H 5.27, N 9.56.
1-(2-furyl)-3,5-bis((E)-4-hydroxy-3-methoxystyryl)-1H-pyrazol (2d)
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL) and to it, was added 2-Furoichydrazide (1.5 mmole, 0.2 g). Produced solution was refluxed for 12 h. Yield 81 (0.52 g), mp 138-141°C, IR: vmax cm-1 3605 (-C-OH), 3030, 1673 (–C=O amido), 1640 ( -C=N), 1590, 1080 (C-O ether), 1520, 1320 (C-O of the five-member ring). 1H-NMR (400 MHz, DMSO-d6) δ: 3.78 (s, 6H, OCH3), 6.01 (s, 2H, OH), 6.50-7.32 (m, 13 H), 7.52 (dd, 1H, J= 16.15Hz). 13C-NMR (400 MHz, DMSO-d6) δ: 56.03, 99.79, 101.4, 111.74, 112.43, 115.18, 116.01, 116.14, 116.37, 120.56, 121.52, 123.67, 126.74, 128.79, 130.06, 141.18, 146.32, 146.67, 148.32, 148.46, 149.89, 157.70, 183.30. Anal. Calcd for C25H22N2O5: C 69.76, H 5.15, N 6.51. Found: C 69.66, H 5.18, N 6.62.
1-carbohydrazidyl-3,5-bis-2-(4-hydroxy-3-methoxystyryl)-1H-pyrazole (2e)
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL), to it was added carbohydrazide (1.5 mmole, 0.135 g). Produced solution was refluxed for 12 h. Yield 88.6 (0.0.56 g), mp 151-154°C, IR: vmax cm-1 3605 (-C-OH), 3320, 3275, 3190 (–C–NH), 3020, 1635 (-C=N), 1600, 1080 (C-O ether).1H-NMR (400 MHz, DMSO-d6) δ: 3.78 (s, 3H, OCH3), 4.32 (s, 2H, NH), 6.01 (s, 2H, OH), 6.50-7.40 (m, 11H), 8.80 (s, H, NH). 13C-NMR (400 MHz, DMSO-d6): 56.07, 56.12, 100.1, 110.6, 116.08, 116.24,120.30, 120.66, 123.16, 123.34, 128.62, 129.81, 130.4, 131.03, 143.78, 144.70, 147.13, 147.51, 147.50, 150.69, 151.13, 161.06. Anal. Calcd for C22H22N4O5: C 62.55, H 5.25, N 13.26. Found: C 62.63, H 5.25, N 13.37.
3,5-bis-2-(4-hydroxy-3-methoxystyryl)-isoxazole ( 3 )
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL) and to it, was added hydroxylamine hydrochloride (1.5 mmole, 0.11g). Produced solution was refluxed for 12 h. Yield 72.0 (0.4 g), mp 116-119°C, IR: 3570 (-C-OH), 3030, 1605, 1586 (-C=N), 1330 (C-O of the five-member ring) cm-1. 1H-NMR (400 MHz, DMSO-d6): 1H-NMR: δ 3.85 (s, 6H, 2OCH3), 6.25 (s, 2H, OH), 6.71 (s, 1H, C4-H), 6.84-7.01 (m, 3H), 7.04-7.15 (m, 4H), 7.26 (m, 3H). 13C-NMR (300 MHz, CDCl3) δ ppm: 56.14, 56.18, 98.33, 110.56, 110.82, 113.12, 113.43, 115.98, 116.11, 116.24, 121.8, 122.16, 127.49, 127.83, 129.22, 135.27, 135.98, 148.22, 148.42, 162.70, 168.84. Anal. Calcd for C21H19NO5: C 69.03, H 5.24, N 3.38. Found: C 68.89, H 5.21, N 3.41.
3,5-bis-2-(4-hydroxy-3-methoxystyryl)-3,6-dihydro-2H-1,4-diazepine ( 4 )
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL) and then, ethylenediamine (1.5 mmole, 0.1 mL) was added to the solution. Produced solution was refluxed for 4 h. Yield 76.3 (0.45), mp 65-68°C (hydrated) IR: vmax cm-1 3605 (-C-OH), 3020, 1640 (-C=N), 1600, 1080 (C-O ether). 1H-NMR (400 MHz, DMSO-d6) δ: 3.16 (s, 2H, CH2), 3.764 (s, 4H, CH2CH2); 3.78 (s, 6H, OCH3), 5.72 (s, 2H, OH), 6.85 (m, 2H), 6.91 (d, 2H, J = 15.2 Hz), 7.05 (d, 2H, J = 12.1 Hz), 7.20 (s, 2H), 7.40- 7.60 (m, 2H). 13C-NMR (400 MHz, DMSO-d6) δ: 24.50, 48.30, 56.40, 112.90, 116.30, 120.60, 122.40, 127.80, 129.30, 148.20, 149.30, 165.80. LC/MS [M + 1] for C23H24N2O4 Calcd 393.0, found: 394.0. Anal. Calcd for C23H24N2O4: C 70.39, H 6.16, N 7.14. Found: C 70.33, H 6.21, N 7.19. The differences in the [M+1] values could be due to the protonation of compound 4 during LC/MS analysis, since mobile phase used in the analysis was an acidic solution of aqueous methanol.
4,4›-((1E,3Z,5Z,6E)-3-(butylamino)-5-(butylimino)hepta-1,3,6,triene-1,7-diyl) bis(2-methoxyphenol) ( 5 )
Curcumin (1.5 mmole, 0.5 g) was dissolved in glacial acetic acid (8.0 mL) and to it was added n-butyl amine (3 mmole, 0.25 mL). Produced solution was refluxed for 10 h. Yield 78% (0.56 g), mp 106-109°C IR: vmax cm-1 3605 (-C-OH), 3335 (–C–NH), 3040, 1640 (-C=N), 1600, 1080 (C- O ether). 1H-NMR (400 MHz, DMSO-d6) δ: 0.79 (m, 6H), 1.23 (m, 4H), 1.32-1.41 (m, 4H),1.82 (s, 1H), 2.52 (t, 2H), 2.72 (t, 2H), 3.78 (s, 6H, OCH3), 6.10 (s, 2H, OH), 6.62 (s, 1H, C4-H), 6.84 (m, 4H, C2-H, C6-H), 7.10 (m, 2H), 7.20 (m, 2H), 7.40 (d, 2H). 13C-NMR (400 MHz, DMSO-d6) δ: 13.60, 13.60, 19.20, 19.20, 35.30, 36.10, 44.50, 46.80, 56.20, 56.43, 97.31, 109.74, 111.95, 115.83, 116.12, 116.11, 121.50, 123.80, 125.31, 129.3, 129.31, 135.55,136.26, 148.23, 148.27, 149.92, 149.93, 152.81, 174.65. Anal Calcd for C29H38N2O4: C 72.77, H 8.00, N 5.85. Found: C 72.54, H 8.12, and N 5.76.
Antibacterial activity of materials
Culture media: Mueller-Hinton, Tryptic Soy Broth was obtained from Hylabs, Israel.
Microorganisms used
Bacterial strains used in the study were clinical isolates of Staphylococcus aureus, Escherichia coli, Proteus mirabilis, and Pseudomonas aeroginosa, were all isolated from patients suffering from bacterial infections with the relevant bacteria.
Procedure
These isolates were tested for their susceptibility to the prepared curcumin-based heterocyclics as follows:
Solutions of these derivatives were prepared at concentration of 4 mg per 1 mL of dimethyl sulfoxide (DMSO) solvent, and then incubated for 24 h at 37°C.
Screening the antimicrobial activity
The antibacterial bioactivities of the prepared heterocyclics were screened using the well diffusion method reported in the literature by Perez et al (55).
Three colonies of bacteria were transferred to sterile tubes each containing 5 mL of Tryptic Soy Broth. Turbidity of the bacterial suspensions was then adjusted to reach an optical density equivalent to a 0.5 McFarland standard to give a bacterial suspension of 108 cfu/mL (cfu: colony forming unit). Mueller-Hinton agar plates were inoculated through streaking bacterial swabs over the entire surface of the plates. Produced Plates were allowed to dry at room temperature and 6 mm wells were punched in each plate. In each plate, 50 μL of 4 mg/mL solutions of each curcumin derivatives were added into duplicate wells. Plates were allowed to stand at room temperature to let the tested derivative be diffused into the agar, and afterwards, they were incubated at 37°C for 18 to 24 h. Then, plates were examined for bacterial growth inhibition and zones of inhibition were measured in millimeters.
Determination of minimum inhibitory concentration (MIC)
Two-fold serial dilutions were prepared from the derivative solution in Tryptic Soy Broth. Duplicate tubes of each dilution were inoculated with 5 × 105 of the bacterial strains. All tubes were incubated at 37°C for 18 to 24 h. The highest dilution of the drug that resulted in inhibition of bacterial growth was considered as the MIC.
Determination of minimal bactericidal concentration (MBC)
Subcultures from the above dilutions were done on Muller-Hinton plates and incubated at 37°C for 18 to 24 h. The highest dilution that resulted in total inhibition of bacterial growth was then determined and considered as MBC.
Acknowledgment
Acknowledgment is made to the Donors of An-Najah National University Fund for support of this research. Authors are gratefully acknowledged Loyola University of Chicago and Hashemite University for the help they provided in the analysis of the produced samples by Varian INOVA-300 NMR and other instruments.
References
- 1.Travis J. Reviving the antibiotics miracle? Science. 1994;264:360–362. doi: 10.1126/science.8153615. [DOI] [PubMed] [Google Scholar]
- 2.Hata K, Hata J, Miki HT, Toyosawa T, Nakamura T, Katsu K. In-vitro and in-vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob. Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugawara T, Shibazaki M, Nakahara H, Suzuki K. YM-47522, a novel antifungal antibiotic produced by Bacillus sp. II. Structure and relative stereochemistry. J. Antibiot. (Tokyo) 1996;49:345–348. doi: 10.7164/antibiotics.49.345. [DOI] [PubMed] [Google Scholar]
- 4.Moellering R C. Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 1998;26:1196–1199. doi: 10.1086/520283. [DOI] [PubMed] [Google Scholar]
- 5.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 6.Thomson J M, Bonomo R A. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: beta-lactams in peril. Curr Opin Microbiol. 2005;8:518–524. doi: 10.1016/j.mib.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Ammon HP, Wahl MA. Pharmacology of curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 8.Eigner DD, Scholz J. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. Ethnopharmacol. Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 9.M Rezvani. Extract of Curcuma longa in the treatment of radiation lesions. Iranian J. Pharm. Res. 2004;Suppl:78–78. [Google Scholar]
- 10.Lodha R, Baga A. Traditional Indian systems of medicine. Ann. Acad. Med. Singapore. 2000;29:37–41. [PubMed] [Google Scholar]
- 11.Aggarwal BB, Kumar A, Bharti AC. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 12.Prusty BK, Das BC. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer. 2005;113:951–960. doi: 10.1002/ijc.20668. [DOI] [PubMed] [Google Scholar]
- 13.Prusty BK, Husain SA, Das BC. Constitutive activation of nuclear factor -kB: preferntial homodimerization of p50 subunits in cervical carcinoma. Front. Biosci. 2005;10:1510–1519. doi: 10.2741/1635. [DOI] [PubMed] [Google Scholar]
- 14.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 15.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–512. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 17.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, Fox LH, Dickens M, Prigent SA, Manson MM. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 2003;65:361–376. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 18.Duvoix R, Blasius S, Delhalle M, Schnekenburger F, Morceau E, Henry M, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Martono S. Inhibitory Effects of Curcumin and its Analogs on In Vitro Rat Liver Glutathione S - Transferases Activity. Chem. Abstract. 1996;128 [Google Scholar]
- 20.Rajakumar DV, Rao MN. Antioxidant properties of dehydrozingerone and curcumin in rat brain homogenates. Mol Cell Biochem. 1994;140:73–79. doi: 10.1007/BF00928368. [DOI] [PubMed] [Google Scholar]
- 21.Kiran B, Tripathy BC, Sharma D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology. 2006;7:81–89. doi: 10.1007/s10522-006-6495-x. [DOI] [PubMed] [Google Scholar]
- 22.Rajkrishnan V, Vishwanathan P, Rajasekharan KN, Menon VP. Neuroprotective role of curcumin from curcuma longa on ethanol-induced brain damage. Phytother Res. 1999;13:571–574. doi: 10.1002/(sici)1099-1573(199911)13:7<571::aid-ptr494>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Herris-White ME, Cole GM. Phenolic anti-inflammatory anti- oxidant reversal of a beta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese V, Butterfield DA, Stella AM. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: Novel targets for neuroprotection in Alzheimer›s disease. Ital. J. Biochem. 2003;52:177–181. [PubMed] [Google Scholar]
- 25.Senior K. FDA approves first drug in new class of antibiotics. The Lancet. 2000;355:1523. doi: 10.1016/S0140-6736(00)02173-5. [DOI] [PubMed] [Google Scholar]
- 26.Lin AH, Murray RW, Vidmar TJ, Marotti KR. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997;41:2127–2131. doi: 10.1128/aac.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diekema DJ, Jones R N. Oxazolidinone antibiotics. The Lancet. 2001;358:1975–1982. doi: 10.1016/S0140-6736(01)06964-1. [DOI] [PubMed] [Google Scholar]
- 28.Hancock RE. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005;5:209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 29.Stevens DL, Dotter B, Madaras K. Review of linezolid: the first oxazolidinone antibiotic. Exp. Rev. Ant. Infect. Ther. 2004;2:51–59. doi: 10.1586/14787210.2.1.51. [DOI] [PubMed] [Google Scholar]
- 30.Mazumder M, Raghavan K, Weinstein J, Kohn KW, Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- 31.Sui Z, Salto R, Li J, Craik C, Ortiz de Montellano PR. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993;1:415–422. doi: 10.1016/s0968-0896(00)82152-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Wang LF, Li YZ, Li QX, Xu ZD, Qu DM. Antitumour activity of transition metal complexes with the thiosemicarbazone derived from 3-acetylumbelliferone. Trans. Met. Chem. 2001;26:307–310. [Google Scholar]
- 33.Yadav LDS, Singh S. Synthesis of antiviral acyclic C-nucleosides incorporating thiazolo-1,3,4-oxa(thia) diazole or thiazolo-1,2,4-triazole structure as a nucleobase Indian J. Chem. 2001;40B:440–442. [Google Scholar]
- 34.Hans N, inventor. Swiss Patent (1977) No 592103. [Chem. Abstr. (1978) 88: 22886]
- 35.Wilson KJ, Utig CR, Subhasinghe N, Hoffman JB, Rudolph NJ, Soll R, Molloy CJ, Bone R, Green D, Randall J. Synthesis of thiophene-2-carboxamidines containing 2-aminothiazoles and their biological evaluation as urokinase inhibitors. Bio-Org Med. Chem. Lett. 2001;11:915–918. doi: 10.1016/s0960-894x(01)00102-0. [DOI] [PubMed] [Google Scholar]
- 36.Berlin KD, Herd MD. Novel 2-Amino-4-Aryl-Substituted-and 2-Amino-4,5-Disubstituted-Thiazoles. Proc. Okala. Acad. Sci. 1991;71:29–33. [Google Scholar]
- 37.Iovu M, Zalaru C, Dumitrascu F, Draghici C, Moraru M, Criste E. New substituted 2-(pyrazol-1-yl)-dialkylacetanilides with potential local anesthetic and antiarrhythmic action. Part II. Farmaco. 2003;58:301–307. doi: 10.1016/S0014-827X(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 38.Mahajan RN, Havaldar FH, Fernandes PS. Synthesis and Biological Activity of Heterocycles Derived from 3-Methoxy-I-Phenyl-IH-Pyrazole-5-Carboxylate. J. Indian Chem. Soc. 1991;68:245–249. [Google Scholar]
- 39.Baraldi PG, Manfredini S, Romagnoli R, Stevanato L, Zaid AN, Manservigi R. Nucleos. Nucleot. Nucleic Acids. 1998;17:2165–2171. [Google Scholar]
- 40.Bruno O, Bondavalli F, Ranise A, Schenone P, Losasso C, Cilenti L, Matera C, Marmo E. 3,5-Diphenyl-1H-pyrazole derivatives. V-1-Acetyl-4-hydroxy-3,5-diphenyl-2-pyrazoline esters, 4-hydroxy-3,5-diphenyl-1H-pyrazole esters and N-substituted 4-(3-amino-2-hydroxy-1-propoxy)-1-methyl-3,5-diphenyl-1H-pyrazoles with antiarrhythmic, sedative and platelet antiaggregating activities. Farmaco. 1990;45:147–166. [PubMed] [Google Scholar]
- 41.Cottineau B, Toto P, Marot C, Pipaud A, Chenault J. Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorg. Med. Chem. Lett. 2002;12:2105–2108. doi: 10.1016/s0960-894x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 42.Smith SR, Denhardt G, Terminelli C. The anti-inflammatory activities of cannabinoid receptor ligands in mouse peritonitis models. Eur. J. Pharmacol. 2001;432:107–119. doi: 10.1016/s0014-2999(01)01477-7. [DOI] [PubMed] [Google Scholar]
- 43.Varshney V, Mishra NN, Shukla PK, Sahu P, Devi P. Synthesis and antibacterial evaluation of isoxazolinyl oxazolidinones: Search for potent antibacterial. Bioorg. Med. Chem. Lett. 2009;19:3573–3576. doi: 10.1016/j.bmcl.2009.04.133. [DOI] [PubMed] [Google Scholar]
- 44.Lin AH, Murray RW, Vidmar TJ, Marotti KR. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997;41:2127–2131. doi: 10.1128/aac.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hancock RE. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005;5:209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 46.Stevens DL, Dotter B, Madaras K. Review of linezolid: the first oxazolidinone antibiotic. Exp. Rev. Ant. Infect. Ther. 2004;2:51–59. doi: 10.1586/14787210.2.1.51. [DOI] [PubMed] [Google Scholar]
- 47.Gonzales RD, Scherkenberger PC, Graham MB, Kelkar S, Denbesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. The Lancet. 2001;357:1179. doi: 10.1016/S0140-6736(00)04376-2. [DOI] [PubMed] [Google Scholar]
- 48.Schutz H. Benzodiazepines. Heidelberg, Germany: Springer; 1982. [Google Scholar]
- 49.Landquist JK. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, C.W. Rees, editors. Oxford, UK: Pergamon; 1984. pp. 166–170. [Google Scholar]
- 50.Fryer RI, Taylor EC, editors. Bicyclic Diazepines. In Comprehensive Heterocyclic Chemistry. New York: Wiley; 1991. [Google Scholar]
- 51.Randall LO, Kappel B. In: Benzodiazepines. Garattini S, Musini E, Randall LO, editors. New York: Raven Press; 1973. p. 27. [Google Scholar]
- 52.Amolins MW, Peterson LB, Blagg BS. Synthesis and evaluation of electron-rich curcumin analogues. J. Bioorg. and Med. Chem. 2009;17:360–367. doi: 10.1016/j.bmc.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bapat BA, Shirote P J, Tanavade SS. Substituted and Annulated 1,4-Diazepines-Synthesis and Characterization. Int. J. of Med. and Pharm. Sci. 2011;1:28–35. [Google Scholar]
- 54.Kumar R, Joshi YC. Synthesis, antimicrobial and antifungal activities of novel 1H-1,4-diazepines containing pyrazolopyrimidinone moiety. J. Chem. Sci. India. 2009;121:497–502. [Google Scholar]
- 55.Perez C, Pauli M, Bazevque P. An antibiotic assay by the agar well diffusion method. Acta Biologiaeet Medicine Experimentalis. 1990;15:113–115. [Google Scholar]