Type 1 diabetes is one of the most common chronic childhood illnesses, affecting 18 to 20 per 100 000 children a year in the United Kingdom.1 The American Diabetes Association committee recommends the term type 1A diabetes for immune mediated diabetes with its destruction of the islet β cells of the pancreas.2 Non-immune mediated diabetes with severe insulin deficiency is termed type 1B. In this review, we will use the term type 1 diabetes to refer to immune mediated type 1A diabetes. At present, the development of type 1 diabetes is a life sentence to a difficult therapeutic regimen that is only partially effective in preventing acute and chronic complications. We will concentrate here on recent advances in our understanding of the epidemiology, pathogenesis, prediction, and prevention of type 1 diabetes and new treatments for the disease.
Sources and selection criteria
This review is based on information obtained from a recent Medline search with type 1 diabetes, pathogenesis, prediction, prevention, and treatment as key words. We also consulted summaries of the literature on type 1 diabetes (available with teaching slides at www.barbaradaviscenter.org).
Epidemiology
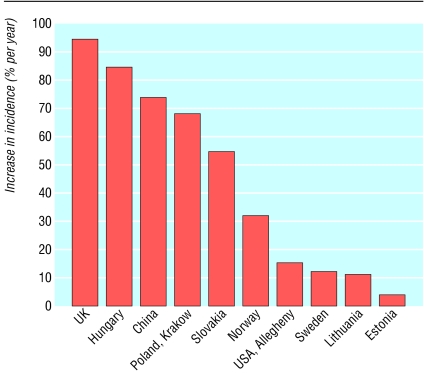
Although most attention has focused on the increase in type 2 diabetes, a parallel rise in type 1 diabetes has occurred (fig 1).1 Type 1 diabetes has always been known as a disease of childhood, but more recent epidemiological studies have indicated that the incidence is comparable in adults.3 The enormous international variation in incidence is now recognised. A child in Finland is almost 40 times more likely to develop type 1 diabetes than a child in Japan and almost 100 times more likely to get the disease than a child in the Zunyi region of China.1 The EURODIAB collaborative study, a registry involving 44 countries in Europe, indicates an annual rate of increase in incidence of type 1 diabetes of 3-4%, with a larger increase in some central and eastern European countries.4 The largest rate of increase is seen in children aged 0-4 years. Type 1 diabetes is associated with other autoimmune conditions; the most common association is with thyroid disease.5 The Belgian Diabetes Registry indicated that the prevalence of thyroid peroxidase autoantibodies is 22% in patients with type 1 diabetes. Approximately 1 in 10 patients with type 1 diabetes express transglutaminase IgA autoantibodies, and more than half of these patients have coeliac disease on intestinal biopsy. Approximately 1 in 50 people with type 1 diabetes have 21-hydroxylase autoantibodies, and approximately 25% of these patients progress to Addison's disease.
Fig 1.
Relative increase in incidence of type 1 diabetes in children aged less than 14 years. Modified from Onkamo et al1 (Available in: Eisenbarth GS, ed. Type 1A diabetes: cellular, molecular and clinical immunology, teaching slides www.barbaradaviscenter.org)
Summary points
The incidence of type 1 diabetes is increasing rapidly worldwide, and it is also presenting at an earlier age
Genetically engineered human insulins have improved care of type 1 diabetes, and devices for continuous glucose monitoring may revolutionise care
An interplay between genetic susceptibility and environmental factors (triggering or suppressive) may account for the pathogenesis of type 1 diabetes
Many associations with various environmental triggers have been found in type 1 diabetes, but so far only congenital rubella syndrome has been conclusively associated with the disease
The expression of diabetes related autoantibodies in young children monitored from birth indicates that these markers are a major risk factor for the future development of type 1 diabetes
No treatment has been shown to safely prevent type 1 diabetes in humans, although islet transplantation and new immunosuppressive regimens show that the disease can be cured
Genes
Alleles or genetic variants associated with type 1 diabetes provide either susceptibility to or protection from the disease. An interplay between genetic susceptibility and environmental factors is thought to provide the fundamental element for disease and provides potential targets for both prediction and prevention of disease.6 The concordance for type 1 diabetes is approximately 50% for monozygotic twins, and the risk to a first degree relative is approximately 5%.7 The major genetic determinant of susceptibility to diabetes lies within the major histocompatibility complex (termed IDDM 1). More than 90% of patients who develop type 1 diabetes have either DR3, DQ2 or DR4, DQ8 haplotypes, whereas fewer than 40% of normal controls have these haplotypes.6 DR3-DR4 heterozygosity is highest in children who develop diabetes before age 5 (50%) and lowest in adults presenting with type 1 diabetes (20-30%), compared with a US population prevalence of 2.4%. Only one non-HLA gene has been identified with certainty—IDDM 2 on chromosome 11p5.5—and this contributes about 10% of the familial aggregation of type 1 diabetes.8 This locus is a polymorphic region that maps to a variable number of tandem nucleotide repeats (VNTR) 5' of the insulin gene. Studies in man indicate that different sizes of this VNTR 5' of the insulin gene are associated with risk for type 1 diabetes. The long form of the VNTR (≥ 100 repeats, class III) is associated with protection from diabetes.9 This influence of the insulin gene locus may relate to variation in expression of insulin within the thymus (greater thymic insulin message with protective VNTR). Table 1 shows a summary of the susceptibility loci for type 1 diabetes.
Table 1.
Inherited susceptibility loci for type 1 diabetes with associated chromosome location and candidate genes or microsatellite markers
| Locus | Chromosome | Candidate genes or microsatellites |
|---|---|---|
| IDDM 1 | 6p21 | HLA-DQ/DR |
| IDDM 2 | 11p15 | InsulinVNTR |
| IDDM 3 | 15q26 | D15s107 |
| IDDM 4 | 11q13 | MDU1, ZFM1, RT6, FADD/MORT1, LRP5 |
| IDDM 5 | 6q24-27 | ESR, MnSOD |
| IDDM 6 | 18q12-q21 | D18s487, D18s64, JK (Kidd locus) |
| IDDM 7 | 2q31 | D2s152, IL-1, NEUROD, GALNT3 |
| IDDM 8 | 6q25-27 | D6s264, D6s446, D6s281 |
| IDDM 9 | 3q21-25 | D3s1303 |
| IDDM 10 | 10p11-q11 | D10s193, D10s208, D10s588 |
| IDDM 11 | 14q24.3-q31 | D14s67 |
| IDDM 12 | 2q33 | CTLA-4, CD28 |
| IDDM 13 | 2q34 | D2s137, D2s164, IGFBP2, IGFBP5 |
| IDDM 14 | ? | NCBI # 3413 |
| IDDM 15 | 6q21 | D6s283, D6s434, D6s1580 |
| IDDM 16 | ? | NCBI # 3415 |
| IDDM 17 | 10q25 | D10s1750-D10s1773 |
Environment
Two major hypotheses exist that may account for the increase in incidence of type 1 diabetes. The first hypothesis is that an environmental agent such as a virus may account for this.10 Seasonality, increasing incidence, and epidemics of type 1 diabetes, as well as many cross sectional and retrospective studies, suggest that certain viruses and some aspects of early childhood diet may influence risk of type 1 diabetes. Many associations with various environmental triggers have been found in type 1 diabetes, but so far only congenital rubella syndrome has been conclusively associated with the disease. Table 2 summarises the studies that have attempted to show an association with type 1 diabetes.
Table 2.
Summary of studies investigating association of environmental factors in type 1 diabetes
| Agent | Type of study | No of participants | Outcome |
|---|---|---|---|
| Enterovirus | Prospectivew1
|
155
|
Associated with diabetes autoantibodies
|
| Case-controlw2
|
260
|
In utero infection associated with type 1 diabetes
|
|
| Prospectivew3 | 65 | No association with type 1 diabetes | |
| Mumps
|
Case-controlw4
|
127
|
All these studies found associations with diabetes autoantibodies
|
| Rubella
|
Retrospectivew5
|
386
|
|
| Rotavirus
|
Case controlw6
|
54
|
|
| Chickenpox
|
Prospectivew7
|
n/a
|
|
| Cow's milk* | Prospectivew8
|
725 | Positive association with autoimmunity |
| Cross sectionalw9
|
253
|
Lack of association with autoimmunity
|
|
| Prospectivew10 | 317 | Lack of association with autoimmunity | |
| Common childhood vaccinations | Case-controlw11
|
3202
|
No association with autoimmunity
|
| Case-controlw12
|
317
|
No association with autoimmunity
|
|
| Prospectivew13
|
823
|
No association with autoimmunity
|
|
| Prospectivew14 | 4400 | Positive association with autoimmunity | |
| Nirates, nitrites, or nitrosamines | Prospectivew15 | 867 | Both these studies showed circumstantial evidence suggesting an association between type 1 diabetes and consumption of food and water containing nitrates |
| Retrospectivew16 | 1280 |
The data on cow's milk are conflicting. The TRIGR study (Finland) is under way to determine if elimination of cow's milk from infants' diet can prevent type 1 diabetes.
The DAISY (diabetes autoimmunity study in the young) study in Denver, Colorado, followed newborns from birth and to date has found no evidence that bovine milk ingestion, enteroviral infection, or vaccination contribute to risk of diabetes; nevertheless, reports about the first two environmental factors have been conflicting.11 Recent reports (including from the DAISY study) that suggest that early ingestion of cereal or gluten increases risk of type 1 diabetes need to be confirmed (see fig A on bmj.com).12,13 The reason why risk of islet autoimmunity is increased by exposure to cereal or gluten is not entirely clear and may result from a mechanism involving an aberrant immune response to cereal antigens in an immature gut immune system in susceptible individuals. Interestingly, several case reports exist of patients developing anti-islet autoantibodies and then type 1 diabetes (as well as other autoimmune endocrine disorders) after treatment with interferon alfa.14 Compounds such as poly-IC (a viral RNA mimic) that induce α interferon can generate insulitis (selective β cell destruction) and diabetes in animal models, strengthening the link between induction of diabetes and α interferon.15 α interferon has therefore been implicated as an important cytokine linking viruses and the initiation of type 1 diabetes, and neutralising this cytokine may potentially prevent the disease.14
The second hypothesis, under the rubric of the “hygiene hypothesis,” indicates that environmental factors can also inhibit the development of autoimmunity. As an oversimplification, our environment for young infants is far too clean, leading to a deficiency in immunoregulation such that “Th2” diseases (for example, asthma) and “Th1” diseases (for example type 1 diabetes) are increasing dramatically.16,17
Pathogenesis
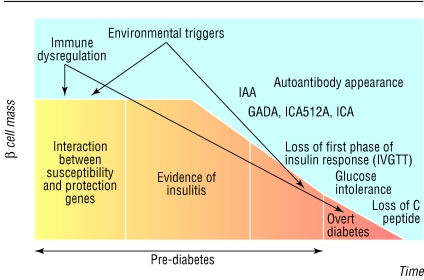
The hallmark of type 1 diabetes is the selective destruction of insulin-producing cells in the pancreas, or insulitis. Studies measuring the expression of diabetes related autoantibodies in young children from birth suggest that the appearance of these markers is a major risk for the future development of type 1 diabetes.18 However, the role of autoantibodies in the actual pathogenesis of type 1 diabetes has not been established in humans. In fact, a recent case report showed the development of type 1 diabetes in a patient with X linked agammaglobulinaemia, suggesting that autoantibodies are not needed for either the initiation or the progression of type 1 diabetes.19 In general, type 1 diabetes is considered primarily a T cell mediated disease, and extensive evidence exists in both man and mouse to support this. Examination of islet tissue obtained from pancreatic biopsy from patients with recent onset type 1 diabetes confirms insulitis, with the presence of an infiltrate composed of CD4 and CD8 T lymphocytes, B lymphocytes, and macrophages, suggesting that these cells have a role in destruction of the β cells.20 Early studies in mice showed that anti-CD3 treatment prevented diabetes, and a trial using humanised anti-CD3 antibody in patients with new onset type 1 diabetes is under way.21 In figure 2 we describe a general model of β cell destruction leading to type 1 diabetes. The initial interaction of genes and environmental factors seem to trigger an immune mediated response, with the appearance of autoantibodies as the first sign of β cell destruction, followed eventually by the loss of the first phase insulin response. The progression to overt diabetes resulting in significant β cell destruction is triggered by the development of a more aggressive T cell phenotype and a change in the Th1 to Th2 balance towards a more proinflammatory milieu. The expression of FasLigand on cytotoxic T cells also marks the progression to overt diabetes. Examination of islets during insulitis suggests that Fas mediated apoptosis occurs and therefore provides one possible mechanism of β cell destruction.22
Fig 2.
Model of pathogenesis and natural history of type 1 diabetes. Modified from Atkinson and Eisenbarth6 (Available in: Eisenbarth GS, ed. Type 1A diabetes: cellular, molecular and clinical immunology, teaching slides www.barbaradaviscenter.org)
Prediction
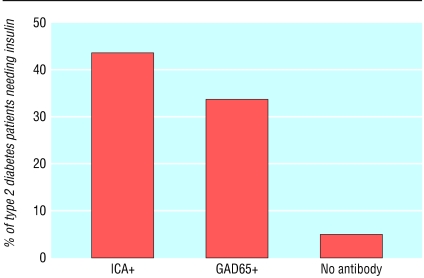
The long prodromal phase preceding the onset of type 1 diabetes suggests a potential to predict the disease and design trials for its prevention. The development of type 1 diabetes in relatives of patients with type 1 diabetes can now be predicted with reasonable accuracy by the detection of islet related autoantibodies (see table on bmj.com). Detection of two or more autoantibodies (GADA, IA-2, or insulin autoantibodies) in relatives of patients with type 1 diabetes has a positive predictive value exceeding 90% (see fig B on bmj.com).23 Insulin autoantibodies are often the first autoantibody to develop, especially in younger children. Although most prediction studies using autoantibody markers have focused mainly on relatives of patients with type 1 diabetes, the presence of multiple diabetes related autoantibodies seems to be similarly predictive in the general population.24 A study in Florida suggested that antibody positive people and their family members became anxious on learning their screening results; this subsided in most but not all people.25 Although relatively good predictions of type 1 diabetes can be obtained by measuring autoantibodies, a successful method of prevention has not yet been discovered. Nevertheless, in the DAISY study high risk children with positive autoantibodies (without any interventions) seemed to have less severe diabetic ketoacidosis at onset of diabetes, better HbA1c at onset, and a lower rate of admission to hospital.26 The use of autoantibody markers has been extended to define subsets of patients thought to have type 2 diabetes. Results from the United Kingdom prospective diabetes study (UKPDS) indicate that as many as 30% of younger “type 2” patients with diabetes may have an autoimmune process and that these patients usually progress to needing insulin within three years (fig 3).27 This subgroup of patients has been termed latent autoimmune diabetes of adults.
Fig 3.
Latent autoimmune diabetes of adults in UK prospective diabetes study. Percentage of type 2 diabetes patients (25-34 years old) with ICA or GAD antibody needing insulin within six years of diagnosis. Adapted from Turner et al27 (In: Eisenbarth GS, ed. Type 1A diabetes: cellular, molecular and clinical immunology, teaching slides www.barbaradaviscenter.org)
Prevention and new treatments
To date no treatment has been shown to prevent type 1 diabetes in humans. More than 100 different treatments prevent type 1 diabetes in the NOD mouse model, and this may indicate that disease prevention in this model is “too” easy.28 Two major trials have been conducted to try to prevent type 1 diabetes. In the United States, the diabetes prevention trial (DPT-1) was started in 1994 with the aim of determining whether antigen based treatment with insulin (oral and parenteral insulin treatment in relatives at high and moderate risk) would prevent or delay diabetes. These treatments did not overall slow the progression to diabetes. The European nicotinamide diabetes intervention trial (ENDIT) also found no difference in protection from diabetes when participants were assigned to either oral nicotinamide or placebo treatment (P Bingley, European Association of the Study of Diabetes, Budapest, September 2002). Many challenges remain in this field; in particular assays for pathogenic human T cells are not yet available. Such assays have the potential to provide surrogate markers to guide evaluation of immunotherapy; in the absence of such markers, the primary outcome of trials today is the preservation of insulin secretion (for example, measurement of C peptide secretion). TrialNet and the Immune Tolerance Network created by the US National Institutes of Health will be focusing not only on the prevention of diabetes but also on preventing further loss of islet β cells in patients with new onset type 1 diabetes.
Insulin remains the main treatment in type 1 diabetes. The diabetes control and complications trial (DCCT) showed the importance of strict metabolic control in delaying and preventing complications.29 The risk of hypoglycaemia is still the major limiting factor in achieving euglycaemia with insulin treatment. The introduction of rapidly absorbed insulin analogues has reduced variability of insulin absorption and allows insulin administration in young children after meals.30 Another recent introduction to the insulin market has been insulin glargine, which functions as a very long acting insulin (peakless basal insulin).31 Combinations of engineered very long acting insulins and rapid acting insulins can provide control and convenience similar to that obtained with insulin pumps.
The use of metformin treatment alongside insulin has increased in patients with type 1 diabetes. Recent studies have suggested that metformin might benefit type 1 diabetes patients who are overweight, are receiving large doses of insulin, or have an HbA1c > 8%.32 The coexistence of insulin resistance in patients with type 1 diabetes is a new area of interest. Islet transplantation with modified immunosuppressive regimens can cure type 1 diabetes. Islet transplantation is a consideration for the limited but important subset of patients with recurrent severe hypoglycaemic episodes not responsive to medical management.33 Inability to control autoimmunity and alloimmunity and a lack of donor organs limit the application of islet transplantation.
Additional educational resources
Diabetes UK (www.diabetes.org.uk)—provides useful links for both patients and healthcare professionals in the United Kingdom
Barbara Davis Center for Childhood Diabetes (www.uchsc.edu/misc/diabetes/books.html)—provides an online teaching guide for healthcare professionals and guidance on type 1 diabetes for patients and their families
American Diabetes Association (www.diabetes.org)—provides useful links for both patients and healthcare professionals in the United States
Immunology of Diabetes Society (www.idsoc.org)—provides an update on current immune intervention trials, links to the Immune Tolerance Network, and current updates on autoantibody assay technology
Supplementary Material
See also p 741
 Additional references, two figures, and a table are on bmj.com
Additional references, two figures, and a table are on bmj.com
Contributors: DD had the idea for the paper, did most of the background research, wrote the text and tables, and referenced the paper. EL helped to plan the content and wrote on the pathogenesis and T cell assays. GSE provided most of the information on new developments, created the figures, and edited the final paper. DD and GSE accept full responsibility for the content of the paper and controlled the decision to publish.
Funding: This work is supported by grants from the National Institute of Health (DK32082, AI39213, DK55969, DK62718, AI50864, AI95380, DK50970, AI46374), Diabetes Endocrine Research Center (P30 DK57516), Clinical Research Centers (MO1 RR00069, MO1 RR00051), American Diabetes Association, Juvenile Diabetes Foundation, and Children's Diabetes Foundation. DD is supported by an Eli Lilly fellowship award, and EL is supported by a NIH grant (DK 06405).
Competing interests: None declared.
References
- 1.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes—the analysis of the data on published incidence trends. Diabetologia 1999;42: 1395-403. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20: 1183-97. [DOI] [PubMed] [Google Scholar]
- 3.Molbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med 1994;11: 650-5. [DOI] [PubMed] [Google Scholar]
- 4.EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000;355: 873-6. [PubMed] [Google Scholar]
- 5.Devendra D, Eisenbarth GS. Immunologic endocrine disorders. J Allergy Clin Immunol 2003;111: 624-36. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MA, Eisenbarth GS. Type 1A diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358: 221-9. [DOI] [PubMed] [Google Scholar]
- 7.Redondo MJ, Rewers M, Yu L, Garg S, Pilcher CC, Elliott RB, et al. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1A diabetes: prospective twin study. BMJ 1999;318: 698-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett ST, Todd JA. Human type 1A diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet 1996;30: 343-70. [DOI] [PubMed] [Google Scholar]
- 9.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15: 289-92. [DOI] [PubMed] [Google Scholar]
- 10.Hyoty H, Taylor KW. The role of viruses in human diabetes. Diabetologia 2002;45: 1353-61. [DOI] [PubMed] [Google Scholar]
- 11.Norris JM, Beaty B, Klingensmith G, Yu L, Hoffman M, Chase HP, et al. Lack of association between early exposure to cow's milk protein and β-cell autoimmunity: diabetes autoimmunity study in the young (DAISY). JAMA 1996;276: 609-14. [DOI] [PubMed] [Google Scholar]
- 12.Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290: 1713-20. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1A diabetes-associated autoantibodies. JAMA 2003;290: 1721-8. [DOI] [PubMed] [Google Scholar]
- 14.Devendra D, Eisenbarth GS. Interferon alpha—a potential link in viral induced diabetes and autoimmunity. Clin Immunol 2004. (in press). [DOI] [PubMed]
- 15.Moriyama H, Wen L, Abiru N, Liu E, Yu L, Miao D, et al. Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci USA 2002;99: 5539-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale EA. A missing link in the hygiene hypothesis? Diabetologia 2002;45: 588-94. [DOI] [PubMed] [Google Scholar]
- 17.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347: 911-20. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, et al. Early expression of anti-insulin autoantibodies of man and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97: 1701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med 2001;345: 1036-40 [DOI] [PubMed] [Google Scholar]
- 20.Imagawa A, Hanafusa T, Itoh N, Waguri M, Yamamoto K, Miyagawa J, et al. Immunological abnormalities in islets at diagnosis paralleled further deterioration of glycaemic control in patients with recent-onset type I (insulin-dependent) diabetes mellitus. Diabetologia 1999;42: 574-8. [DOI] [PubMed] [Google Scholar]
- 21.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1A diabetes mellitus. N Engl J Med 2002;346: 1692-8. [DOI] [PubMed] [Google Scholar]
- 22.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type I diabetes (insulin dependent) mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 1986;29: 267-74. [DOI] [PubMed] [Google Scholar]
- 23.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45: 926-33. [DOI] [PubMed] [Google Scholar]
- 24.LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, et al. Successful prospective prediction of type 1A diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State diabetes prediction study. Diabetes Care 2002;25: 505-11. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SB. Screening programs to identify children at risk for diabetes mellitus: psychological impact on children and parents. J Pediatr Endocrinol Metab 2001;14: 653-9. [DOI] [PubMed] [Google Scholar]
- 26.Barker J, Klingensmith G, Barriga K, Rewers M. Clinical characteristics of type 1 diabetic children identified by a genetic screening and intensive follow-up program. Diabetes 2003;52(suppl 1): A188. [Google Scholar]
- 27.Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997;350: 1288-93. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson MA, Leiter EH. The NOD mouse model of type 1A diabetes: as good as it gets? Nat Med 1999;5: 601-4. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1A diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342: 381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vajo Z, Duckworth WC. Genetically engineered insulin analogs: diabetes in the new millennium. Pharmacol Rev 2000;52: 1-9. [PubMed] [Google Scholar]
- 31.Bolli GB, Owens DR. Insulin glargine. Lancet 2000;356: 443-5. [DOI] [PubMed] [Google Scholar]
- 32.Meyer L, Guerci B. Metformin and insulin in type 1A diabetes: the first step. Diabetes Care 2003;26: 1655-6. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1A diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343: 230-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.