Abstract
Background
Acute lung injury (ALI) is associated with high mortality. Low tidal volume (Vt) ventilation has been shown to reduce mortality in ALI patients in the Intensive Care Unit.
Anesthesiologists do not routinely provide lung protective ventilation strategies to patients with ALI in the operating room. We hypothesized an alert recommending lung protective ventilation regarding patients with potential ALI would result in lower Vt administration.
Methods
We conducted a randomized controlled trial on anesthesia providers caring for patients with potential ALI. Patients with an average or last collected ratio of partial pressure of arterial oxygen to inspired fraction of oxygen < 300 were randomized to providers being sent an alert with a recommended Vt of 6 cc/kg predicted body weight or conventional care. Primary outcomes were Vt and Vt/kg predicted body weight administered to patients. Secondary outcomes included ventilator parameters, length of postoperative ventilation and death.
Results
The primary outcome was a clinically significant reduction in mean Vt from 508 to 458 cc (p=0.033), with a reduction in Vt when measured in cc/kg predicted body weight from 8 to 7.2 cc/kg predicted body weight (p=0.040). There were no statistically significant changes in other outcomes or adverse events associated with either arm.
Conclusions
Automated alerts generated for patients at risk of having ALI resulted in a statistically significant reduction in Vt administered when compared to a control group. Further research is required to determine if a reduction in Vt results in decreased mortality and/or postoperative duration of mechanical ventilation.
Introduction
Acute lung injury (ALI) is a devastating condition with significant mortality. The clinical syndromes of ALI and acute respiratory distress syndrome (ARDS) are defined by descriptive clinical findings, regardless of the specific etiology of acute pulmonary dysfunction. The American-European Consensus Committee in 1994 defined clinical ALI to require 1) respiratory failure of acute onset with a ratio of partial pressure of arterial oxygen (PaO2) to inspired fraction of oxygen (FiO2) (PaO2/FiO2 or P/F) ratio ≤300 mmHg (regardless of the level of positive end expiratory pressure (PEEP), 2) bilateral infiltrates on frontal chest radiograph, and 3) a pulmonary capillary wedge pressure <18 mmHg (if measured) or no evidence of left atrial hypertension.1 ARDS was defined identically except for a lower limiting value of <200 mmHg for PaO2/FiO2.1 ALI and ARDS affect a large number of patients and have a poor prognosis. The incidence of ALI/ARDS has been variably reported to be 50,000–190,000 cases per year in the United States.1–7
The only ventilator intervention to date that has clearly demonstrated a survival benefit in controlled studies in adults with ARDS has been the adoption of a low tidal volume ventilation strategy (6ml/kg predicted body weight (PBW))with plateau pressures < 30 cmH2O).8 Additionally, the use of recruitment maneuvers, higher levels of PEEP, and judicious administration of fluids have been shown to reduce ventilator days and increase PaO2 while avoiding potential hyperoxia induced lung injury.9–12 Together, these methods comprise a lung protective ventilation strategy. A number of animal and clinical studies have shown that resorting to a non-protective strategy by adoption of high tidal volumes or higher plateau pressures can result in serious lung injury and, in some cases, parallel the course of patients with severe ARDS.13,14
Despite dissemination of this management strategy, we have recently found that lung protective ventilation strategies were not specifically undertaken in patients with ALI in the operating room setting.15 In 1,286 patients that underwent procedural anesthesia with a PaO2/FiO2 ratio < 300, 242met the criteria for diagnosis of ALI prior to the procedure. Intraoperative lung protective ventilator management was not routinely performed on these patients. Patients, on average, received 8.5 cc/kg PBW ventilation and approximately 5cm H2O of PEEP with high FiO2. Typically, these patients had higher PaO2and tidal volumes in the operating room compared to the pre-anesthetic setting.
In the present study, we tested the hypothesis that informing anesthetic providers of a recommended ventilation strategy for potential ALI in patients with low preoperative P/F ratios would result in an increased use of lower tidal volumes and higher PEEP, consistent with a lung protective ventilation strategy.
Materials and Methods
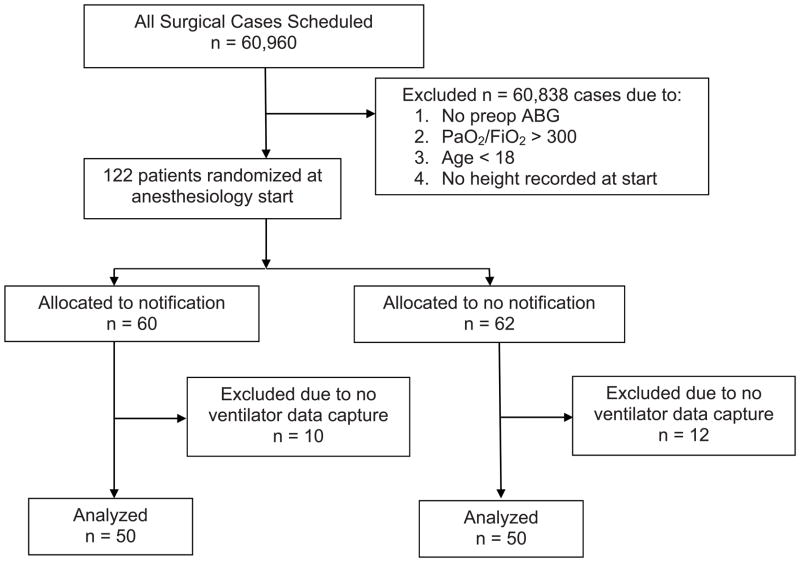
Institutional Review Board approval was obtained for this randomized, open-label, parallel arm clinical trial from the Institutional Review Boards of the University of Michigan Medical School (Ann Arbor, MI) under waiver of consent without use of a data safety monitoring board due to the low-risk nature of the study. Figure 1 depicts the flow of patients through the trial. The study was conducted at a single site, the University of Michigan Medical Center. Patients were recruited using a “just-in-time” automated anesthesia information management system (Centricity, Wishahaka, WI) script that was created to screen all patients undergoing an anesthetic at the time of the start of anesthesia or entry into the operating room with central laboratory arterial blood gases collected within the past two days. From these arterial blood gases, the average P/F ratio was calculated for each patient. If the patient was ≥ 18 years old, the average P/F ratio was < 300 or the last P/F ratio was < 300, and a valid history and physical including the patient’s height was present, patients were enrolled and randomized using a real-time electronic pseudorandom number generator to intervention or no intervention (control) in a 1:1 allocation ratio to enroll two equal groups of 50 patients. No system of stratification was used during randomization. If randomized to intervention, the height of the patient was obtained from the electronic history and physical, and the PBW was calculated. An automated text page was sent to the attending anesthesiologist’s and/or in-room provider’s hospital pager, depending on “signed in” status, at either anesthesia start or at the time the patient was in the operating room stating:
“Patient: LAST, FIRST has a P/F ratio consistent with Acute lung injury. If this patent has ALI, recommended tidal volume is <XXX>cc. Recommended PEEP and FiO2 are shown on http://anes.med.umich.edu/ARDStable.html.”
Figure 1.
There was no protocolized care in this study. All clinical decisions were left to the discretion of the anesthetic care team, who could decide whether to follow the recommendations of the automated alert. The anesthesia machine used in this study was the Aisys (General Electric Healthcare, Milwaukee, WI). This machine provides basic volume control ventilation in addition to advanced pressure control modes, pressure control mode with a volume guarantee, pressure support, and inverse ratio ventilation. Only basic ventilator information was collected by the anesthesia information management system including delivered low tidal volume, PEEP, peak inspiratory pressure, respiratory rate, and FiO2. No recommendation on ventilator mode was included in the alert, and the ventilator mode was not recorded.
The study was intended to demonstrate the superiority of the alert group with a reduction in the total cc/kg PBW administered. The primary outcome measure was median tidal volume in cc/kg PBW between the two groups, from surgical incision to dressing complete. Secondary outcomes included median total tidal volumes, PEEP, and peak inspiratory pressure. Finally, we calculated what would have been the recommended lung protective ventilation strategy (LPVS) tidal volumes for the patients not receiving the alert (control group) and compared these tidal volumes with the actual tidal volumes administered to determine the mean difference. After the completion of enrollment and initial statistical analysis, the preoperative anesthetic records were reviewed for common conditions that have been associated with increased morbidity and mortality. Additionally, preoperative chest radiographs were examined, if available, to look for the presence of bilateral infiltrates that would be consistent with a diagnosis of acute lung injury. X-ray review was completed using a consensus process by the investigators while they were blinded to additional information about the patients. Finally, the hospital course of each patient was examined for postoperative complications that could be associated with low tidal volume ventilation including prolonged intubation, stroke/herniation, myocardial infarction, new onset renal failure, and death.
Statistics
The study was designed with a sample size of 50patients in each group to have 80% power to detect a difference in means of 1.0 cc/kg PBW, assuming the common standard deviation was1.00cc/kg PBW using a two-sided independent t-test (α=0.05). Comparison between groups was made with SPSS version 18 (SPSS Software, Chicago, IL) and R version 2.16 (R Foundation for Statistical Research, Vienna, Austria) using the two-tailed Student’s t-test and/or Mann-Whitney U for continuous variables or chi-square analysis for dichotomous variables. P values less than 0.05 were considered to be statistically significant.
Results
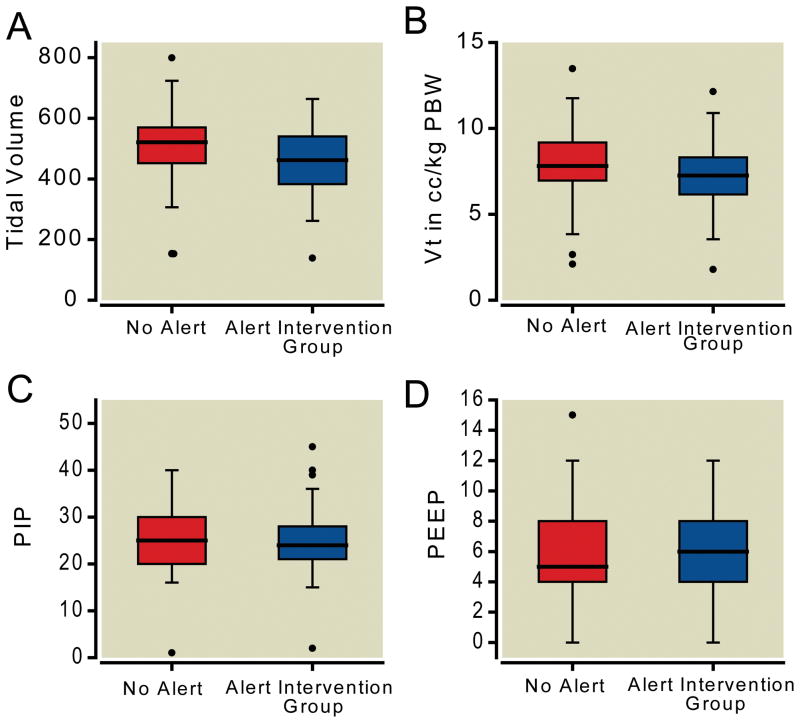
We successfully enrolled and collected data on 100 patients (figure 1). The study enrolled from July 8, 2010 until February 21, 2011. Twenty-two patients were excluded from the dataset after randomization due to lack of ventilator data capture. No other data was missing in the dataset that was collected. There were no statistically significant differences between comorbidities in the control and intervention groups (table 1). In the 50 patients enrolled in the intervention group, the average recommended tidal volume was 390 cc. The mean PBW in both populations was 65 kg. The intervention group received lower total tidal volumes (458 cc vs. 508 cc, p=0.033) and lower tidal volumes per kilogram of PBW (7.19 cc/kg vs. 7.97 cc/kg, p=0.040) (figure 2). The distribution of the low tidal volume cc/kg PBW was not entirely normal, as such we examined it using both the Mann-Whitney U and t-test, which provided p values of 0.040 and 0.058 respectively.
Table 1.
Demographics and Comorbidities between intervention and control groups.
| Intervention n=50
|
Control n=50
|
|||
|---|---|---|---|---|
| Age | 60.5 | (53.0, 67.0) | 56.5 | (44.3, 64.8) |
| Predicted Body Weight | 63.9 | (57.0, 72.4) | 68.4 | (55.0, 73.0) |
| ASA Status | 4 | (3, 4) | 4 | (3, 4) |
| Preoperative P/F Ratio | 198.8 | (168.3, 231.7) | 184.5 | (139.5, 215.4) |
| Male | 30 | 60% | 33 | 66% |
| Emergent Surgery | 17 | 34% | 21 | 42% |
| Hypertension | 29 | 58% | 25 | 50% |
| CAD | 20 | 40% | 15 | 30% |
| CHF | 8 | 16% | 16 | 32% |
| Pneumonia | 7 | 14% | 6 | 12% |
| COPD | 11 | 22% | 9 | 18% |
| Asthma | 2 | 4% | 2 | 4% |
| OSA | 10 | 20% | 11 | 22% |
| Hepatic Disease | 8 | 16% | 13 | 26% |
| Renal Failure | 17 | 34% | 18 | 36% |
| Diabetes | 16 | 32% | 14 | 28% |
| Bilateral Infiltrates on CXR | 37 | 74% | 33 | 66% |
There were no statistically significant differences between groups in any parameter. Values are represented as median (IQR) or n (%). ASA = American Society of Anesthesiologists, CAD = coronary artery disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CXR = chest x-ray, P/F = ratio of partial pressure of arterial oxygen to inspired fraction of oxygen, OSA = obstructive sleep apnea.
Figure 2.
There was no statistical difference in the PEEP or peak inspiratory pressure between the two groups. These data are summarized in table 2 and figure 2. Patients in the intervention group received total tidal volumes closer to the recommended tidal volumes than those who did not (mean difference 67 cc (95% CI 33, 101) vs 117 cc (95% CI 81, 153), p=0.046). The absolute value of the difference in patients who received the alert was 109 cc (95% CI 86, 133), which was lower than the absolute value of 147 cc (95% CI 121, 173) in those patients who did not receive the alert (p=0.034). There were also more patients in the intervention group who received tidal volumes <6.5 cc/kg PBW (17 patients vs 8 patients, p=0.065), although this did not meet statistical significance. Upon review of all cases there was no statistically or clinically increased rate of complications associated with the intervention group. This included a statistically identical number of postoperative ventilator days, strokes, myocardial infarctions, and deaths. These data are further illustrated in table 2.
Table 2.
Intraoperative differences and Postoperative Outcomes
| Intervention n = 50 | Control n = 50 | p | |||
|---|---|---|---|---|---|
|
|
|||||
| Median Vt | 462 | (383.5, 539.3) | 522.5 | (455.3, 573.8) | 0.014 |
| Median Weight-Adjusted Vt (cc/kg PBW) | 7.3 | (6.2, 8.3) | 7.8 | (7.0, 9.1) | 0.032 |
| Median PIP | 24 | (21, 27.5) | 25 | (20, 29.8) | 0.849 |
| Median PEEP | 6 | (4.0, 8.0) | 5 | (4.3, 8.0) | 0.652 |
| Postoperative Ventilator Days | 3 | (1, 5) | 3 | (1, 9) | 0.325 |
| Mortality at 28 days | 8 | 16% | 6 | 12% | 0.774 |
| Postoperative Stroke/Herniation | 1 | 2% | 1 | 2% | 1.000 |
| Postoperative MI | 0 | 0% | 2 | 4% | 0.495 |
| Postoperative New AKI/Dialysis | 4 | 8% | 6 | 12% | 0.741 |
|
|
|
||||
Values are represented as average (IQR) or n (%). AKI = acute kidney injury, MI = myocardial infarction, PBW = predicted body weight, PEEP = positive end expiratory pressure, PIP = peak inspiratory pressure, Vt = tidal volume
Upon review of the initial dataset, three patients were found to have profoundly low tidal volumes of < 3 cc/kg PBW and three were found to be of The American Society of Anesthesiologists Physical Status 6. A sensitivity analysis was completed excluding these patients from the dataset (tables 3 and 4). Overall, there were no statistically significant changes in the results.
Table 3.
Demographics and Comorbidities between intervention and control groups.
| Intervention n = 47 | Control n = 47 | ||||
|---|---|---|---|---|---|
|
|
|||||
| Age | 63 | (53.5, 67.5) | 57 | (45.5, 64.5) | |
| Predicted Body Weight | 63.9 | (57.0, 71.9) | 68.4 | (55.3, 74.2) | |
| ASA Status | 4 | (3, 4) | 4 | (3, 4) | |
| Preoperative P/F Ratio | 201 | (176.1, 230.9) | 184.8 | (140.2, 221.3) | |
| Male | 29 | 62% | 30 | 64% | |
| Emergent Surgery | 15 | 32% | 18 | 38% | |
| Hypertension | 27 | 57% | 25 | 53% | |
| CAD | 20 | 43% | 15 | 32% | |
| CHF | 8 | 17% | 16 | 34% | |
| Pneumonia | 7 | 15% | 6 | 13% | |
| COPD | 11 | 23% | 9 | 19% | |
| Asthma | 2 | 4% | 2 | 4% | |
| OSA | 10 | 21% | 10 | 21% | |
| Hepatic Disease | 6 | 13% | 12 | 26% | |
| Renal Failure | 16 | 34% | 17 | 36% | |
| Diabetes | 15 | 32% | 14 | 30% | |
| Bilateral Infiltrates on CXR | 36 | 72% | 31 | 62% | |
There were no statistically significant differences between groups in any parameter. Values are represented as median (IQR) or n (%). ASA = American Society of Anesthesiologists, CAD = coronary artery disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CXR = chest x-ray, P/F = ratio of partial pressure of arterial oxygen to inspired fraction of oxygen, OSA = obstructive sleep apnea.
Table 4.
Intraoperative differences and Postoperative Outcomes
| Intervention n = 47 | Control n = 47 | p | |||
|---|---|---|---|---|---|
|
|
|||||
| Median Vt | 464 | (391, 539) | 525 | (488, 580) | 0.003 |
| Median Weight-Adjusted Vt (cc/kg PBW) | 7.3 | (6.2, 8.3) | 7.8 | (7.0, 9.0) | 0.02 |
| Median PIP | 24 | (21, 27) | 25 | (20, 30) | 0.674 |
| Median PEEP | 6 | (4, 8) | 5 | (4,8) | 0.571 |
| Postoperative Ventilator Days | 3 | (1, 5) | 3 | (1, 9) | 0.945 |
| Mortality at 28 days Postoperative | 6 | 13% | 5 | 11% | 1.000 |
| Stroke/Herniation | 1 | 2% | 1 | 2% | 1.000 |
| Postoperative MI | 0 | 0% | 2 | 4% | 0.495 |
| Postoperative New AKI/Dialysis | 4 | 9% | 5 | 11% | 1.000 |
|
|
|
||||
Values are represented as average (IQR) or n (%). AKI = acute kidney injury, MI = myocardial infarction, PBW = predicted body weight, PEEP = positive end expiratory pressure, PIP = peak inspiratory pressure, Vt = tidal volume.
Discussion
In this prospective randomized controlled trial an automated intraoperative notification alerting clinicians to the potential presence of ALI/ARDS led to statistically significant reduction in delivered total median tidal volumes and in volumes measured in cc/kg PBW. There were no differences in the PEEP between the two groups. This is the first trial we are aware of that changed clinician behaviors in patients with possible ALI in the perioperative environment.
Acute lung injury is an established syndrome that has profound effects on numerous patients each year. The incidence of ALI in two recent studies has been estimated at 22–86 cases per 100,000 persons per year,5,7 with 40–43 percent of these patients having ARDS.7 Survival statistics for patients with ALI/ARDS vary with lung injury etiology and age, but overall mortality rates in both adult and pediatric patients remain very substantial at 20–50% despite sophisticated intensive care.2,3,5–7,16–19 The significance of distinguishing between the two clinical syndromes in a practical sense is uncertain, since a meta-analysis of 102 studies prior to 1996 suggested little or no difference in mortality rates between patients meeting criteria for ALI compared to ARDS.20 This was also the conclusion in the recent NEJM article by Rubenfeld et al,7 which reported mortality rates of 38.5% for ALI and 41% for ARDS, with an estimated 74,500 deaths per year and an aggregate 3.6 million hospital days of care in the United States.
Over the past two decades, a variety of interventions and intensive care strategies have been used in treating patients with ALI/ARDS. Historically, ALI/ARDS was treated with large tidal volumes and high peak pressures in an attempt to improve oxygenation. However, it has since been shown that such efforts actually add to ventilator induced lung injury through multiple mechanisms, including alteration of the pulmonary cytoskeleton, disturbed alveolar fluid balance, and increased inflammatory response.21–25 Current standard of care for ARDS includes mechanical ventilation with adoption of lung protective strategies, judicious fluid management, adjunct nutritional support, and more importantly the diagnosis and treatment of the underlying cause. Despite this care, the only multicenter randomized controlled evidence for reducing ARDS-associated mortality consists of a reduction in tidal volumes from 12 cc/kg PBW to 6 cc/kg PBW and the administration of neuromuscular blockade for 48 hours after the onset of the syndrome.26
The application of relatively low tidal volumes during anesthesia is not a new concept and has been advocated in thoracic anesthesia where ALI is a devastating and common diagnosis. In work by Licker, it was determined that increased tidal volumes were associated with the development of ALI.27 Subsequent work showed that protocolled care with reduced tidal volumes in thoracic oncologic surgery during one lung ventilation reduced the incidence of ALI from 3.7% to 0.9%.28 However, Licker’s prevention trial was conducted in non-randomized patients without ALI undergoing only a single type of procedure, and did not address the management of patients with pre-existing ALI.
Recently we have shown that patients meeting criteria for ALI preoperatively are managed in a similar format to those that undergo anesthesia with hypoxia for another reason.15 This care frequently mirrors the care provided in the Intensive Care Unit prior to their procedure, with the important exception that patients receiving LPVS with tidal volumes ≤6 cc/kg PBW received significantly increased tidal volumes intraoperatively. While the reason for this difference in practice in unknown, the present study shows that an automated alert triggered by P/F ratios can increase compliance with a LPVS for patients who may have ALI. This is especially important for patients undergoing non-thoracic surgeries, where ALI may not be a foremost concern for anesthetic providers and is likely under-diagnosed.
We have demonstrated that a single pager notification that simply suggests the institution of a LPVS can impact provider behavior with regards to intraoperative management of patients at risk for ALI. Notifications, such as those trialed in this pilot study, may be an effective way to intervene and reduce the potential for further injury in patients with ALI. However, it is important to allow clinicians the flexibility to selectively implement a set of recommendations, rather than protocolize care, as situations where it is either impractical or unsafe to follow a set of predefined recommendations may occur. For example, in a patient at risk for ALI with underlying pulmonary dysfunction as well as concomitant head injury and elevated intracranial pressures, low tidal volumes with permissive hypercapnea may be contraindicated. These complex clinical situations may have contributed to the non-adherence to strict LPVS in this study. For example, this may be the reason for lower PEEP values than were recommended by the table on the website. As increasing PEEP may cause hemodynamic compromise, providers may have been concerned about applying PEEP above a certain level in these potentially critically ill patients.
Our study has several limitations which are important to discuss. First, the trial was undertaken at a single center with a relatively small number of patients, and these results and the changes in provider behavior may not be generalizable to other settings. In particular, the study relied on the relatively advanced anesthesia information management system architecture in place at the study hospital, although most centers with an anesthesia information management system should be able to replicate the notifications. This is notable, as this study design introduces a new method of studying the impact of low risk clinical reminder on the impact of care in ALI and other conditions that is consistent with the highest level of medical evidence, the randomized controlled trial. This minimizes the concerns frequently associated with pre/post analysis that is common in quality improvement literature.
Second, in this study we enrolled patients based on only one of the three criteria for ALI; because the study was performed with a “just-in-time”, automated screening and enrollment strategy that would be replicable at other centers, it was not possible to obtain intravascular volume status or chest radiograph information to confirm a diagnosis of ALI prior to the anesthetic. However, previous work has shown that lower tidal volumes can also help prevent the development of ALI.27,28 Therefore, interventions that lower tidal volumes may be beneficial to any patient with a low P/F ratio, regardless of whether the other criteria for ALI are met.
Finally, while there was an attempt made to determine if the outcomes were different between the two groups, the study was distinctly underpowered to detect a subtle increase in complications from implementation of the reminder intervention. Further studies assessing the impact of these types of interventions are therefore warranted.
In conclusion, we have demonstrated that anesthesiologists will modify their intraoperative ventilation strategies when notified about the potential risk for ALI underlying preoperative hypoxia. This relatively simple alert system is a potentially useful tool to bring provider behavior more in-line with the most recent standards of care from the literature. It would be reasonable to undertake a multi-center prospective trial in the future to determine the influence this type of notification can have on morbidity and mortality.
Final Boxed Summary Statement.
What we already know about this topic
Low tidal volume ventilation (6 ml/kg) improves survival in patients with acute respiratory distress syndrome (ARDS), yet when such individuals receive anesthesia, higher tidal volumes are typically used
What this article tells us that is new
In an open-label parallel arm study of 100 patients with ARDS receiving low tidal volume ventilation, sending the anesthesia providers an alert with a recommended tidal volume of 6 ml/kg resulted in a significant reduction in tidal volume delivered during anesthesia compared to conventional care
Complications and major morbidity did not differ between groups
Acknowledgments
Financial support for the preparation of this manuscript was provided from University of Michigan CTSA award KL2 Scholar Grant #F025965 from the National Institutes of Health, Bethesda, Maryland, USA, as well as by department funds of the Department of Anesthesiology, University of Michigan Medical Center, Ann Arbor, Michigan, USA.
Footnotes
This work should be attributed to the Anesthesiology Department, University of Michigan Medical Center, Ann Arbor, Michigan, USA.
References
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–48. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–8. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 5.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–11. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31:S276–84. doi: 10.1097/01.CCM.0000057904.62683.2B. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, Clemmer T, Lanken PN, Schoenfeld D. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 2003;31:2592–7. doi: 10.1097/01.CCM.0000090001.91640.45. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1807–14. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AL, Dechert RE, Park PK, Bartlett RH. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 12.Stewart RM, Park PK, Hunt JP, McIntyre RC, Jr, McCarthy J, Zarzabal LA, Michalek JE. Less is more: Improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. J Am Coll Surg. 2009;208:725–35. doi: 10.1016/j.jamcollsurg.2009.01.026. discussion 735–7. [DOI] [PubMed] [Google Scholar]
- 13.Pavone LA, Albert S, Carney D, Gatto LA, Halter JM, Nieman GF. Injurious mechanical ventilation in the normal lung causes a progressive pathologic change in dynamic alveolar mechanics. Crit Care. 2007;11:R64. doi: 10.1186/cc5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106:1226–31. doi: 10.1097/01.anes.0000267607.25011.e8. [DOI] [PubMed] [Google Scholar]
- 15.Blum JM. A description of intraoperative ventilator management in patients with acute lung injury and the use of lung protective ventilation strategies. Anesthesiology. 2011;115:75–82. doi: 10.1097/ALN.0b013e31821a8d63. [DOI] [PubMed] [Google Scholar]
- 16.Bindl L, Dresbach K, Lentze M. Incidence of acute respiratory distress syndrome in German children and adolescents: A population based study. Crit Care Med. 2005;33:209–12. doi: 10.1097/01.ccm.0000151137.76768.08. [DOI] [PubMed] [Google Scholar]
- 17.Manzano F, Yuste E, Colmenero M, Garcia-Horcajadas A, Rivera R, Fernandez-Mondejar E. Granada Respiratory Failure Study Group: Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care. 2005;20:274–80. doi: 10.1016/j.jcrc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury. Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 19.The Paediatric Study Group of the Australian and New Zealand Intensive Care Society (ANZICS) Acute lung injury in pediatric intensive care in Australia and New Zealand - A prospective, multicentre, observational study. Pediatr Crit Care Med. 2007;8:317–23. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 20.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, Hammerle AF, Steltzer H. The acute respiratory distress syndrome: Definitions, severity, and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–29. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 21.Finigan JH. The coagulation system and pulmonary endothelial function in acute lung injury. Microvasc Res. 2009;77:35–8. doi: 10.1016/j.mvr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Walker MG, Yao LJ, Patterson EK, Joseph MG, Cepinskas G, Veldhuizen RA, Lewis JF, Yamashita CM. The effect of tidal volume on systemic inflammation in Acid-induced lung injury. Respiration. 2011;81:333–42. doi: 10.1159/000323609. [DOI] [PubMed] [Google Scholar]
- 23.Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, van der Poll T, Vroom MB, Hollmann M, Schultz MJ. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 24.Choi G, Wolthuis EK, Bresser P, Levi M, van der Poll T, Dzoljic M, Vroom MB, Schultz MJ. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006;105:689–95. doi: 10.1097/00000542-200610000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Bolin RW, Pierson DJ. Ventilatory management in acute lung injury. Crit Care Clin. 1986;2:585–99. [PubMed] [Google Scholar]
- 26.Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal J-M, Perez D, Seghboyan J-M, Constantin J-M, Courant P, Lefrant J-Y, Gurin C, Prat G, Morange S, Roch A. Neuromuscular blockers in early acute respiratory distress syndrome. N Eng J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 27.Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, Tschopp JM. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–65. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 28.Licker M, Diaper J, Villiger Y, Spiliopoulos A, Licker V, Robert J, Tschopp JM. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009;13:R41. doi: 10.1186/cc7762. [DOI] [PMC free article] [PubMed] [Google Scholar]