Abstract
Objectives
CRS is accompanied by evidence of a vigorous adaptive immune response, and emerging studies demonstrate that some nasal polyps manifest a polyclonal autoantibody response. We previously found that antibodies against BP180, a component of the hemidesmosome complex and the dominant epitope in autoimmune bullous pemphigoid, were found at elevated levels in nasal polyp tissue. Given the critical role of hemidesmosomes in maintaining epithelial integrity, we sought to investigate the distribution of BP180 in nasal tissue and evaluate for evidence of systemic autoimmunity against this antigen in CRS.
Study Design
Case-control experimental study
Methods
The expression and distribution of BP180 in cultured nasal epithelial cells and normal nasal tissue were confirmed using real-time PCR, Western immunoblotting, immunofluorescence and immunohistochemistry. Sera were collected from three groups: control, CRSsNP, and CRSwNP. A commercially available ELISA was utilized to compare anti-BP180 autoantibody levels in sera.
Results
BP180 is expressed in nasal epithelium, but is not confined to the basement membrane as it is in human skin. In cultured nasal epithelial cells, confocal immunofluoresence showed a punctate distribution of BP180 along the basal surface, consistent with its distribution in epithelial keratinocytes. There are significantly higher levels of circulating nonpathologic anti-BP180 autoantibodies in CRS patients compared with normal controls (p<0.05).
Conclusions
BP180 is more widely expressed in nasal epithelium versus skin, although it appears to play a similar role in formation of hemidesmosomes along the basement membrane. Further investigations are ongoing to characterize the pathogenicity of the anti-epithelial antibody response in CRS.
Keywords: Chronic rhinosinusitis, sinusitis, nasal polyps, autoimmunity, autoantibodies, biomarker, bullous pemphigoid
Introduction
Chronic rhinosinusitis (CRS) is a prevalent chronic inflammatory condition of the paranasal sinuses affecting approximately eight percent of the US population. Clinical experience and analysis of the inflammatory infiltrate associated with CRS suggests that it manifests phenotypically in two different forms – one with nasal polyps (CRSwNP) and another without (CRSsNP)1,2. Previous studies by our group have highlighted that B-cells play an important role in CRSwNP, with locally increased levels of B-cell activating factor (BAFF); IL6, a cytokine involved in B-cell maturation and plasma cell differentiation; chemokines associated with B-cell recruitment; and elevated levels of class-switched immunoglobulins such as IgA and IgG when compared with CRSsNP and normal controls3,4. The systemic overexpression of some of these pro-inflammatory factors in mouse models results in autoimmunity, and it has been hypothesized that a similar effect could occur, at least locally, in CRSwNP.
Recent studies in our lab utilized autoantigen microarrays to make a novel observation that most nasal polyps manifest a polyclonal autoantibody response5. Furthermore, this polyclonal antibody response includes elevated levels of anti-nuclear autoantibodies in polyp specimens from patients with CRSwNP, but not CRSsNP. This class-switched IgA and IgG autoantibody response was not correlated with total immunoglobulin content of the nasal polyp tissue, but it was found that significantly higher levels of anti-dsDNA were correlated with recurrent nasal polyps requiring revision surgery. These data suggest that a previously undescribed autoimmune response is present in CRSwNP, and suggest a novel role for autoimmunity in CRSwNP. The antigenic targets of these autoantibodies have only begun to be characterized, and it remains unclear if this represents a local or systemic autoimmune response.
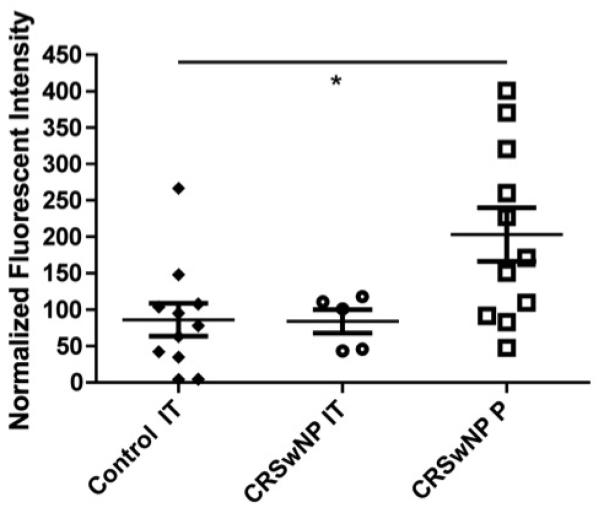
The microarray analysis also suggested that increased levels of anti-BP180 IgG autoantibodies are present in nasal polyp tissue (Fig. 1). BP180 (BPA2/collagen XVII) is a transmembrane glycoprotein that is associated with the hemidesmosome complex on the basal surface of epidermal keratinocytes, and functions to maintain adhesion of the stratified epithelia to the basement membrane6,7. This protein is known to be a primary target of IgG autoantibodies in several autoimmune blistering diseases, including bullous pemphigoid8,9,10. The expression of BP180 and its localization to the epithelial-ECM junction is best characterized in skin, but has also been shown in cultured human bronchial epithelial cells11. Since bullous pemphigoid is not known to have nasal or airway manifestations, the presence, distribution and function of BP180 in the nose has direct relevance to the potential pathogenicity of anti-BP180 autoantibodies in nasal polyps.
Figure 1.
Autoantibody microarray data demonstrating the fluorescent intensity of extracts from control inferior turbinate (IT) tissue, IT tissue from patients with CRSwNP and polyps (P) from patients with CRSwNP. Presented results are normalized to the fluorescent intensity of IgG.
IT = inferior turbinate; P = polyps; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps
Previous studies in our lab have demonstrated a reduction in several molecules of the epidermal differentiation complex including psoriasin, calprotectin and SPINK5 in CRS, suggesting an underlying epithelial barrier defect that may contribute to disease pathogenesis1,12,13. An anti-epithelial antibody response affecting basement membrane integrity in CRSwNP would have important implications for the barrier dysfunction observed in this disease. This study investigated the expression and distribution of BP180 in sinonasal tissue and nasal epithelial cells, and the presence of systemic anti-BP180 autoantibodies in patients with CRS.
Methods
All protocols were reviewed and approved by a Northwestern University Feinberg School of Medicine Institutional Review Board. Informed consent was obtained from all subjects from whom clinical information was collected. De-identified specimens obtained from subjects during routine surgical care were deemed exempt from informed consent.
Cell Culture
Primary nasal epithelial cells (NECs) were collected and cultured as previously described3. NECs were maintained in serum-free bronchial epithelial growth medium (Cambrex, Walkersville, MD). NECs were plated in 10cm culture plates coated with collagen (Vitrogen; Collagen Biomaterials, Palo Alto, CA). An immortalized line of human epidermal keratinocytes (HEKs) derived from neonatal foreskin (a gift from Dr. Lou Laimins, Northwestern University, Chicago, IL) and known to express BP180 was used as a positive control14. HEKs were maintained in defined keratinocyte serum-free medium (DKSFM)(0.07mM CaCl2) supplemented with a proprietary growth factor mixture (serum and bovine pituitary extract free) (Invitrogen, Carlsbad, CA). When cells reached 90% confluence, they were lysed for protein and RNA extraction or transferred to glass coverslips for cellular immunofluorescence.
Nasal protein extraction and Western blot
BP180 protein expression was assessed in whole-cell extracts of cultured cells as described previously11. Briefly, cells were lysed in a sample buffer consisting of 8M urea, 1% sodium dodecyl sulfate (SDS) in 10mM Tris-HCl, pH 6.8, and 15% β-mercaptoethanol. Proteins in the preparation were resolved on a 3 to 8% NuPAGE Tris-Acetate gel (Invitrogen, Carlsbad, CA), and transferred to a polyvinylidene difluoride membrane for immunoblotting (Bio-Rad Laboratories, Hercules, CA). The membranes were incubated with AB79878, a mouse monoclonal IgG antibody against BP180 (Abcam, Cambridge, MA) at a concentration of 1:1000 overnight at 4°C. After washing, they were treated with a goat anti-mouse IgG secondary antibody (Li-Cor Biosciences, Lincoln, NE). Immunoblots were digitalized and quantified with Odyssey imaging software (Li-Cor Biosciences, Lincoln, NE). Densitometry values for BP180 were normalized to those of the housekeeping gene β-actin.
Real-time PCR
HEKs and NECs were lysed in RNA lysis buffer supplemented with β-mercaptoethanol and RNA was extracted according to the manufacturer’s protocol (Clontech, Mountain View, CA). Real-time PCR was performed using the TaqMan method, as described previously15. TaqMan primer and probe sets for BP180 were purchased from ABI (4331182, COL17A1, Hs00166711_m1). The mRNA expression levels of BP180 were normalized to the median expression of the housekeeping gene β-actin and expressed as copies/ng RNA.
Immunofluorescence and Immunohistochemisty
Cells on coverslips were processed for immunofluorescence as previously described and treated with 1804b, a mouse monoclonal IgM antibody against BP180 (a gift from Dr. Jonathan Jones, Northwestern University, Chicago, IL) at a concentration of 1:112. After washing, cells were incubated with a FITC-conjugated goat anti-mouse IgM secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA). All preparations were viewed with a confocal laser scanning microscope (LSM 510, Zeiss, Thornwood, NY).
Normal nasal tissue was harvested from individuals without a history of CRS who were undergoing sinonasal surgery for unrelated reasons (e.g., endoscopic skull-base tumor excisions, intranasal procedures for obstructive sleep apnea). Normal human facial skin, redundant after cosmetic surgery procedures was obtained as a positive control. Tissue specimens were prepared and processed for immunofluorescence and immunohistochemistry as previously described16,3.
For immunofluorescence, tissue sections were incubated with J17, a rabbit polyclonal IgG antibody against BP180 (a gift from Dr. Jonathan Jones, Northwestern University, Chicago, IL) at a concentration of 1:3000. The slides were gently washed in PBS and incubated with goat anti-rabbit IgG Alexa-Fluor conjugated secondary antibody. For immunohistochemistry, tissue sections were incubated with J17 at a concentration of 1:5000 for 1 hour at room temperature. Sections were rinsed and then incubated in biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. All preparations were viewed using an Olympus IX71 Inverted Microscope (Olympus America, Inc, Center Valley, PA).
Patients and Sera
Patients with CRS were recruited from a tertiary care allergy and otolaryngology practice at the Northwestern University Feinberg School of Medicine. The diagnosis of CRSwNP or CRSsNP in this study was based on clinical criteria, as defined by the American Academy of Otolaryngology—Head and Neck Surgery Chronic Rhinosinusitis Task Force17. Sera were collected from CRS patients and normal controls without CRS at the time of endoscopic sinus surgery; collection was limited to serum alone, and whole blood was not available for any further hematological assessment.
The population was limited to otherwise healthy adults between the ages of 18 and 80 years. Subjects taking anticoagulants, who had received an organ transplant, or who had taken oral corticosteroids or antibiotics within the previous 2 weeks were excluded from the study. None of the patients enrolled in this study had a history of autoimmune disease, as determined by a thorough review of each patient’s electronic medical record. Details of subjects’ characteristics are included in Table I.
TABLE I.
Patient Characteristics
| Control | CRSsNP | CRSwNP | |
|---|---|---|---|
| Median age (range), yr | 54 (27-78) | 38 (18-70) | 48 (20-74) |
| Gender ratio (F/M) | 0.85 (6/7) | 1.1 (22/20) | 0.56 (15/27) |
| Asthma, no. (%) | 0 (0) | 8 (19) | 19 (45) |
| Atopy, no. (%) | 0 (0) | 25 (60) | 20 (48) |
|
| |||
| Total | 13 | 42 | 42 |
F/M = female/male
Patients’ sera were screened for IgG antibodies against BP180 using a standard validated enzyme-linked immunosorbent assay (ELISA) (MBL Diagnostics, Nagoya, Japan) according to the manufacturer’s instructions for diagnosis of bullous pemphigoid18,19. Briefly, patient serum was diluted 1:101 with diluent, then incubated on plates coated with recombinant purified BP180 NC16a antigen. After washing, plates were incubated with horseradish peroxidase conjugated goat anti human IgG, followed by development with the substrate solution. Absorbance was read at 450nm, and results calculated as instructed using the provided standard curve and negative controls. Any positive results were verified by performing indirect immunofluorescence on monkey esophagus (Immco Diagnostics, Buffalo, NY). Tissue sections were incubated with serum at a concentration of 1:40 for 45 minutes, followed with a goat anti-human IgG FITC conjugated secondary antibody (Immco Diagnostics, Buffalo NY). Sections were rinsed in PBS, mounted with 50% glycerol in PBS and viewed as above.
The relationship between serum levels of anti-BP180 and age and total IgG were assessed using linear regression and the R2 correlation coefficient was determined. Multigroup comparisons were performed using the Kruskal-Wallis test since the distribution of autoantibodies in sera was non-Gaussian and a post-hoc Dunn’s test was used to evaluate the binary comparisons driving the positive Kruskal-Wallis test. Binary comparisons were carried out using a Mann-Whitney U test. All analyses were performed using software obtained from GraphPad Prism (La Jolla, CA). A 2-tailed p-value of less than 0.05 was considered statistically significant.
Results
BP180 expression in nasal epithelial cells
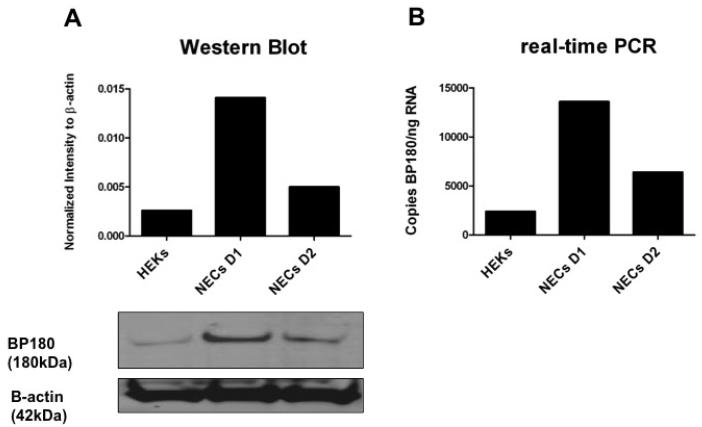
We first performed Western blot analyses to confirm that BP180 is expressed in nasal epithelial cells. We compared BP180 levels in HEKs (expressed as normalized intensity to β-actin) to those in NECs from two normal donors without a history of CRS (Fig. 2). NECs from both donors had higher levels of BP180 mRNA compared with keratinocytes, although this was not statistically significant. We next confirmed these results using real-time PCR, in which we compared BP180 mRNA levels in HEKs (expressed in copies BP180/ng RNA) to those in NECs from the same normal donors (Fig. 2). We again found markedly higher levels of BP180 mRNA in NECs compared to HEKs, demonstrating that the BP180 antigen is in fact expressed in NECs.
Figure 2.
Expression of BP180 in human epidermal keratinocytes and nasal epithelial cells. (A) Western blot results, where BP180 protein expression was measured in normalized intensity to β-actin, with the corresponding immunoblot of HEK and NEC whole-cell extracts. (B) Real-time PCR results, where BP180 mRNA expression was measured in copies BP180/ng RNA.
HEK = human epidermal keratinocytes; NEC D1 = nasal epithelial cell donor 1; NEC D2 = nasal epithelial cell donor 2
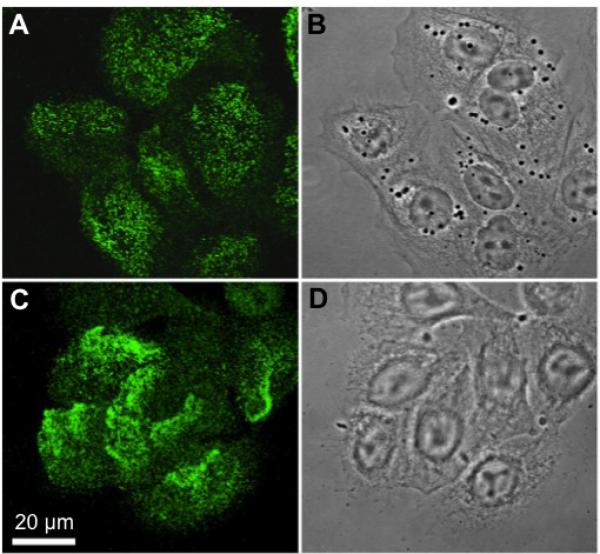
We next evaluated the distribution of BP180 within NECs using immunofluorescence and confocal microscopy. We found that BP180 is localized to the basilar surface in cultured NECs grown in submersion, in the region where cells contact the underlying extracellular matrix (Fig. 3). Similar to its distribution in cultured HEKs, BP180 is distributed in punctate complexes consistent with localization to hemidesmosomes.
Figure 3.
Immunofluorescent localization of BP180 in human epidermal keratinocytes and nasal epithelial cells (A and C, respectively) viewed with confocal microscopy, with the focal plane being at the site of cell-substrate interaction. (B,D) Phase-contrast images of the cells.
Distribution of BP180 in nasal epithelium
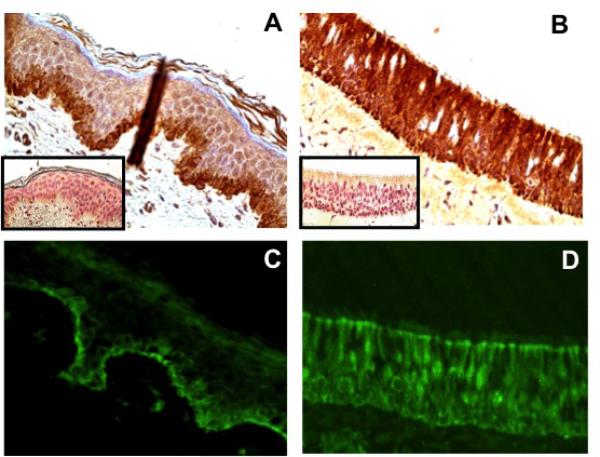
We compared the distribution of BP180 in normal nasal epithelium to that in normal human skin using immunohistochemistry and immunofluorescence. In human skin we found the expected distribution of BP180, with staining localized to the basal keratinocytes and basement membrane (Fig. 4). In nasal epithelium, however, we found diffuse staining present throughout all layers of the epithelium. These results suggest that BP180 is distributed very differently in pseudostratified nasal epithelium versus skin, and may thus serve a different role aside from cellular adhesion to the basement membrane.
Figure 4.
BP180 expression in skin and control nasal epithelium. In skin, localization of BP180 to the basement membrane is demonstrated by immunohistochemistry and immunofluorescence (A and C, respectively). In control uncinate tissue, diffuse localization of BP180 throughout all layers of the epithelium is seen (B and D). The corresponding immunohistochemistry isotype controls are shown in the lower left-hand corner of each image. Skin and nasal tissue samples were assessed for autofluorescence, which was negative (results not shown).
Anti-BP180 autoantibodies in the serum of patients with CRS
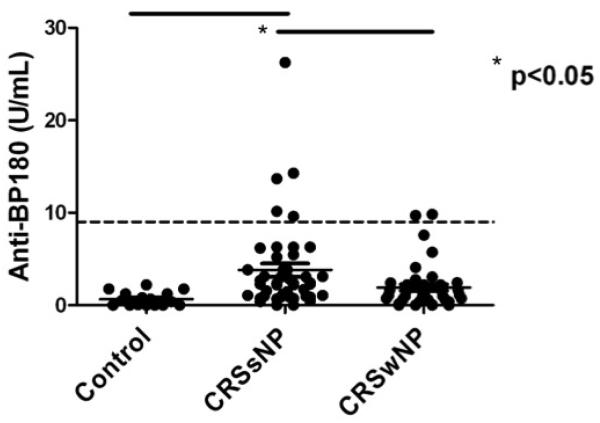
We used a commercially available ELISA (MBL Diagnostics, Nagoya, Japan), routinely used to diagnose bullous pemphigoid, to screen the serum of patients with CRS for the presence of systemic anti-BP180 autoantibodies. Interestingly, we found an elevated level of anti-BP180 autoantibodies in the serum of patients with CRS (Fig. 5). There was a significantly higher level of circulating anti-BP180 autoantibodies in CRSsNP compared with CRSwNP and normal controls (p<0.05). Even more surprising, we found that several patients actually tested positive for bullous pemphigoid according to the kit’s specifications, despite having no clinical manifestations of disease. However, when we assessed ELISA-positive samples for specific antibodies using indirect immunofluorescence on monkey esophagus, the serum showed no characteristic binding along the basal surface (data not shown).
Figure 5.
Serum levels of anti-BP180 autoantibodies in patients with CRS and normal controls, measured in U/mL. There was a significantly elevated level of serum anti-BP180 in CRSsNP compared to CRSwNP and normal controls. The dotted line represents the cut-off value of 9U/mL, above which the ELISA is considered positive for bullous pemphigoid.
CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps
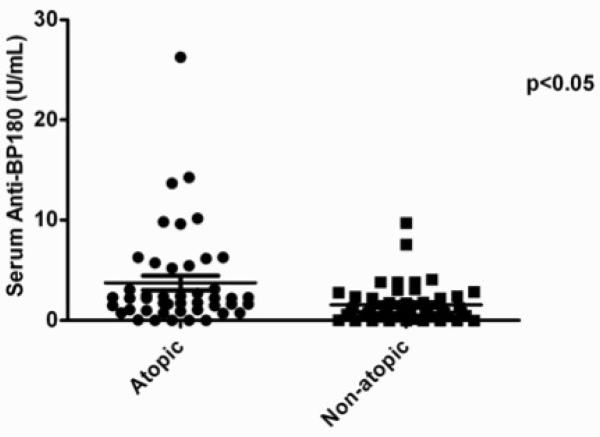
In analyzing the clinical and laboratory correlates of serum anti-BP180 autoantibody levels, we found no correlation with total IgG (R2 = 0.0227), age (R2 = 0.0001), gender (p = 0.381) or asthma status (p = 0.666). There was, however, a significant correlation between serum anti-BP180 autoantibody levels and atopy, defined as at least one positive skin test response, with atopic patients having significantly higher levels of serum anti-BP180 than non-atopic patients (p < 0.05) (Fig. 6). Overall, 13.3% of atopic patients with CRS tested tested positive for bullous pemphigoid using the commercially available ELISA assay.
Figure 6.
Serum levels of anti-BP180 autoantibodies by atopic status. There was a significant elevation of serum anti-BP180 in patients with atopy compared to nonatopic patients.
Discussion
The goal of this study was to characterize the expression and distribution of BP180 in NECs and nasal tissue, and to assess for evidence of systemic autoimmunity against this antigen in CRS. We first performed Western blot analyses and real-time PCR on whole cell extracts of cultured NECs, and confirmed that BP180 is highly expressed at both the protein and mRNA level within the cells. We then used immunofluorescence and confocal microscopy to demonstrate that BP180 localizes to the basal surface of cultured, submerged NECs in a punctate distribution, as it does in keratinocytes and bronchial epithelial cells11. This implies that BP180 likely plays a similar role in the formation of hemidesmosomes and anchoring of NECs to the basement membrane. However, we next performed immunofluorescence and immunohistochemistry on nasal tissue, and found that BP180 is diffusely distributed throughout all layers of the epithelium. This is in contrast to the linear distribution of BP180 along the basement membrane that is seen in human skin, and suggests that BP180 may serve a different role and function in the nose in addition to that of epithelial anchoring. Finally, we previously showed an increased level of anti-BP180 autoantibodies in nasal polyp tissue5. Interestingly, we also found that a significant percentage of CRS patients have increased levels of systemic anti-BP180 autoantibodies. These antibodies were judged to be nonpathogenic by the indirect immunofluorescence assay used to confirm a diagnosis of bullous pemphigoid, and therefore may recognize a distinct epitope or represent a distinct isotype from the anti-BP180 autoantibodies classically associated with bullous pemphigoid.
BP180 is known to be a critical structural adhesion protein in skin7. In bullous pemphigoid, autoantibodies against BP180 lead to separation of the stratified epithelium from the underlying basement membrane, resulting in the characteristic subepidermal blisters seen in the disease8,9,10 However, previous studies have also established that the hemidesmosome has many roles beyond that of an anchoring complex. Components of the hemidesmosome have been shown to transduce extracellular signals and thereby regulate cell motility, differentiation, proliferation and even apoptosis20. Recent studies have further identified BP180 as a critical component necessary for the activation of these signaling pathways21. Aberrant expression of BP180 has been demonstrated in dysplastic and cancerous epithelial lesions, where distribution of BP180 is seen diffusely throughout all layers of the epithelium22,23. It is therefore possible that the diffuse distribution of BP180 in nasal epithelium reflects altered properties of cell migration and differentiation compared with other epithelial tissues. A unique distribution and function of BP180 in nasal tissue may also explain why there are no known nasal manifestations of bullous pemphigoid.
It has been previously demonstrated that pathogenic anti-BP180 autoantibodies in bullous pemphigoid bind the NC16a noncollagenous domain of the BP180 protein, and it is this epitope that is the target antigen in the commercially available ELISA used for diagnosis24. Sakuma-Oyama et al. tested the sensitivity and specificity of the ELISA used in this study, and found that using a cut-off value of 9U/mL was associated with a sensitivity of 89% and a specificity of 98%18. Only 3% of normal subjects in this study falsely tested positive for bullous pemphigoid, which is in keeping with the 1.2% false positive rate reported by the manufacturer. These results are echoed by those of Wieland et al, who assessed the prevalence of systemic anti-BP180 antibodies in the normal population25. Again, only 3% of the study population falsely tested positive for bullous pemphigoid. Our study found that 13.3% of atopic patients with CRS tested positive for bullous pemphigoid, despite having no systemic manifestations of the disease. All patients that tested positive by ELISA were found to be negative by indirect immunofluorescence, demonstrating that these circulating antibodies are not pathologic in the traditional sense. To our knowledge, ours is the first study to demonstrate significantly elevated levels of circulating anti-BP180 autoantibodies that bind the NC16a noncollagenous domain in a population without history of autoimmune blistering disease.
Previous studies in our lab have demonstrated that increased levels of B-cell attractant chemokines are present in nasal polyp tissue26. B-cell accumulation in CRSwNP is associated with increased tissue concentrations of IgG, IgA and IgE antibodies compared with normal controls, but these increases are not reflected the systemic circulation27,28. It has also been determined that local B-cell class switch recombination to IgD occurs constitutively in nasal mucosa and evidence for class switching to IgE can be found in the nasal mucosa of patients with allergic rhinitis, as well as in the bronchial mucosa of patients with asthma and in the esophageal mucosa of patients with eosinophilic esophagitis29,30,31,32. Furthermore, local IgE antibodies against Altenaria can be found at elevated levels in the homogenates of nasal polyps without associated elevations in serum28. Similarly, our recent report demonstrated local production of IgG and IgA anti-dsDNA antibodies in nasal polyps, in addition to increased levels of anti-BP180 autoantibodies5. This local autoreactivity is unlikely to correlate with a systemic autoimmune response, given that our previous observations of local autoantibodies were confined to the CRSwNP population, and given the lack of systemic manifestations of autoimmunity in patients with CRS.
The systemic elevation of anti-BP180 autoantibodies seen in CRS appears associated with atopy but is of unclear clinical significance as these antibodies are not classically pathologic, resulting in clinical bullous pemphigoid or a specific staining pattern on indirect immunofluorescence. It is possible that atopy may trigger the development of autoreactivity due to epithelial damage and liberation of epitopes. Alternatively, it has been shown that nasal mucosa and tonsils contain resident IgD+IgM-B-cells that produce immunoglobulins that are intrinsically poly- and autoreactive even in normal, healthy individuals32,33. Thus, these B-cells have been proposed as a “sink” for autoreactive B-cells and a nasal inflammatory process may result in spillover autoreactive immunoglobulins into the systemic circulation. Studies by Faller et al have described a similar phenomenon in H. pylori gastritis, in which a subset of patients was found to have increased levels of serum anti-gastric autoantibodies that correlated with the degree of gastric mucosal atrophy34. Further investigation is thus necessary to define the pathologic implications of anti-epithelial autoantibodies in CRS.
Due to the low prevalence of systemic anti-BP180 autoantibodies in CRS, and the failure of these antibodies to bind the basement membrane in studies of indirect immunofluorescence, it is unlikely that antibodies against this epitope will be of value for diagnosis. It is, however, important to note that a subset of patients with CRS may have elevated levels of circulating autoantibodies, and may falsely test positive for autoimmune disorders such as bullous pemphigoid. The presence of autoreactive antibodies to BP180 in CRS patients raises questions as to whether yet undescribed autoantibodies directed against more specific nasal antigens may be present or detected at higher levels in patients with CRS, and might possibly play a role in the pathogenesis of disease. Experimental models for autoimmune diseases, such as systemic lupus erythematosus, propose that cellular damage or failure to clear self antigens leads to the breakdown of tolerance and generation of autoimmunity35. The presence of systemic, class-switched, self-reactive antibodies against BP180, a critical anchoring protein expressed in nasal mucosa, suggests that similar processes may occur in CRS. However, further studies are needed to answer these questions and to determine whether the systemic autoantibodies detected in CRS play have implications for the efficacy of existing therapies or the customization of new therapies for treating specific CRS patients.
Conclusion
BP180, a critical anchoring protein in keratinocytes, is highly expressed in nasal epithelium and likely serves a similar function in cellular anchoring to the basement membrane. However, its distribution in nasal tissue is more diffuse throughout the pseudostratified epithelial layer. Thus, BP180 is a relevant epithelial antigen for the local autoantibody response seen in CRSwNP. Surprisingly, non-pathologic anti-BP180 antibodies were elevated in CRS patients, although this association may be linked to atopy. The role of anti-epithelial antibodies in CRS needs further investigation.
Acknowledgments
Financial disclosure: Supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute RO1 HL78860, National Institutes of Health/National Institute of Allergy and Infectious Diseases RO1 AI072570 and the Ernest S. Bazley grant to Northwestern Memorial Hospital and Northwestern University (R.P.S.); the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases 1K99AR060242 (K.J.H.); the National Institutes of Health/National Institute of Deafness and Communications Disorders 1K23DC012067 and the Triological Society (B.K.T.), and the Department of Otolaryngology, Northwestern University Feinberg School of Medicine (B.K.T. and J.S.J.).
Abbreviations
- BAFF
B-cell activating factor of the TNF family
- CRS
Chronic rhinosinusitis
- CRSsNP
Chronic rhinosinusitis without nasal polyps
- CRSwNP
Chronic rhinosinusitis with nasal polyps
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–1392. 1392, e1381–1382. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters AT, Kato A, Zhang N, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e310. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–1206. e1191. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Franzke CW, Bruckner P, Bruckner-Tuderman L. Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem. 2005;280:4005–4008. doi: 10.1074/jbc.R400034200. [DOI] [PubMed] [Google Scholar]
- 8.Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- 9.Mutasim DF, Takahashi Y, Labib RS, Anhalt GJ, Patel HP, Diaz LA. A pool of bullous pemphigoid antigen(s) is intracellular and associated with the basal cell cytoskeleton-hemidesmosome complex. J Invest Dermatol. 1985;84:47–53. doi: 10.1111/1523-1747.ep12274684. [DOI] [PubMed] [Google Scholar]
- 10.Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–1235. [PubMed] [Google Scholar]
- 11.Michelson PH, Tigue M, Jones JC. Human bronchial epithelial cells secrete laminin 5, express hemidesmosomal proteins, and assemble hemidesmosomes. J Histochem Cytochem. 2000;48:535–544. doi: 10.1177/002215540004800411. [DOI] [PubMed] [Google Scholar]
- 12.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richer SL, Truong-Tran AQ, Conley DB, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–234. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehgal BU, DeBiase PJ, Matzno S, et al. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poposki JA, Uzzaman A, Nagarkar DR, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81. e74. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 18.Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126–131. doi: 10.1111/j.1365-2133.2004.06082.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224–232. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- 20.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamill KJ, Hopkinson SB, DeBiase P, Jones JC. BPAG1e maintains keratinocyte polarity through beta4 integrin-mediated modulation of Rac1 and cofilin activities. Mol Biol Cell. 2009;20:2954–2962. doi: 10.1091/mbc.E09-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada T, Endo R, Tsukagoshi K, et al. Aberrant expression of a hemidesmosomal protein, bullous pemphigoid antigen 2, in human squamous cell carcinoma. Lab Invest. 1996;75:589–600. [PubMed] [Google Scholar]
- 23.Parikka M, Kainulainen T, Tasanen K, Vaananen A, Bruckner-Tuderman L, Salo T. Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. J Histochem Cytochem. 2003;51:921–929. doi: 10.1177/002215540305100707. [DOI] [PubMed] [Google Scholar]
- 24.Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol. 1993;151:5742–5750. [PubMed] [Google Scholar]
- 25.Wieland CN, Comfere NI, Gibson LE, Weaver AL, Krause PK, Murray JA. Anti-bullous pemphigoid 180 and 230 antibodies in a sample of unaffected subjects. Arch Dermatol. 2010;146:21–25. doi: 10.1001/archdermatol.2009.331. [DOI] [PubMed] [Google Scholar]
- 26.Patadia M, Dixon J, Conley D, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:11–16. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–1847. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 28.Sabirov A, Hamilton RG, Jacobs JB, Hillman DE, Lebowitz RA, Watts JD. Role of local immunoglobulin E specific for Alternaria alternata in the pathogenesis of nasal polyposis. Laryngoscope. 2008;118:4–9. doi: 10.1097/MLG.0b013e3181567a7a. [DOI] [PubMed] [Google Scholar]
- 29.Smurthwaite L, Durham SR. Local IgE synthesis in allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2002;2:231–238. doi: 10.1007/s11882-002-0024-z. [DOI] [PubMed] [Google Scholar]
- 30.Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Xu W, Wilson M, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng NY, Wilson K, Wang X, et al. Human immunoglobulin selection associated with class switch and possible tolerogenic origins for C delta class-switched B cells. J Clin Invest. 2004;113:1188–1201. doi: 10.1172/JCI20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faller G, Steininger H, Eck M, Hensen J, Hann EG, Kirchner T. Antigastric autoantibodies in Helicobacter pylori gastritis: prevalence, in-situ binding sites and clues for clinical relevance. Virchows Arch. 1996;427:483–486. doi: 10.1007/BF00199508. [DOI] [PubMed] [Google Scholar]
- 35.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–8. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]