Abstract
Objective
To investigate the prevalence of polycystic ovary syndrome (PCOS) in adolescents and its association with obesity.
Design
Cross-sectional study using electronic medical records.
Setting
Integrated health care delivery system in Southern California.
Patients
Adolescents aged 15–19 years (n= 137,502).
Interventions
None.
Main Outcome Measures
PCOS diagnosed or defined according to NIH criteria.
Results
The prevalence of a confirmed diagnosis of PCOS was 0.56%, and increased to 1.14% when undiagnosed cases with documented symptoms qualifying for PCOS according to NIH criteria were included. Compared to normal/underweight girls, the ORs (95% CI) for confirmed PCOS diagnosis were 3.85 (3.04–4.88), 10.25 (8.16–12.84) and 23.10 (18.66–28.61) for overweight, moderately obese, and extremely obese adolescents, respectively (P-trend<0.001) after adjusting for potential confounders. When adolescents with two or more supportive diagnoses were included (diagnosed and undiagnosed PCOS-NIH) the ORs (95% CI) for PCOS-NIH by weight class were significantly attenuated to 2.95 (2.53–3.44), 6.73 (5.78–7.83), and 14.65 (12.73–16.86) for overweight, moderately obese, and extremely obese adolescents, respectively.
Conclusions
Overweight and obesity were associated with higher odds of PCOS in adolescents. Studies based solely on diagnosis codes may underestimate the prevalence of PCOS and overestimate the magnitude of the association between obesity and PCOS.
Keywords: Obesity, epidemiology, body weight, childhood, adolescence, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a very common endocrine disorder that is present in approximately 7% of reproductive-age women (1). It is a heterogeneous syndrome that usually presents during adolescence and is characterized by features of anovulation (amenorrhea, oligomenorrhea, irregular menstrual cycles) combined with symptoms of androgen excess (hirsutism, acne, alopecia) (2). In the U.S., PCOS is the single most common endocrine cause of annovulatory infertility and a major risk factor for the metabolic syndrome and type 2 diabetes (3, 4).
Population-based studies investigating the prevalence of PCOS in adolescents are scant (5) with an estimated prevalence between 0.8% based on clinical data from the U.S. (6) and 3% based on survey data assessing PCOS symptoms in Iran (7). In adults, the prevalence of PCOS varies slightly by race with non-Hispanic White women at the highest risk (6).
PCOS occurs in both normal-weight and overweight women, and obesity is a frequent comorbidity that often precedes the development of PCOS (8). There is sufficient evidence that excessive body weight amplifies the clinical severity of PCOS and increases the risk of several metabolic and cardiovascular complications associated with PCOS, such as insulin resistance, hyperlipidemia, hypertension, and subclinical atherosclerosis (9). Moreover, the prevalence of impaired glucose tolerance (IGT) in obese young women with PCOS has estimated to be as high as 30–40%, with an additional 5–10% having diabetes (10, 11).
The recent childhood obesity epidemic (12) including an increasing proportion of children becoming extremely obese (13–15) give rise to numerous concerns about short and long-term health consequences of obesity in youth and young adults - including concerns about PCOS, given the role of obesity in its etiology. The prevalence of PCOS in adolescents with obesity has not been reported.
The goal of the present study is to provide population-based estimates for the prevalence of PCOS in adolescent girls based on clinical data of PCOS diagnosis and symptoms consistent with PCOS. We also evaluated the association between body mass index (BMI)-for-age and PCOS in a large, population-based cohort of adolescent girls enrolled in an integrated prepaid health plan in Southern California.
Materials and Methods
Study design and subjects
Kaiser Permanente Southern California (KPSC) provides health care service to over 3.4 million members including almost 1.3 million children and adolescents 2–19 years of age. This reflects about 16% of the underlying total population of the service area (16). KPSC members are largely representative of the population in the service area according to most demographic and socio-economic factors (16). For this crosssectional study, we used a subset of patients enrolled in a large population-based cohort study, the KPSC Children’s Health Study 2007–2009 (n= 920,034) which has been described in detail elsewhere(17). Briefly, subjects in this study were members of a pre-paid integrated health plan between January 1, 2007 and December 31, 2009. Members received their care in medical offices and hospitals owned by KPSC. Comprehensive electronic health records are used to capture all aspects of clinical care. The study protocol was reviewed and approved by the Institutional Review Board of KPSC.
For the present study, we identified 144,426 adolescent girls who were 15 years of age and older at study inclusion. After exclusion of pregnant members (n=6,775), 137,651 adolescent females ages 15 through 19 years were identified. We then excluded patients with congenital adrenal hyperplasia (n=30), Cushing’s syndrome (n=23), adrenal, ovarian and pituitary cancer (n=6), and prolactinoma or hyperprolactenemia (defined as at least one prolactin >60 ng/dL) (n=90), resulting in a final study sample of 137,502 adolescent females.
Outcome ascertainment
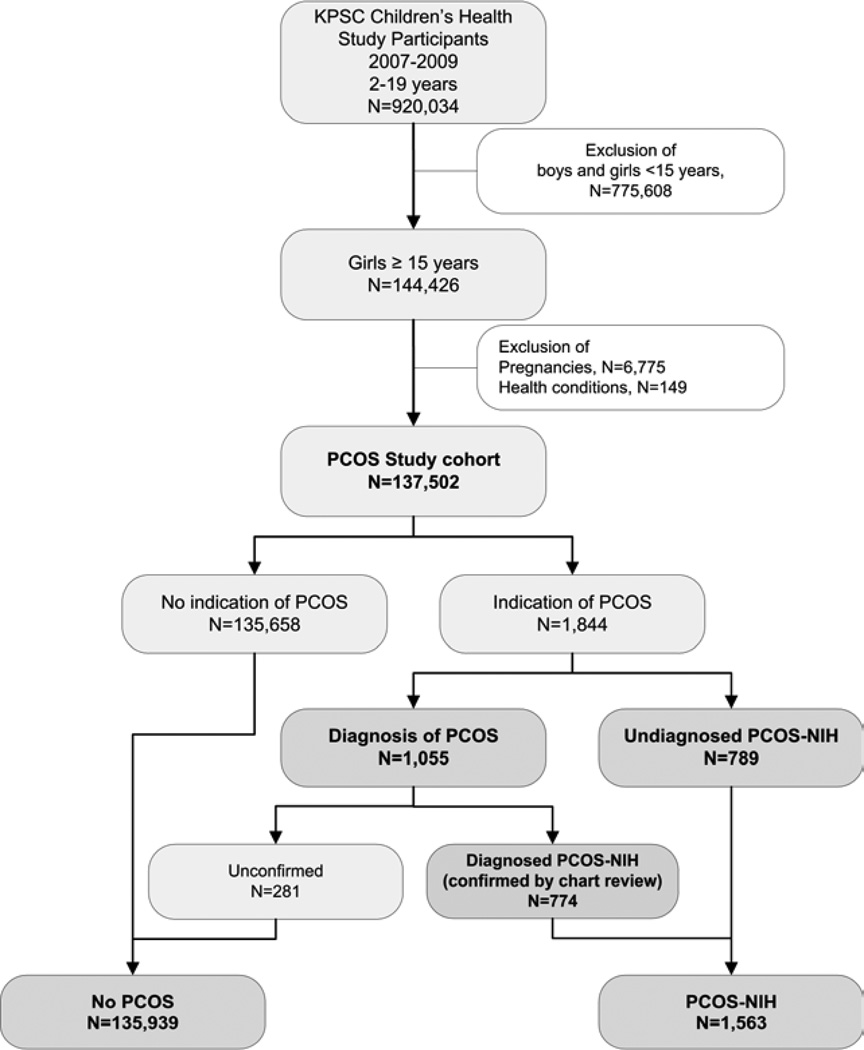
According to the 1990 NIH criteria (2), PCOS is defined as having 1) hyperandrogenism and/or hyperandrogenemia, 2) oligoovulation, and 3) exclusion of known disorders. To identify adolescents with PCSO according to NIH criteria and to allow comparison to previously published estimates of the prevalence of PCOS, we used several approaches to identify all adolescents with PCOS using information from electronic medical records (Figure 1).
Figure 1.
Study flow chart
First, we identified adolescents who received one or more diagnoses of PCOS [International Classification of Diseases, Ninth Revision (ICD-9) code 256.4] from all inpatient and outpatient encounters since enrollment into the health plan (n=1,055; diagnosed PCOS group).
Second, among these patients with a diagnosis of PCOS (n=1,055), a validation of PCOS diagnosis was performed by confirming diagnosis of PCOS from physician’s notes, laboratory data, and pharmacy records in the electronic medical record. Trained research staff validated all PCOS diagnoses by confirming that at least two of the following criteria were present: clinical and/or biochemical hyperandrogenism, oligoanovulation, and exclusion of other causes or these conditions. An additional review of different PCOS phenotypes was not performed because ultrasound results were only available in a subgroup (n=411). The diagnosis for PCOS meeting NIH criteria was confirmed in 774 (73.4%) patients with a PCOS diagnosis. Unconfirmed cases (n=289) were considered as not having PCOS.
Third, we performed additional searches to identify potentially undiagnosed or uncoded patients with PCOS using diagnosed symptoms consistent with 1990 NIH criteria (2) which include 1) chronic oligoanovulation and 2) clinical and/or signs of hyperandrogenism. Hyperandrogenism was defined by a diagnosis of hirsutism [ICD-9 code 704.1], other ovarian hyperfunction [ICD-9 code 256.1], or laboratory evidence of elevated testosterone. Chronic oligoanovulation was defined by a diagnosis of amenorrhea [ICD-9 code 626.0], oligomenorrhea [ICD-9 code 626.1], or irregular menstrual cycle [ICD-9 code 626.4]. Through this search, we identified an additional 789 adolescents who met the NIH definition of PCOS.
For the main analyses, all cohort members who were included in the study (n = 137,502) were categorized into one of two groups: adolescents with PCOS according to NIH criteria (PCOS-NIH, n = 1,563) and patients without an indication of PCOS according to NIH criteria (n = 135,939, Figure 1). Adolescents in the PCOS-NIH group were further stratified into diagnosed (n = 774) and undiagnosed PCOS-NIH (n = 789).
Body weight and height
Body weight and height were extracted from electronic health records, and body mass index (BMI) was calculated as weight (kilograms) divided by the square of the height (meters). For patients enrolled into the study in years 2007, 2008, and 2009, the median BMI-for-age of all encounters in the year of study enrollment for a patient was used for analysis. Based on a validation study including 15,000 patients with 45,980 medical encounters, the estimated error rate in body weight and height data was <0.4% (18).
Overweight and obesity for children and adolescents are defined based on the sex-specific BMI-for-age growth charts developed by the Centers of Disease Control and Prevention and the World Health Organization definitions for overweight and obesity in adults (13, 19, 20). Adolescents were categorized as underweight (BMI-forage <5th percentile), normal weight (BMI-for-age ≥ 5th and < 85th percentile), overweight (BMI-for-age ≥85th percentile or a BMI ≥25 kg/m2), moderately obese (BMI-for age ≥95th percentile or a BMI ≥30 kg/m2), and extremely obese (BMI-for age ≥1.2 × 95th percentile or a BMI ≥35 kg/m2).
Race/ethnicity and socioeconomic status
Race and ethnicity information were obtained from health plan administrative records and birth certificates. Race/ethnicity were categorized as non-Hispanic White, Hispanic White, Black, Asian or Pacific Islander, other or multiple race/ethnicity, and unknown due to missing information based on health plan administrative records and birth certificate information. For subjects with unknown race/ethnicity (31.7%), administrative record information was supplemented by an imputation algorithm based on surname lists and address information derived from the U.S. Census Bureau (21–23). The specificity and positive predictive values were >98% for all races/ethnicities (24).
Two measures of socioeconomic status were used: neighborhood education and participation in Medi-Cal (Medicaid) or other state-subsidized health care coverage programs. Neighborhood education was estimated on the basis of the linkage of health plan members’ addresses via geocoding (Geospatial Entity Object Coding) with U.S. Census block data (16, 25).
Oral contraceptive use
Information on oral contraceptive use was extracted from pharmacy records and was defined as any dispensed prescription of oral contraceptives within 24 months prior to the study enrollment.
Statistical analysis
Differences in the distribution of basic demographics for adolescent girls with and without diagnosed PCOS were assessed using chi-squared tests for categorical variables and independent two-sided Student’s t tests for continuous variables. Prevalence of PCOS and corresponding 95% confidence intervals (CI) were calculated. Multiple logistic regression models were used to examine the association between weight class and PCOS adjusted for age, race/ethnicity, low neighborhood education (yes/no), and Medi-Cal use status (yes/no). The outcome variables were PCOS-NIH as well as subgroups diagnosed and undiagnosed PCOS-NIH. The main exposure variable was weight class (categorized as normal/underweight, overweight, moderately obese, and extremely obese). We estimated odds ratios and their 95% CI for each weight class category with normal/underweight as the reference group and preformed a linear trend test. We also evaluated the potential interaction by race/ethnicity by performing logistic regression models for each race/ethnicity separately and including the appropriate interaction term in the multiple logistic regression model and stratification if applicable. Sensitivity analyses were performed to control for the potential effect of oral contraceptives by 1) adjusting for oral contraceptive use and 2) excluding girls without PCOS who are taking oral contraceptives from the analysis. Because the estimates were essentially unaltered, oral contraceptive use was not included in the main analysis. All analyses were conducted using STATA version 11 (College Station, TX). P values <0.05 were considered to be significant.
Results
Cases of PCOS and supporting diagnoses
We initially identified 1,055 adolescents with at least one diagnosis of PCOS from all inpatient and outpatient encounters since enrollment into the health plan (Figure 1). A total of 774 (73.4%) patients with a PCOS diagnosis were confirmed by manual chart review according to NIH criteria (referred to as “diagnosed PCOS-NIH”). The remaining patients with a diagnosis of PCOS but no evidence of PCOS in the medical record (including supportive diagnosis, physician’s notes, and laboratory data, n =281) were assigned to patients without PCOS. A total of 408 (52.7%) diagnosed PCOS-NIH patients received two or more separate inpatient or outpatient diagnoses of PCOS.
Most patients with diagnosed PCOS-NIH patients (n=774) received at least one other additional diagnosis code supportive of PCOS (89.4%), about half of the patients (53.5%) received additional diagnoses indicative of both clinical and/or biochemical hyperandrogenism and oligoanovulation. Of the 774 patients with diagnosed PCOS-NIH (Table 1), an additional diagnosis of hyperandrogenism and/or oligoanovulation was present in the majority of patients (69.6% and 67.6%, respectively).
Table 1.
Demographic and clinical characteristics of adolescent girls (N=137,502)
| PCOS-NIH | ||||||
|---|---|---|---|---|---|---|
| Characteristic | No PCOS-NIH | All PCOS-NIH | Diagnosed PCOS-NIH |
Undiagnosed PCOS-NIH |
||
| n | 135,939 | 1,563 | 774 | 789 | ||
| Age (years, mean ± SD ) | 17.0 ± 1.5 | 17.7 ± 1.4a | 17.8 ± 1.4a | 17.5 ± 1.4a | ||
| Race/ethnicity (n,%) | a | a | ab | |||
| Non-Hispanic White | 35,748 (26.3) | 341 (21.8) | 195 (25.2) | 146 (18.5) | ||
| Black | 11,289 (8.3) | 146 (9.3) | 55 (7.1) | 91 (11.5) | ||
| Hispanic White | 61,317 (45.1) | 809 (51.8) | 393 (50.8) | 416 (52.7) | ||
| Asian/Pacific Islander | 7,716 (5.7) | 84 (5.4) | 40 (5.2) | 44 (5.6) | ||
| Other/Multiple races | 2,565 (1.9) | 36 (2.3) | 15 (1.9) | 21 (2.7) | ||
| Unknown | 17,304 (12.7) | 147 (9.4) | 76 (9.8) | 71 (9.0) | ||
| Neighborhood education (n,%) | a | a | ab | |||
| Less than high school | 37,247 (27.4) | 491 (31.4) | 227 (29.3) | 264 (33.5) | ||
| High school graduate | 29,227 (21.5) | 338 (21.6) | 167 (21.6) | 170 (21.6) | ||
| Some college or associate degree | 41,869 (30.8) | 456 (29.2) | 234 (30.2) | 222 (28.2) | ||
| Bachelor degree or higher | 27,596 (20.3) | 278 (17.8) | 146 (18.8) | 132 (16.7) | ||
| Neighborhood income (n,%) | a | a | a | |||
| < $25,000 | 25,557 (18.8) | 327 (20.9) | 156 (20.2) | 170 (21.5) | ||
| $25,000 to $49,999 | 31,810 (23.4) | 383 (24.5) | 187 (24.1) | 197 (25.0) | ||
| $50,000 to $74,999 | 26,508 (19.5) | 306 (19.6) | 152 (19.6) | 154 (19.5) | ||
| $75,000 to $99,999 | 19,167 (14.1) | 208 (13.3) | 104 (13.5) | 104 (13.2) | ||
| $100,000 or more | 32,897 (24.2) | 339 (21.7) | 175 (22.6) | 164 (20.8) | ||
| Beneficiary of Medi-Cal or other state subsidized support programs (n,%) | 9,806 (7.2) | 112 (7.2) | 50 (6.5) | 62 (7.9) | ||
| Weight class (n,%) | a | a | ab | |||
| Underweight | 2,957 (2.2) | 12 (0.8) | 3 (0.4) | 9 (1.1) | ||
| Normal | 83,004 (61.1) | 327 (20.9) | 122 (15.8) | 205 (26.0) | ||
| Overweight | 27,596 (20.3) | 329 (21.1) | 155 (20.0) | 174 (22.1) | ||
| Moderately obese | 13,451 (9.9) | 356 (22.8) | 192 (24.8) | 164 (20.8) | ||
| Extremely obese | 8,931 (6.6) | 539 (34.5) | 302 (39.0) | 237 (30.0) | ||
| Diagnosis of hirsutism (n,%) | 366 (0.3) | 413 (26.4)a | 199 (25.7)a | 214 (27.1)a | ||
| Diagnosis of ovarian hyperfunction (n,%) | 76 (0.06) | 253 (16.2)a | 77 (10.0)a | 176 (22.3)ab | ||
| Elevated testosterone (n,%) | 326 (0.2) | 1,048 (67.1)a | 437 (56.5)a | 611 (77.4)ab | ||
| Diagnosis of hyperandrogenismc (n,%) | 630 (0.5) | 1,328 (85.0)a | 539 (69.6)a | 789 (100.0)ab | ||
| Diagnosis of oligoanovulationd (n,%) | 10,234 (7.5) | 1,312 (83.9)a | 523 (67.6)a | 789 (100.0)ab | ||
| Diagnosis of alopecia (n,%) | 1,545 (1.1) | 57 (3.7)a | 36 (4.7)a | 21 (2.7)ab | ||
Adolescents were characterized into three mutually exclusive PCOS categories: having a PCOS diagnosis that was confirmed by clinical and/or biochemical hyperandrogenism and oligoanovulation, or by having a diagnosis of two PCOS symptoms (oligoanovulation and clinical and/or biochemical hyperandrogenism) but without a PCOS diagnosis, and having no diagnosis of PCOS or PCOS symptoms.
P<0.05 vs. no PCOS.
P<0.05 vs. diagnosed PCOS-NIH diagnosis
Defined as diagnosis of hirsutism [ICD-9 code 704.1], other ovarian hyperfunction [ICD-9 code 256.1] or laboratory evidence of elevated testosterone.
Defined as a diagnosis of amenorrhea [ICD-9 code 626.0], oligomenorrhea [ICD-9 code 626.1] or irregular menstrual cycle [ICD-9 code 626.4].
Additional searches were performed to identify potentially undiagnosed patients with PCOS using a search algorithm for patients with a prevalent diagnosis of hyperandrogenism and oligoanovulation. Through this search, an additional 789 adolescents (referred to as “undiagnosed PCOS-NIH”) were identified who met these criteria, resulting in 1,563 adolescents with either confirmed diagnosis of PCOS or PCOS with related symptomatology (together referred to as “PCOS-NIH”).
Prevalence of diagnosed and undiagnosed PCOS-NIH
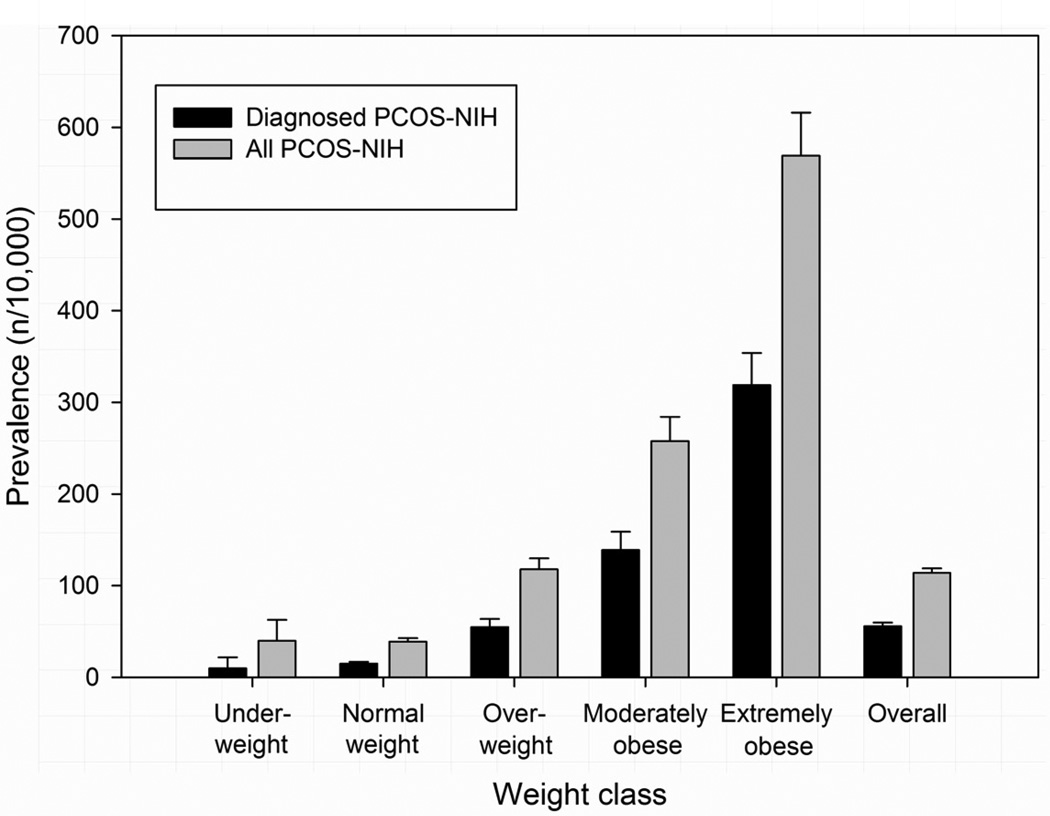
The prevalence of diagnosed (and confirmed) PCOS-NIH among adolescent girls aged 15–19 years was 0.56% (95% CI: 0.52%–0.60%). If all cases with a diagnosis of PCOS were included, regardless of manual confirmation by chart review, the prevalence of PCOS was 0.78% (95% CI: 0.72%–0.81%). The point prevalence of diagnosed PCOS-NIH varied greatly by weight class with 0.10% (0.01–0.22%) in underweight, 0.15% (0.12–0.17%) in normal weight, 0.55% (0.47–0.64%) in overweight, 1.39% (1.20–1.59%) in moderately obese, and 3.19% (2.84–3.54%) in extremely obese adolescent girls. When additional 789 adolescent girls with undiagnosed PCOS-NIH were included, the prevalence of PCOS was 1.14% (1.08%–1.19%) suggesting that a PCOS prevalence estimate based on diagnosed cases only (0.56%) is likely to be very conservative. Including all PCOS-NIH patients, the prevalence of PCOS was 0.40% (0.18–0.63%) in underweight, 0.39% (0.35–0.43%) in normal weight, 1.18% (1.05–1.30%) in overweight, 2.58% (2.31%–2.84%) in moderately obese, and 5.69% (5.23–6.16%) in extremely obese adolescent girls (Figure 2).
Figure 2.
Prevalence of diagnosed (black) and undiagnosed PCOS according to NIH criteria (grey) in adolescents aged 15 to 19 years of age.
Association between PCOS and weight class
The characteristics of adolescent girls aged 15–19 with and without PCOS-NIH, and for diagnosed versus undiagnosed PCOS-NIH are shown in Table 1. Girls with PCOS-NIH were more likely to be older (P<0.001). Adolescents with PCOS also included a slightly higher proportion of Hispanic Whites and lower proportion of Blacks.. Adolescents with PCOS-NIH were substantially more likely to be moderately or extremely obese than adolescents without PCOS-NIH (63.1 vs. 16.5%). Diagnosed hyperandrogenism and oligoanovulation were common in adolescents diagnosed with PCOS, 52.9% and 64.7% respectively. Alopecia was also significantly more prevalent in adolescents diagnosed with PCOS-NIH (4.2 vs. 1.1%; P<0.001).
After adjusting for potential confounders, the ORs (95% CI) for diagnosed PCOSNIH were 3.85 (3.04–4.88), 10.25 (8.16–12.84) and 23.10 (18.66–28.61) for overweight, moderately obese, and extremely obese adolescents, respectively, compared to normal/underweight adolescents (P-trend<0.001, Table 2). When adolescents with two or more supportive diagnoses were included (diagnosed and undiagnosed PCOS-NIH) the ORs (95% CI) for PCOS-NIH by weight class were significantly attenuated to 2.95 (2.53–3.44), 6.73 (5.78–7.83), and 14.65 (12.73–16.86) for overweight, moderately obese, and extremely obese adolescents, respectively. The association between weight class and PCOS-NIH varied marginally by race/ethnicity groups when unknown race/ethnicity was excluded although this interaction was not statistically significant (Pinteraction <=0.130 for both PCOS definitions).
Table 2.
Odds ratiosafor PCOS according to body weight class, overall and by race/ethnicity
| Weight Class | ||||||
|---|---|---|---|---|---|---|
| Underweight/Normal | Overweight | Moderately Obese | Extremely Obese | P-trendb | P-interactionc | |
| Diagnosed PCOS-NIH | ||||||
| Overall | 1.0 (reference) | 3.85 (3.04–4.88) | 10.25 (8.16–12.84) | 23.10 (18.66–28.61) | <0.001 | |
| By Race/Ethnicity | 0.133 | |||||
| Non-Hispanic White | 1.0 (reference) | 4.53 (2.89–7.11) | 13.72 (8.86–21.25) | 29.62 (19.58–44.81) | <0.001 | |
| Black | 1.0 (reference) | 2.79 (1.23–6.34) | 2.98 (1.15–7.73) | 10.14 (4.95–20.77) | <0.001 | |
| Hispanic White | 1.0 (reference) | 3.64 (2.56–5.17) | 10.55 (7.60–14.65) | 20.38 (14.85–27.97) | <0.001 | |
| Asian/Pacific Islander | 1.0 (reference) | 1.32 (0.44–3.95) | 7.27 (3.09–17.14) | 21.09 (9.66–46.05) | <0.001 | |
| Other/Multiple races | 1.0 (reference) | 2.49 (0.55–11.24) | 4.99 (1.10–22.72) | 12.03 (3.15–45.87) | <0.001 | |
| All PCOS-NIH | ||||||
| Overall | 1.0 (reference) | 2.95 (2.53–3.44) | 6.73 (5.78–7.83) | 14.65 (12.73–16.86) | <0.001 | |
| By Race/Ethnicity | 0.126 | |||||
| Non-Hispanic White | 1.0 (reference) | 2.87 (2.13–3.87) | 6.14 (4.48–8.43) | 14.28 (10.73–19.02) | <0.001 | |
| Black | 1.0 (reference) | 3.78 (2.29–6.34) | 3.78 (2.09–6.83) | 11.77 (7.36–18.82) | <0.001 | |
| Hispanic White | 1.0 (reference) | 2.76 (2.21–3.46) | 7.30 (5.91–9.01) | 13.76 (11.23–16.87) | <0.001 | |
| Asian/Pacific Islander | 1.0 (reference) | 2.46 (1.33–4.56) | 7.69 (4.22–14.01) | 17.32 (9.62–31.18) | <0.001 | |
| Other/Multiple races | 1.0 (reference) | 1.16 (0.37–3.62) | 4.62 (1.85–11.52) | 10.36 (4.52–23.72) | <0.001 | |
Odds ratios, 95% confidence intervals, and P-values estimated from multivariate logistic regression models that adjust for age (years), proportion of individuals with an education below high school in the neighborhood, and beneficiary status of Medi-Cal or other state subsidized support programs (yes or no). Overall estimates also adjust for race/ethnicity.
P-trend evaluated on categorical weight class variable using likelihood ratio test.
P-interaction evaluated using likelihood ratio test for race/ethnicity * weight class interaction term excluding unknown race/ethnicity from data.
Discussion
In this large, community-based population study, about 1 out of 200 adolescent girls aged 15–19 years old had a confirmed diagnosis of PCOS. The actual prevalence of PCOS is likely to be closer to 2 out of 200 adolescents - as suggested by the large number of adolescents with clinical features and phenotypes consistent with PCOS but without a diagnosis of PCOS.
Although PCOS is a common gynecologic condition with an estimated prevalence of 7% in women of reproductive age (1), it is often not diagnosed for several years (26, 27). Hence, reports investigating the prevalence of PCOS in adolescents are rare. In a population-based study in patients enrolled in a Northern California health plan, the prevalence of diagnosed PCOS in adolescents 15–19 years of age was 0.81% (6). In an Iranian study that screened school girls, the prevalence of PCOS regardless of diagnosis was estimated to be 3% based on a questionnaire assessing PCOS symptoms (7). In our population, the prevalence was 0.76% for diagnosed PCOS and 0.56% for diagnosed and manually confirmed PCOS. However, the prevalence of PCOS was 1.14% when combining diagnosed and undiagnosed PCOS with clinical features and phenotypes based on NIH criteria. Our study suggests that PCOS in adolescent girls is underdiagnosed.
Excessive body weight was significantly associated with PCOS. Interestingly, the association was strongest when only diagnosed cases of PCOS were included suggesting that obese girls are more likely than normal weight girls to receive a diagnosis by their care provider. The association between excessive body weight and PCOS was significantly attenuated when all patients of PCOS (regardless of diagnosis) were analyzed. This may be indicative of an overestimation of the strength of association between body mass and PCOS for studies based on a diagnosis of PCOS due to a large number of undiagnosed cases with lower body mass. In a similar study of Kaiser health plan members in Northern California, the odds ratio of being obese or extremely obese defined by a BMI ≥30 kg/m2 was 4.21 (95% CI: 3.96–4.47) for women with diagnosed PCOS compared to women without PCOS (6), and the odds ratios for obesity estimated from our study in adolescents were much higher. Findings from some small clinical studies suggest that the metabolic risk associated with PCOS is mostly due to obesity and that PCOS does not independently increase the metabolic risk of adolescents (28, 29). Obese adolescents or women may be more likely to receive a diagnosis of PCOS leading to a potential bias in studies based on diagnosis codes that may result in an overestimation of the association with obesity. On the other hand, women without obesity – and a potentially different metabolic risk - seem to be less likely to be diagnosed which may bias studies aiming to identify potential high metabolic risk phenotypes of PCOS if criteria other than diagnosis codes cannot be used.
Another finding of the study was that the association between body mass and PCOS varied marginally by race/ethnicity although the interaction was not statistically significant. Our results suggest that obesity may have a smaller impact on the development of PCOS in Black girls than in other races. These suggestions have to be interpreted carefully considering the cross-sectional nature of the data and require confirmation by prospective studies. However, the results may also be explained by difference in body composition, body fat distribution, and adipose tissue-related inflammation. Black women have a lower prevalence of PCOS overall but Black women with PCOS were more likely to be obese compared to non-Hispanic White women (6). Abdominal visceral adipose tissue has been identified as a better marker of metabolic health than body weight (30) and mouse models of PCOS suggest that adipose tissue and tissue-specific inflammation play a crucial role in the development of PCOS (31). Because White women tend to have significantly higher visceral fat than Black women (32), it can be speculated that differences in abdominal fat accumulation and subsequent inflammatory processes may explain the lower prevalence of PCOS and attenuated association between obesity and PCOS in Black women.
A strength of our study is the inclusion of a very large, ethnically diverse population who receive medical care within a large integrated health care delivery system in a seven-county region of Southern California. Therefore, these data are likely to be representative of a typical clinical population and reflect the burden of this disease within health care systems.
Underdiagnosis of mostly silent diseases is a concern. In contrast to systematic screening of selected populations to identify both diagnosed and undiagnosed cases, fewer adolescents would be classified within the context of clinical care where routine screenings are not performed. In order to address the issue of potential under diagnosis of PCOS, we expanded our case identification beyond the standard approach of using diagnosis codes alone and identified additional cases with diagnoses of clinical and/or biochemical hyperandrogenism and oligoanovulation. The classification of PCOS phenotypes, however, was limited because sufficient ultrasound, other diagnostic criteria, and their documentation were lacking. Ultrasound results are not required for the diagnosis and may not affect the decision on how to manage the condition. Hence, the decision to order an ultrasound is in the hands of the treating physician and often not performed. Our study suggests that underdiagnosis is a serious issue and that about half of patients with PCOS symptomatology remained undiagnosed. We also addressed the issue of misdiagnosis by indication, such as obesity, and manually reviewed and confirmed all patients with a diagnosis of PCOS. Only cases of PCOS with documented clinical features according to NIH criteria were included in our analysis.
Our study also benefits from measured weight and height from medical office visits as opposed to self-reported weight and height. All children were members of a large pre-paid managed care system, and standardized screening guidelines for weight and height in all children in the health plan were routine. However, no general calibration protocol for scales has been followed, data are manually entered into a computer system, and inaccurate measurements may occur because of seasonal differences in clothing or measurement taken with shoes.
The cross sectional design of this study does not allow causal conclusions, and as such, the interpretability of our findings is limited. Because both exposure and outcome have been simultaneously assessed, no conclusions pertaining to causality can be made. Therefore, results on PCOS and body weight must be interpreted with caution. The relationship between PCOS and obesity is bi-directional because obesity can trigger hyperandrogenism, which in turn triggers obesity (3). Obesity is also a driving factor in insulin resistance, which has been linked to anovulation, one of the symptoms of PCOS (3).
In summary, the present study shows that obesity, especially extreme obesity is significantly associated with PCOS. Our study also suggests that a high number of patients with PCOS symptomatology may remain undiagnosed and that the likelihood of receiving a diagnosis is higher in obese patients. As a result, future studies on PCOS in adolescent girls should consider including clinical information on PCOS symptomatology to supplement diagnosis codes.
Acknowledgements
The authors gratefully thank Julie Stern, Britta Amundsen, and Theresa Im for their outstanding support.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Disorders (NIDDK, R21DK085395) and Kaiser Permanente Direct Community Benefit Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no financial conflicts of interest to disclose.
REFERENCES
- 1.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 2.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 3.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes. 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN Group PCTS. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:66–71. doi: 10.1210/jc.2004-0229. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E. PCOS in adolescents. Best Pract Res Clin Obstet Gynaecol. 2010;24:173–183. doi: 10.1016/j.bpobgyn.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 7.Hashemipour M, Faghihimani S, Zolfaghary B, Hovsepian S, Ahmadi F, Haghighi S. Prevalence of polycystic ovary syndrome in girls aged 14–18 years in Isfahan. Iran. Horm Res. 2004;62:278–282. doi: 10.1159/000081842. [DOI] [PubMed] [Google Scholar]
- 8.Motta AB. The role of obesity in the development of polycystic ovary syndrome. Curr Pharm Des. 2012;18:2482–2491. doi: 10.2174/13816128112092482. [DOI] [PubMed] [Google Scholar]
- 9.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS. Diabetes prevalence and risk factors in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:99–109. doi: 10.1016/s0889-8545(05)70188-1. [DOI] [PubMed] [Google Scholar]
- 11.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diab Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index for age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 15.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157:26–31. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koebnick C, Langer-Gould AM, Gould MK, Chun CR, Iyer RL, Smith N. Sociodemographic Characteristics of Members of a Large, Integrated Health Care System: Comparison with US Census Bureau Data. Perm J. 2012;13:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koebnick C, Coleman KJ, Black MH, Smith N, Der-Sarkissian JK, Jacobsen SJ. Cohort Profile: The KPSC Children's Health Study, a population-based study of 920 000 children and adolescents in southern California. Int J Epidemiol. 2012;41:627–633. doi: 10.1093/ije/dyq252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith N, Coleman KJ, Lawrence JM, Quinn VP, Getahun D, Reynolds K. Body weight and height data in electronic medical records of children. Int J Pediatr Obes. 2010;5:237–242. doi: 10.3109/17477160903268308. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva: World Health Organization; 2000. Technical Report Series 894: Obesity: Preventing and managing the global epidemic. ISBN 92-4120894-5. [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 21.Bureau of the Census. Census 2000 surname list. Washington D.C: Bureau of Census; 2009. [Google Scholar]
- 22.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41:1482–1500. doi: 10.1111/j.1475-6773.2006.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Word DL, Perkins RC. Building a Spanish surname list for the 1990's - A new approach to an old problem. Washington, DC: US Bureau of the Census; 1996. Technical Working paper No. 13. [Google Scholar]
- 24.Smith N, Iyer RL, Langer-Gould A, Getahun DT, Strickland D, Jacobsen SJ. Health plan administrative records versus birth certificate records: quality of race and ethnicity information in children. BMC Health Serv Res. 2010;10:316. doi: 10.1186/1472-6963-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Petitti DB, Enger S. Limitations and potential uses of census-based data on ethnicity in a diverse community 4. Ann Epidemiol. 2004;14:339–345. doi: 10.1016/j.annepidem.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 26.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 27.Shannon M, Wang Y. Polycystic ovary syndrome: a common but often unrecognized condition. J Midwifery Womens Health. 2012;57:221–230. doi: 10.1111/j.1542-2011.2012.00161.x. [DOI] [PubMed] [Google Scholar]
- 28.Glueck CJ, Morrison JA, Friedman LA, Goldenberg N, Stroop DM, Wang P. Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism. 2006;55:508–514. doi: 10.1016/j.metabol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Rossi B, Sukalich S, Droz J, Griffin A, Cook S, Blumkin A. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–4786. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 31.Marino JS, Iler J, Dowling AR, Chua S, Bruning JC, Coppari R. Adipocyte Dysfunction in a Mouse Model of Polycystic Ovary Syndrome (PCOS): Evidence of Adipocyte Hypertrophy and Tissue-Specific Inflammation. PLoS One. 2012;7:e48643. doi: 10.1371/journal.pone.0048643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]