Abstract
Cross-sectional studies have found that obesity is associated with low intellectual ability and neuroimaging abnormalities in adolescence and adulthood. Some have interpreted these associations to suggest that obesity causes intellectual decline in the first half of the life course. We analyzed data from a prospective longitudinal study to test whether becoming obese was associated with intellectual decline from childhood to midlife. We used data from the ongoing Dunedin Multidisciplinary Health and Development Study, a population-representative birth cohort study of 1,037 children in New Zealand who were followed prospectively from birth (1972–1973) through their fourth decade of life with a 95% retention rate. Intelligence quotient (IQ) was measured in childhood and adulthood. Anthropometric measurements were taken at birth and at 12 subsequent in-person assessments. As expected, cohort members who became obese had lower adulthood IQ scores. However, obese cohort members exhibited no excess decline in IQ. Instead, these cohort members had lower IQ scores since childhood. This pattern remained consistent when we accounted for children's birth weights and growth during the first years of life, as well as for childhood-onset obesity. Lower IQ scores among children who later developed obesity were present as early as 3 years of age. We observed no evidence that obesity contributed to a decline in IQ, even among obese individuals who displayed evidence of the metabolic syndrome and/or elevated systemic inflammation.
Keywords: cognitive aging, intellectual decline, intelligence quotient, IQ decline, life course, longitudinal study, metabolic syndrome, obesity
Obesity is a global public health challenge and a leading cause of preventable death (1). Obesity is a well-established risk factor for both cardiovascular and metabolic diseases. Recent research has suggested that obesity may damage not only the body but also the brain.
Two streams of research have given rise to the hypothesis that obesity can harm the brain and compromise performance on intelligence tests. The first stream of research comprises brain imaging studies that document structural and functional abnormalities in obese individuals that are apparent as early as adolescence (2–5) and studies that show that obese children and adults have lower intelligence quotients (IQs) than do lean controls (6, 7). Together, these findings have been interpreted to suggest that obesity may harm key brain regions and functions that support the IQ (8). This interpretation has entered popular discourse through media headlines such as “Extreme obesity in tots tied to low IQ” (9); “Obesity can lower children's IQ” (10); “Obesity might lower teens' thinking skills, study suggests” (11); and “The greater your weight, the lower your IQ, say scientists” (12). The conclusion that obesity damages the IQ during the first half of the life course is premature. Because extant studies were cross-sectional, they could not establish the temporal ordering of obesity and low IQ. To advance research and refine causal inferences about the obesity–IQ association, life-course studies are needed that use individuals as their own controls. Such studies can test within-individual changes in IQ from childhood, before the onset of obesity, to adulthood, after obesity develops (2, 13).
The second stream of research comprises studies that follow-up cohorts established in midlife. Most of these studies found that adults who were obese in midlife were more likely to suffer cognitive decline and develop dementia than were their lean peers (14–16). This finding has given rise to theories that prevention and treatment of obesity may protect older adults from cognitive decline (17–19). This conclusion is also premature. Low IQ in childhood is a risk factor for both obesity and later-life cognitive decline, and it may account for some of the observed association between the 2 factors (20–25). To advance research and refine causal inferences about the obesity–dementia association in the second half of the life course, it is important to understand the interplay between cognitive function and cardiometabolic processes in the first half of the life course. For this reason, Epidemiologic Reviews recently noted that to “better understand the direction of the association between obesity and cognitive aging, longitudinal studies are needed … preferably starting in early life” (26, p. 30).
We conducted a prospective life-course study of the association between obesity and IQ decline using data from a birth cohort of 1,037 individuals followed prospectively through 38 years of age. Cohort members completed tests of cognitive ability at ages 3, 7, 9, and 11 years and again in adulthood at age 38 years. Obesity was assessed at regular intervals across the 4 decades of follow-up. To advance research on the association between obesity and low IQ, we used these data to test the hypothesis that cohort members who developed obesity between the ages of 11 and 38 years would experience a decline in IQ greater than that of their peers who were never obese. Measures of weight and cognitive ability between birth and 3 years of age were also used to push back the age window for the ascertainment of temporal ordering. Measures of metabolic and inflammatory abnormalities were used to differentiate severe obesity cases from persons who had elevated body mass indexed (BMI) but not other clinical features of obesity.
MATERIALS AND METHODS
Study sample
Participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (n = 1,037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible for the longitudinal study based on residence in the province at 3 years of age and who participated in the first follow-up assessment at 3 years of age. The cohort represents the full range of socioeconomic statuses in the general population of New Zealand's South Island and is primarily white (27). Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and 38 years, at which time 95% of the 1,007 study members who were still alive took part. At each assessment wave, study members were brought to the Dunedin research unit for a full day of interviews and examinations. The Otago Ethics Committee approved each phase of the study and informed consent was obtained from all study members. The study protocol was approved by the institutional ethical review boards of the participating universities.
Measures
Intelligence quotient
IQ is a highly reliable measure of general intellectual functioning that captures overall ability across differentiable cognitive functions. Participants’ IQ scores were assessed in childhood at ages 7, 9, and 11 years and again in adulthood at age 38 years. We measured IQ decline by comparing scores from the individually administered Wechsler Intelligence Scale for Children-Revised (averaged across ages 7, 9, and 11 years) (28) with those from the Wechsler Adult Intelligence Scale-IV (29). Both had a mean score of 100 and a standard deviation of 15. In addition to the Wechsler Intelligence Scale for Children-Revised and Wechsler Adult Intelligence Scale-IV, we administered the Rey Auditory Verbal Learning Test (30), the Trail Making Test (31), and the Grooved Pegboard Test at ages 13 and 38 years to assess memory, executive functioning, and motor functioning, respectively (30).
Early childhood cognitive ability measure
Cohort members completed the Peabody Picture Vocabulary Test (32) during their assessment at 3 years of age. The Peabody test prompts children with vocabulary words and asks them to identify the corresponding picture from an array during a roughly 30-minute assessment. Peabody IQ scores were strongly predictive of subsequent IQ test performance (r = 0.52).
Obesity phenotypes
Obesity
Cohort members' heights and weights were measured at each assessment. Height was measured to the nearest millimeter using a portable Harpenden Stadiometer (Holtain, Crymych, United Kingdom). Weight was recorded to the nearest 0.1 kg at ages 3, 5, 7, 9, 11, 13, 15, 21, 26, 32, and 38 years using calibrated scales. Individuals were weighed in light clothing. BMI was measured as weight in kilograms divided by height in meters squared. Through 15 years of age, obesity was defined using the 90th percentile of the US Centers for Disease Control and Prevention reference BMI distributions for boys and girls (http://www.cdc.gov/growthcharts/). Subsequently, obesity was defined as a BMI of 30 or higher. During the 25-year interval between IQ measurements (ages 13–38 years), 28% of the cohort became obese (n = 289). This prevalence is in line with the general New Zealand population (in the most recent Ministry of Health report, obesity prevalence was 28% for New Zealanders 15 years of age or older) (33).
Severe obesity
We defined cases of severe obesity at ages 32 and 38 years using assessments of metabolic syndrome and systemic inflammation, which are established clinical features of severe obesity (34). Metabolic syndrome was assessed using measurements of 5 biomarkers: 1) high waist circumference (≥88 cm for women, ≥102 cm for men), 2) high blood pressure (≥130/85 mm Hg), 3) low high-density lipoprotein cholesterol level (<50 mg/dL for women, <40 mg/dL for men), 4) high glycated hemoglobin (≥5.7%), and 5) high triglyceride level (≥200 mmol/L). Biomarker assessments have been described in detail previously (35, 36). Cohort members with high-risk values for 3 or more biomarkers were defined as having the metabolic syndrome (37).
Elevated systemic inflammation was assessed using assays of high-sensitivity C-reactive protein (hsCRP) in blood. As previously described (36), hsCRP was measured with a Hitachi 917 analyzer (Roche Diagnostics, GmbH, D-68298, Mannheim, Germany) using a particle-enhanced immunoturbidimetric assay. The Centers for Disease Control and Prevention/American Heart Association definition of high cardiovascular risk (hsCRP >3 mg/L) was used to identify our risk group (38).
Of the original cohort, 92% had available data on severe obesity phenotypes (n = 969). Within this group, 18% had severe obesity, defined as having a BMI of 30 or higher and either the metabolic syndrome or a hsCRP level >3 mg/L (n = 181).
Early childhood weight measures
Individual differences in obesity risk emerge during gestation and are further established during infancy and childhood through accelerated growth trajectories (39, 40). We assessed rapid early life growth that predisposed participants to obesity using data on 1) weight at birth; 2) rate of weight gain in early childhood assessed as the difference between weight at birth (from hospital records) and weight at 3 years of age; and 3) age and BMI at adiposity rebound, calculated as the nadir of each cohort member's childhood BMI growth curve fitted over ages 3–13 years (41). Collectively, these measures explained 18% of BMI variance at the end of follow-up and predicted obesity onset with moderate sensitivity and specificity (C statistic = 0.75, 95% confidence interval (CI): 0.71, 0.79).
Obesity risk factors
The socioeconomic statuses of cohort members' families were measured using a 6-point scale that assessed parents' occupational statuses, defined based on average income and educational levels derived from the New Zealand Census. The highest occupational status of either parent was averaged across the childhood assessments (42). Family history of obesity was derived from heights and weights of the cohort members' parents that were assessed via parent report when cohort members were 11 years of age. BMIs were calculated and standardized by sex, and maternal and paternal standardized scores were averaged to create a single family history score (41).
Analysis
All analyses included the 913 cohort members with available childhood and adulthood IQ data. Surviving cohort members for whom we did not have IQ data at either period did not differ from the analysis sample in terms of obesity or their available IQ measurement. Because the IQ is an age-standardized measurement, we were able to compare scores in childhood and adulthood to quantify IQ decline. We used linear regression models to test the hypothesis that developing obesity caused a decline in IQ. We regressed adult IQ scores on obesity status and childhood IQ to estimate the change in IQ associated with becoming obese. The regression model tested whether, after adjustment for baseline IQ, becoming obese was associated with having a lower adult IQ. This approach yielded identical results to so-called “differences in differences” models that tested a time-by-obesity interaction to predict IQ. These models used panel datasets that included 2 observations per individual to test for differences in IQ change from 11 to 38 years of age in the group of participants who developed obesity as compared with the group who remained lean. The regression models included a main effect term for the time elapsed between IQ measurements, a main effect term for a dummy variable that coded whether the study member became obese during follow-up, and a product term that tested the interaction between these 2 variables. Because we did not find that obesity was associated with a decline in IQ, we next tested the hypothesis of reverse causation, that is, that having a low IQ predisposes children to develop obesity. We used Cox models to regress time to obesity onset on childhood IQ and covariates to derive hazard ratios. We used multinomial logit models to regress obesity outcomes on childhood IQ and covariates to derive relative risks. The multinomial logit model showed the probabilities that children would 1) remain lean, 2) develop nonsevere obesity, or 3) develop severe obesity with metabolic syndrome or elevated systemic inflammation. Relative risks were derived using the group who remained lean as the reference group. Effect sizes for analyses of the cognitive testing measure are presented in standardized IQ points (mean, 100; standard deviation, 15). For analyses of obesity outcomes, effect sizes are reported for a 1-standard-deviation change in IQ score. Because the sample is a representative birth cohort, it forms its own test norms for the purpose of estimating effect sizes. Regression models were adjusted for sex to account for differences in obesity prevalence. Statistical analyses were conducted using Stata, version 12.0 (Stata Corp. LP, College Station, Texas).
RESULTS
Is becoming obese associated with IQ decline?
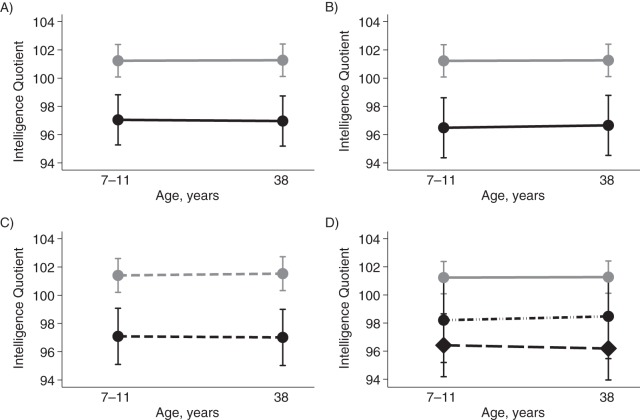
Similar to what was seen in prior cross-sectional studies of adults, we found that cohort members who became obese had lower adult IQ scores than did their peers who remained lean during follow-up (for the obese group, adult IQ score = 96.93 (standard error (SE), 0.92) and for the lean group, adult IQ score = 101.28 (SE, 0.58); P = 0.001 for difference). However, cohort members who developed obesity did not have a greater change in IQ score than those who remained lean over the 25-year period; in both groups, the average change in IQ was less than 0.10 IQ points (P = 0.831 for difference). Instead, cohort members who became obese had lower IQ scores at childhood baseline than did their lean peers (for the group who became obese, childhood IQ score = 97.01 (SE, 0.93) and for the lean group, childhood IQ score = 101.25 (SE, 0.58); P = 0.001 for difference) (Figure 1A).
Figure 1.
Association of obesity and longstanding cognitive deficit from childhood, Dunedin Multidisciplinary Health and Development Study, 1972–2012. A) Change in intelligence quotient (IQ) from childhood to adulthood in Dunedin cohort members who remained lean (n = 644; gray dots) and cohort members who became obese during follow-up between 11 and 38 years of age (n = 269; black dots). B) The same data, excluding cases in whom the onset of obesity occurred by 11 years of age (gray dots, lean group; black dots, the group who first became obese after 11 years of age; n = 187). C) IQ change from childhood to adulthood in the lean (gray dots) and obese (black dots) groups with adjustment for early growth factors that predispose persons to obesity (measured by weight at birth, weight gain between birth and 3 years of age, and age and body mass index at adiposity rebound). D) IQ change in 3 groups: those who remained lean (gray dots); those who became obese but who did not manifest the metabolic syndrome or elevated systemic inflammation (n = 94; black dots); and those who developed severe obesity with metabolic syndrome or elevated systemic inflammation (n = 170; black diamonds). Data in D exclude 5 obese cases for whom the metabolic syndrome and systemic inflammation could not be evaluated because of missing phlebotomy procedures.
It was possible that early-onset obesity had already affected IQ levels by the time of childhood baseline testing (at ages 7–11 years). To rule this out, we repeated our analysis, excluding cases with obesity onset through age 11 years (n = 120). Results were unchanged. Compared with their peers who remained lean, cohort members in whom the onset of obesity occurred for the first time during the ages of 13–38 years had lower IQ scores at childhood baseline (IQ = 96.41, SE, 1.12) and at adult follow-up (IQ = 96.58, SE, 1.08), but there was no evidence of a larger change in IQ (Figure 1B).
It was possible that children on a developmental trajectory to become obese were already suffering IQ decline by the time of the childhood baseline assessment. To rule this out, we performed a statistical adjustment to our analysis to take into account each child's developmental predisposition to obesity as measured by their weight at birth, their weight gain between birth and age 3 years, and their age and BMI at adiposity rebound. Results were unchanged by statistical adjustment for these indicators of early growth predisposing to obesity: Cohort members who became obese had childhood IQs that were 4.27 (SE, 1.27) points lower and adult IQs that were 4.35 (SE, 1.24) points lower than those of cohort members who remained lean (Figure 1C). As a final check, we also examined scores on the Peabody Picture Vocabulary IQ test, which cohort members had completed at 3 years of age. Cohort members who later became obese already had IQs at 3 years of age that were lower than those of their peers who would remain lean (for the group that became obese, Peabody IQ = 97.80 (SE, 0.96); for the never-obese group, Peabody IQ = 100.91 (SE, 0.60); P = 0.006).
It was possible that we did not observe obesity cases of sufficient severity to cause IQ decline. To rule this out, we compared childhood and adulthood IQ scores of cohort members who never developed obesity with those of cohort members who developed severe obesity, defined as having a BMI of 30 or higher in addition to the metabolic syndrome or elevated systemic inflammation (hsCRP >3 mg/L). Figure 1D shows that children who later developed severe obesity had lower IQ scores at childhood baseline and at adult follow-up but exhibited no excess IQ decline (childhood IQ = 96.41 (SE, 1.21) and adult IQ = 96.18 (SE, 1.14)). Results were similar for tests of memory, executive function, and motor function. Cohort members who developed obesity scored lower on neuropsychological functioning tests at baseline (13 years of age) and at follow-up (38 years of age) but exhibited no excess neuropsychological functioning decline (Table 1).
Table 1.
Association of Obesity With Change in Intelligence Quotient and Neuropsychological Functioning, Dunedin Multidisciplinary Health and Development Study, 1972–2012
| Measure | Mean Childhood and Adulthood IQ and Neuropsychological Test Scores by Body Composition in Standardized IQ Units |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean (n = 644)a |
Obese (n = 269)b |
Severely Obese (n = 170)c |
|||||||
| Child | Adult | Changed | Child | Adult | Changed | Child | Adult | Changed | |
| IQ | 101.25 | 101.28 | 0.04 | 97.01*** | 96.93*** | −0.08 | 96.41*** | 96.18*** | −0.23 |
| Verbal IQ | 100.94 | 101.05 | 0.11 | 97.74** | 97.47** | −0.27 | 97.15** | 96.82** | −0.33 |
| Performance IQ | 101.31 | 101.24 | −0.08 | 96.85*** | 97.03*** | 0.19 | 96.35*** | 96.42*** | 0.07 |
| Neurocognitive tests | |||||||||
| Rey Delayed Recall | 100.70 | 100.41 | −0.29 | 98.38* | 99.05 | 0.67 | 97.73* | 98.13 | 0.40 |
| Trails B | 100.80 | 100.89 | 0.09 | 98.14* | 97.94* | −0.21 | 96.53** | 97.77* | 1.24 |
| Grooved Peg Board | 101.68 | 101.40 | −0.28 | 96.10*** | 96.76*** | 0.66 | 94.46*** | 94.96*** | 0.49 |
Abbreviation: IQ, intelligence quotient.
*P < 0.05; **P < 0.01; ***P < 0.001 versus the lean group.
a Cohort members in the lean group did not have a body mass index (weight (kg)/height (m)2) in excess of the cutoff for obesity during the course of follow-up.
b Cohort members in the obese group had a body mass index of 30 or higher at 1 or more assessments during follow-up.
c Cohort members in the severely obese group had a body mass index of 30 or higher at 1 or more measurements during follow-up and were diagnosed with the metabolic syndrome or showed evidence of having elevated systemic inflammation (high-sensitivity C-reactive protein level >3 mg/L).
d One point = 1/15 of 1 standard deviation.
Is lower childhood IQ associated with becoming obese?
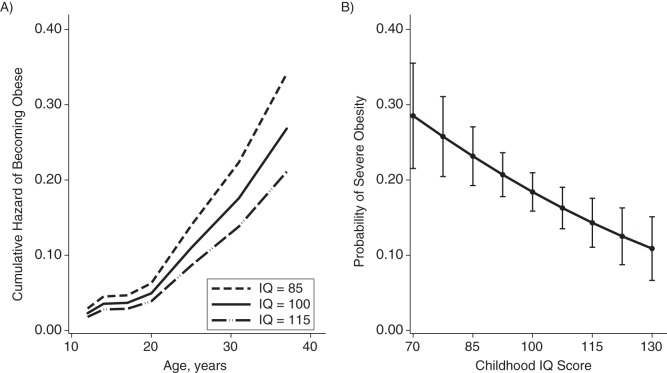
Having found that lower IQ temporally preceded the onset of obesity, we turned to the question of whether having a lower IQ predisposed children to become obese. Children with lower IQ scores were more likely to become obese over the following 3 decades; a 15-point (1 standard deviation) difference in IQ between children predicted a 27% difference in their hazard of becoming obese (hazard ratio = 1.28, 95% CI: 1.10, 1.48). Specifically, children with lower IQs were at an increased risk of developing severe obesity with metabolic syndrome or elevated inflammation (relative risk (RR) = 1.40, 95% CI: 1.15, 1.71). This association was not fully explained by the children's developmental predisposition to obesity (adjusted RR = 1.47, 95% CI: 1.19, 1.82), socioeconomic characteristics of the households in which the children grew up (adjusted RR = 1.30, 95% CI: 1.05, 1.61), children's family histories of obesity (adjusted RR = 1.34, 95% CI: 1.09, 1.65), or adjustment for all of these risk factors (adjusted RR = 1.34, 95% CI: 1.06, 1.70). Figure 2 shows the associations between childhood IQ and obesity onset (Figure 1A) and risk for developing severe obesity (Figure 1B).
Figure 2.
Association of lower IQs with becaming obese earlier in life and developing severe obesity with metabolic syndrome or elevated systemic inflammation, Dunedin Multidisciplinary Health and Development Study, 1972–2012. A) Cumulative hazard functions for Dunedin cohort children with low intelligence quotients (IQ = 85), average IQs (IQ = 100), and high IQs (IQ = 115). Hazards were estimated using a Cox regression model that was adjusted for sex. Children with lower IQs were more likely to develop obesity and became obese earlier in life (for each 1-standard-deviation decrease in IQ, hazard ratio = 1.28, 95% confidence interval: 1.10, 1.48). B) Predicted probability of obesity as function of childhood IQ. Predicted probabilities were estimated using a probit regression model that was adjusted for sex. Children with lower IQs had an increased risk of developing severe obesity with metabolic syndrome or elevated inflammation (for each 1-standard-deviation decrease in IQ, relative risk = 1.40, 95% confidence interval: 1.15, 1.71). Bars, 95% confidence intervals.
DISCUSSION
Reports based on cross-sectional study designs have suggested that developing obesity increases risk of a decline in IQ among adolescents and adults. These findings have raised alarm bells, and not just in the public-health community. If obesity does cause a decline in IQ, it could represent a threat to the ability of today's children to compete in tomorrow's information-intensive economy. There is no doubt that combating the obesity epidemic is a critical national priority. However, our data, which are from a population-representative cohort of 1,000 children followed for 4 decades, are not consistent with the hypothesis that obesity causes a decline in IQ in the first half of the life course. Instead, they show that the IQ deficits of obese adults were already present in childhood.
Children in our cohort who later developed obesity showed no IQ decline, but they did have lower IQs at baseline as compared with their peers who remained lean over 3 decades of follow-up. This pattern remained consistent when we restricted our sample to only lean children and when we accounted for differences in children's birth weight and in their growth during the first years of life. Differences in mental ability between children who remained lean and those who later developed obesity were present as early as 3 years of age. Even when we restricted our obese group to comprise only participants with severe obesity and the metabolic syndrome or elevated systemic inflammation, we observed no evidence that obesity contributed to IQ decline.
Our data are consistent with the hypothesis that cross-sectional associations between obesity and IQ arise because children with lower IQs are at greater risk of becoming obese (43, 44). Prior reports on correlations between low childhood IQ and later obesity risk included only a single childhood measurement of IQ and could not rule out the possibility that children's IQs declined following the onset of obesity (21–23). We followed a population-representative birth cohort for whom we had measurements of IQ in childhood before obesity onset and who were retested in midlife after some cohort members had become obese. Therefore, unlike those from previous studies, our data show that becoming obese in adolescence or young adulthood does not cause IQ decline as of midlife.
We acknowledge some limitations of our study. First, although our study shows a temporal precedence of low IQ over obesity, it does not establish causation. The apparent relationship between low childhood IQ and increased risk of obesity could arise from a common cause (45). Second, our study is right-hand censored at age 38 years. There are reports of obesity as a contributor to cognitive decline observed in elderly patients and those with dementing illnesses (16, 46), which implicates metabolic or inflammatory mechanisms (47, 48). Our cohort is not old enough for us to try to determine whether obesity poses risk for dementia. However, our observation that individuals with metabolic/inflammatory obesity in midlife have already had a 5-point IQ deficit since childhood means that it is necessary to control statistically for childhood IQ before estimating the effect size for the attributable risk of obesity to late-life cognitive decline (49), lest that risk be over-estimated. Third, our study lacked neuroimaging data, and we were limited to neuropsychological testing to assess brain functioning. However, our finding suggests that studies of structural and functional brain abnormalities in obese adults should also consider the question of temporal order (2).
Despite these limitations, our study has important implications for obesity research and policy. After reports from cross-sectional studies that obesity is associated with a lower IQ, much research attention has been invested to elucidate the pathophysiology by which obesity causes decline in mental ability (50–55). Our findings suggest that similar attention should be focused on understanding why children with lower IQs have an increased risk of obesity. A further implication of our results is that research is needed to investigate whether premorbid differences in intellectual functioning account for the link between midlife obesity and late-life dementia. Evidence from prospective studies has shown that children with lower IQs have a greater risk of dementia (24, 25). To date, studies linking midlife obesity with dementia have not accounted for differences in premorbid IQ scores (13, 26). Theories of why obese adults are at greater risk for dementia focus on metabolic dysregulation and elevated systemic inflammation (56, 57). Our results indicate that, at least in the first half of the life course, these exposures do not harm the IQ, but they do occur more often in individuals who have had low IQ since childhood. Collectively, this evidence suggests the hypothesis that intellectual functioning in childhood indexes risk for poor midlife physical health and accelerated cognitive aging and may confound associations between the 2. Future studies should test for such confounding and seek to better understand brain health as driver of the aging process, not just an outcome.
ACKNOWLEDGMENTS
Author affiliations: Center for the Study of Aging and Human Development, Duke University Medical Center, Durham, North Carolina (Daniel W. Belsky); Department of Psychology and Neuroscience, Duke University, Durham, North Carolina (Daniel W. Belsky, Avshalom Caspi, Sidra Goldman-Mellor, Madeline Hogan Meier, Terrie E. Moffitt); Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, North Carolina (Daniel W. Belsky, Avshalom Caspi, Terrie E. Moffitt); Institute for Genome Sciences & Policy, Duke University, Durham, North Carolina (Daniel W. Belsky, Avshalom Caspi, Terrie E. Moffitt); Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, Kings College London, London, United Kingdom (Avshalom Caspi, Terrie E. Moffitt); Carolina Consortium for Human Development, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Sidra Goldman-Mellor); Duke Transdisciplinary Prevention Research Center, Center for Child & Family Policy, Duke University, Durham, North Carolina (Madeline H. Meier); and Dunedin Multidisciplinary Health and Development Research Unit, Department of Preventive and Social Medicine, School of Medicine, University of Otago, Dunedin, New Zealand (Sandhya Ramrakha, Richie Poulton).
The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from the US National Institute on Aging (grants AG032282 and T32-AG000029), the UK Medical Research Council (grant G0601483), the US National Institute on Drug Abuse (grant P30 DA023026), and the US National Institute of Child Health and Development (grant HD061298). Additional support was provided by the Jacobs Foundation.
We thank the Dunedin Study unit research staff and founder Dr. Phil Silva.
Conflict of interest: none declared.
REFERENCES
- 1.Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29(1):1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 2.Convit A. Obesity is associated with structural and functional brain abnormalities: where do we go from here? Psychosom Med. 2012;74(7):673–674. doi: 10.1097/PSY.0b013e3182662c56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yau PL, Castro MG, Tagani A, et al. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller K, Sacher J, Arelin K, et al. Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults: a combined MRI, serum marker and gene expression study. Transl Psychiatry. 2012;2:e200. doi: 10.1038/tp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16(1):119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 6.Miller J, Kranzler J, Liu Y, et al. Neurocognitive findings in Prader-Willi syndrome and early-onset morbid obesity. J Pediatr. 2006;149(2):192–198. doi: 10.1016/j.jpeds.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Cournot M, Marquie JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 8.Yu ZB, Han SP, Cao XG, et al. Intelligence in relation to obesity: a systematic review and meta-analysis. Obes Rev. 2010;11(9):656–670. doi: 10.1111/j.1467-789X.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 9.Hitti M. Extreme obesity in tots tied to low IQ. WebMD. August 31, 2006 http://www.webmd.com/parenting/news/20060831/extreme-obesity-in-tots-tied-to-low-iq. (Accessed March 15, 2013)
- 10.Nordqvist C. Obesity can lower children's IQ. Medical News Today. September 3, 2012 http://www.medicalnewstoday.com/articles/249799.php. (Accessed March 15, 2013)
- 11.Mozes A. Obesity might lower teens’ thinking skills, study suggests. US News and World Report. September 3, 2012 http://health.usnews.com/health-news/news/articles/2012/09/03/obesity-might-lower-teens-thinking-skills-study-suggests. (Accessed March 15, 2013)
- 12.Goswami N. The greater your weight, the lower your IQ, say scientists. The Telegraph. October 15, 2006 http://www.telegraph.co.uk/news/uknews/1531487/The-greater-your-weight-the-lower-your-IQ-say-scientists.html. (Accessed March 15, 2013)
- 13.Elias MF, Goodell AL, Waldstein SR. Obesity, cognitive functioning and dementia: back to the future. J Alzheimer's Dis. 2012;30(suppl 2):S113–S125. doi: 10.3233/JAD-2011-111175. [DOI] [PubMed] [Google Scholar]
- 14.Singh-Manoux A, Czernichow S, Elbaz A, et al. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II Cohort Study. Neurology. 2012;79(8):755–762. doi: 10.1212/WNL.0b013e3182661f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anstey KJ, Cherbuin N, Budge M, et al. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 17.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 18.Mirowsky J. Cognitive decline and the default American lifestyle. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i50–i58. doi: 10.1093/geronb/gbq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhalter TM, Hillman CH. A narrative review of physical activity, nutrition, and obesity to cognition and scholastic performance across the human lifespan. Adv Nutr. 2011;2(2):201S–206S. doi: 10.3945/an.111.000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith E, Hay P, Campbell L, et al. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 21.Batty GD, Gale CR, Mortensen LH, et al. Pre-morbid intelligence, the metabolic syndrome and mortality: the Vietnam Experience Study. Diabetologia. 2008;51(3):436–443. doi: 10.1007/s00125-007-0908-5. [DOI] [PubMed] [Google Scholar]
- 22.Chandola T, Deary IJ, Blane D, et al. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) Study. Int J Obes (Lond) 2006;30(9):1422–1432. doi: 10.1038/sj.ijo.0803279. [DOI] [PubMed] [Google Scholar]
- 23.Richards M, Black S, Mishra G, et al. IQ in childhood and the metabolic syndrome in middle age: extended follow-up of the 1946 British Birth Cohort Study. Intelligence. 2009;37(6):567–572. doi: 10.1016/j.intell.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whalley LJ, Starr JM, Athawes R, et al. Childhood mental ability and dementia. Neurology. 2000;55(10):1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 25.McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71(14):1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- 26.Dahl AK, Hassing LB. Obesity and cognitive aging. Epidemiol Rev. 2012;35(1):22–32. doi: 10.1093/epirev/mxs002. [DOI] [PubMed] [Google Scholar]
- 27.Moffitt TE, Caspi A, Rutter M, et al. Sex Differences in Antisocial Behavior: Conduct Disorder, Delinquency, and Violence in the Dunedin Longitudinal Study. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 28.Wechsler D. Manual for the Wechsler Intelligence Scale for Children—Revised. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 30.Lezak MD. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 31.Battery A. Manual & Directions for Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 32.Dunn L. The Peabody Picture Vocabulary Test. Minneapolis, MN: American Guidance Service; 1965. [Google Scholar]
- 33.University of Otago and Ministry of Health. A Focus on Nutrition: Key findings of the 2008/09 New Zealand Adult Nutrition Survey. Wellington, UK: Ministry of Health; 2011. [Google Scholar]
- 34.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 35.Caspi A, Harrington H, Moffitt TE, et al. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160(8):805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- 36.Danese A, Pariante CM, Caspi A, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 38.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 39.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 40.Frongillo EA, Lampl M. Early identification of children at risk of developing obesity. Arch Pediatr Adolesc Med. 2011;165(11):1043–1044. doi: 10.1001/archpediatrics.2011.193. [DOI] [PubMed] [Google Scholar]
- 41.Belsky DW, Moffitt TE, Houts R, et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med. 2012;166(6):515–521. doi: 10.1001/archpediatrics.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulton R, Caspi A, Milne BJ, et al. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottfredson LS. Intelligence: is it the epidemiologists’ elusive “fundamental cause” of social class inequalities in health? J Pers Soc Psychol. 2004;86(1):174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- 44.Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ and later mortality risk: systematic review. Ann Epidemiol. 2007;17(4):278–288. doi: 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Xu WL, Atti AR, Gatz M, et al. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76(18):1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siervo M, Arnold R, Wells JC, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12(11):968–983. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- 47.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 48.Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging. 2009;24(1):154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring) 2013;21(1):E51–E55. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 50.Gonzales MM, Tarumi T, Eagan DE, et al. Indirect effects of elevated body mass index on memory performance through altered cerebral metabolite concentrations. Psychosom Med. 2012;74(7):691–698. doi: 10.1097/PSY.0b013e31825ff1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 52.Kosari S, Badoer E, Nguyen JC, et al. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res. 2012;235(1):98–103. doi: 10.1016/j.bbr.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Sellbom KS, Gunstad J. Cognitive function and decline in obesity. J Alzheimer's Dis. 2012;30(suppl 2):S89–S95. doi: 10.3233/JAD-2011-111073. [DOI] [PubMed] [Google Scholar]
- 54.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53(11):2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanek KM, Grieve SM, Brickman AM, et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- 56.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5(12):649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 57.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]