Abstract
Given that the primordial ovarian follicular pool is established in utero, it may be influenced by parental characteristics and the intrauterine environment. Anti-Müllerian hormone (AMH) levels are increasingly recognized as a biomarker of ovarian reserve in females in adulthood and adolescence. We examined and compared associations of maternal and paternal prenatal exposures with AMH levels in adolescent (mean age, 15.4 years) female offspring (n = 1,399) using data from the Avon Longitudinal Study of Parents and Children, a United Kingdom birth cohort study that originated in 1991 and is still ongoing (data are from 1991–2008). The median AMH level was 3.67 ng/mL (interquartile range: 2.46–5.57). Paternal but not maternal smoking prior to and during pregnancy were inversely associated with AMH levels. No or irregular maternal menstrual cycles before pregnancy were associated with higher AMH levels in daughter during adolescence. High maternal gestational weight gain (top fifth versus the rest of the distribution) was associated with lower AMH levels in daughters. Parental age, body mass index, and alcohol intake during pregnancy, child's birth weight, and maternal parity and time to conception were not associated with daughters' AMH levels. Our results suggest that some parental preconceptual characteristics and environmental exposures while the child is in utero may influence the long-term ovarian development and function in female offspring.
Keywords: anti-Müllerian hormone, maternal-paternal comparisons, prenatal risk factors
During female fetal development and well into adulthood, a remarkable dynamic in the number of primordial (resting) ovarian follicles takes place. By 18 weeks of gestation, approximately 7 million follicles are present in fetal ovaries. However, by the time of a full-term birth, only approximately 1 million remain, and these follicles continue to undergo atresia so that by puberty, only approximately 400,000 follicles remain. The rate of depletion of the follicle pool varies, with women in whom it is greater being more likely to experience infertility and menopause at a younger age (1).
The reproductive lifespan of the woman is therefore largely determined by factors that regulate the number of follicles laid down during fetal development and their subsequent rate of atresia during adult life. Although there is a strong concordance between monozygotic twins and between mothers and offspring with respect to age at menopause (2–7), which suggests that genetic factors have a strong influence, additional factors such as the intrauterine environment may also contribute. To date, this potential impact has principally been assessed using age at menopause as a surrogate for ovarian reserve. Maternal characteristics, including an age less than 35 years, prepregnancy diabetes, and smoking during pregnancy, together with having either a high or low birth weight and being born large for gestational age have all been associated with earlier age at menopause (8–12).
In adults, circulating anti-Müllerian hormone (AMH) level is increasingly recognized as a biomarker of the ovarian reserve in adults because of its strong correlation with primordial follicle number (13), follicular recruitment rates (14), response to exogenous gonadotrophins (15), and ability to predict the duration of the reproductive lifespan (16). In children, similar associations between AMH levels and the ovarian reserve have been observed. Specifically, girls with a reduced ovarian reserve and shorter reproductive lifespan due to Turners syndrome have low AMH levels (17); in prepubertal and peripubertal girls, AMH levels reflects follicular recruitment rates (14, 18); and as observed in adults, AMH is negatively associated with follicle-stimulating hormone levels in girls from 5 to 15 years of age (19). Collectively, these studies suggest that AMH level may be a useful surrogate for the ovarian reserve throughout life.
The aim of the present study was to examine the associations of a range of prenatal exposures (parental age, body mass index (BMI, measured as weight in kilograms divided by height in meters squared), smoking, and alcohol intake and maternal gestational weight gain (GWG)), other parental reproductive characteristics (time to conception, maternal parity, regularity of maternal menstrual periods), and size at birth with AMH levels in female adolescents aged 14–16 years. Where possible, we compared estimates for maternal exposures with estimates for equivalent paternal exposures. In this approach, the paternal association acts as a negative control, because a direct intrauterine mechanism should result in a considerably stronger estimate for the maternal exposure than for the paternal exposure (20).
MATERIALS AND METHODS
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a longitudinal, population-based birth cohort study that recruited 14,541 pregnant women residing in Avon, United Kingdom, who had expected dates of delivery between April 1, 1991, and December 31, 1992 (http://www.alspac.bris.ac.uk.) (21, 22). Since 7 years of age, surviving offspring participants have been invited to regular follow-up clinics. A total of 2,915 adolescent females attended the clinic conducted when they were 14–16 years of age (2008; hereafter referred to as the 14–16–year clinic), of whom 2,838 were singletons; only singletons are considered here. Of these, 1,751 (62%) provided a blood sample. Ethical approval was obtained from the ALSPAC Law and Ethics Committee (international review board 00003312) and the local research ethics committee.
Anti-Müllerian hormone
At the 14–16–year clinic, participants were asked to fast overnight, for those attending in the morning, or for a minimum of 6 hours, for those attending after lunch. After venepuncture, blood samples were immediately spun and frozen at −80°C. AMH was assayed on serum using the commercial AMH Generation II ELISA kit (Beckman Coulter UK Ltd, High Wycombe, United Kingdom) as previously described (23). Inter- and intra-assay coefficients of variation for the cohort were less than 5%. Values are reported in ng/mL; for conversion to pmol/L, multiply by 7.14.
Exposures
Smoking before and during pregnancy was self-reported in questionnaires using the following categories: 0, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, or ≥30 cigarettes per day (except for paternal smoking before pregnancy, which was reported as yes or no smoking). Frequency of alcohol intake in the first trimester was self-reported at 18 weeks as never, <1 drink per week, ≥1 per week but <1 per day, 1–2 per day, 3–9 per day, or ≥10 drinks per day. Maternal and paternal prepregnancy weight and height were reported at enrollment and at 12 weeks of gestation, respectively. Maternal weight was also measured at the first antenatal clinic visit (median gestational age, 10 completed weeks, interquartile range, 9–12 weeks). Because measured gestational weight and self-reported prepregnancy weight were highly correlated (r = 0.97; P < 0.001), self-reported prepregnancy weight was used because of the higher numbers available.
Information on gestational age, maternal parity (categorized as 0, 1, 2 or ≥3), maternal weight during pregnancy, and birth weight was obtained from medical records. Size for gestational age was categorized as small (a birth weight lower than the 10th percentile of birth weight for gestational age), appropriate (between the 10th and 90th percentiles for gestational age), or large (higher than the 90th percentile for gestational age) using the study population centiles. Preterm birth was defined as less than 37 completed weeks of gestation. GWG was defined as the difference between the first and last recorded antenatal weight measures (24), provided that the first was taken before week 18 of gestation and the last was taken after week 27 of gestation. Information on time to conception (<6 months, 6–12 months, or >12 months) for planned pregnancies, physician consultation for infertility, and regularity (yes vs. no) of menstrual periods in the 12 months prior to pregnancy were reported by mothers in questionnaires.
Other variables
The highest parental occupation was used to allocate the children to family social class groups (classes I (professional/managerial) to V (unskilled manual workers), using the 1991 British Office of Population and Census Statistics classification). Pubertal stage was assessed using the Tanner stage for breast development and pubic hair growth through a self- or parental-completed questionnaire (25). For participants who reported different stages on the 2 scores, the higher stage was used. Age at menarche was reported on the same questionnaire. Offspring cotinine levels (assessed at the same time as AMH levels) were measured on plasma using the Cozart cotinine microplate enzyme immunoassay (Cozart Biosciences Ltd, Abingdon, United Kingdom).
Statistical analysis
Values of AMH were log-transformed to normalize the distribution. We tested all associations for departure from linearity by examining graphs of mean levels of AMH across natural categories for categorical variables (e.g., a time to conception of <6 months, 6–12 months, and >12 months) and across fifths of the distribution for continuously measured exposures. We further compared a model in which the exposure categories were entered as single-ordered categorical variables with one in which they were added as indicator variables using a likelihood ratio test. Coefficients obtained from multivariable linear regression models of the logged values were exponentiated (back transformed) and are therefore ratios of geometric means per unit/category change of the exposure and should be interpreted as percentage change per exposure with a null value of 1.
In the basic model (model 1), we display the crude association between the exposure of interest and AMH level. We considered maternal age and household social class as potential confounders (model 2 was adjusted for these factors). We did not consider offspring age at AMH measurement to be a potential confounder because there is no reason it would be associated with the early-life exposures. In the final model (model 3), we also added terms for gestational age, offspring pubertal stage, and BMI as potential mediators. In analyses of parental smoking, we also added offspring cotinine levels into the final model because offspring of parents who smoke may be more likely to smoke themselves, and there is evidence that smoking affects reproductive health (26). We consider model 2 in which we adjusted for potential confounders to be our main model testing the association of prenatal exposures with AMH levels in adolescence.
Analyses of parental measures were restricted to mother-father-offspring trios with complete data, with the sample size allowed to vary across the different exposures in order to maximize study power. Numbers included in analyses are presented in Figure 1 and Tables 1–4. Differences between maternal and paternal estimates were tested using a Wald test in a model in which both maternal and paternal estimates were included. In a sensitivity analysis, we relaxed the restriction to those trios with data on both parents and included all parent-offspring duos so that numbers for the maternal and paternal analyses were allowed to differ. Results were unchanged from those presented (results available from authors on request). To ensure that females with exceptionally high AMH values were not driving any of the observed associations, we repeated all analyses excluding those postpubertal females within the highest 20% of the AMH distribution (n = 279).
Figure 1.
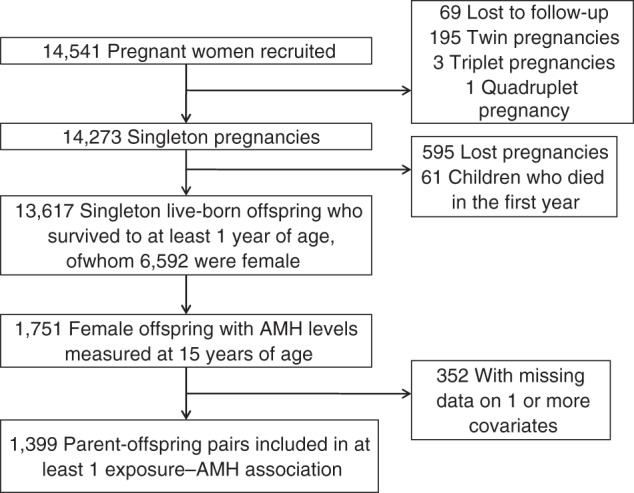
Study population flow chart, The Avon Longitudinal Study of Parents and Children, Avon, United Kingdom, 1991–2008. AMH, anti-Müllerian hormone.
Table 1.
Characteristics of Included and Excluded Eligible Participants Who Attended the Clinic When They Were 14–16 Years of Age, The Avon Longitudinal Study of Parents and Children, Avon, United Kingdom, 1991–2008
| Characteristic | Included |
Excluded |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing Data on Exposures or Covariates |
Missing Data on Exposures or Covariates or AMH |
|||||||||||||
| No. With Data | Mean (SD) | No. | % | No. With Data | Mean (SD) | No. | % | P Valuea | No. With Data | Mean (SD) | No. | % | P Valuea | |
| Offspring characteristics | ||||||||||||||
| AMH, ng/mLb | 1,399 | 3.67 (2.46, 5.57) | 259 | 3.67 (2.50, 5.55) | 0.61 | 259 | 3.67 (2.50, 5.55) | 0.61 | ||||||
| Age at AMH measurement, years | 1,399 | 15.4 (0.3) | 352 | 15.7 (0.6) | <0.001 | 1,449 | 15.6 (0.5) | <0.001 | ||||||
| BMIc | 1,399 | 21.9 (3.9) | 238 | 22.6 (4.2) | 0.01 | 1,245 | 21.8 (3.8) | 0.41 | ||||||
| Postpubertald | 1,399 | 741 | 53.0 | 219 | 123 | 56.2 | 0.38 | 1,179 | 642 | 54.5 | 0.45 | |||
| Cotinine level, ng/mLb | 1,391 | 0.82 (0, 1.3) | 343 | 0.97 (0.3, 2.2) | <0.001 | 351 | 0.95 (0.32, 2.2) | <0.001 | ||||||
| Parental characteristics | ||||||||||||||
| Maternal age, years | 1,399 | 29.2 (4.5) | 259 | 27.8 (5.3) | <0.001 | 1,300 | 28.8 (4.7) | 0.02 | ||||||
| Paternal age, years | 1,049 | 31.3 (5.4) | 134 | 30.0 (5.3) | 0.01 | 854 | 31.0 (5.4) | 0.28 | ||||||
| Maternal smoking before pregnancy | 1,388 | 324 | 23.3 | 228 | 75 | 32.9 | 0.002 | 1,252 | 301 | 24.0 | 0.67 | |||
| Paternal smoking before pregnancy | 1,146 | 257 | 22.4 | 160 | 58 | 36.3 | <0.001 | 983 | 268 | 27.3 | 0.01 | |||
| Maternal smoking in pregnancy | 1,327 | 176 | 13.3 | 164 | 44 | 26.8 | <0.001 | 1,117 | 169 | 15.1 | 0.19 | |||
| Paternal smoking in pregnancy | 1,114 | 310 | 27.8 | 71 | 71 | 45.5 | <0.001 | 950 | 326 | 34.3 | 0.001 | |||
| No maternal alcohol intake | 1,386 | 593 | 42.8 | 226 | 108 | 47.8 | 0.16 | 1,245 | 553 | 44.4 | 0.40 | |||
| No paternal alcohol intake | 1,151 | 31 | 2.7 | 158 | 9 | 5.7 | 0.04 | 989 | 40 | 4.0 | 0.08 | |||
| Maternal BMIc | 1,294 | 22.7 (3.6) | 197 | 23.0 (3.8) | 0.28 | 1,136 | 22.8 (3.6) | 0.37 | ||||||
| Paternal BMIc | 1,081 | 25.1 (3.3) | 139 | 25.2 (4.1) | 0.58 | 875 | 25.1 (3.4) | 0.77 | ||||||
| Manual household social class | 1,399 | 187 | 13.4 | 132 | 38 | 28.8 | <0.001 | 1,099 | 164 | 14.9 | 0.27 | |||
| Fertility and pregnancy characteristics | ||||||||||||||
| Irregular periods in past year | 1,375 | 281 | 20.4 | 213 | 47 | 22.1 | 0.59 | 1,217 | 251 | 20.6 | 0.91 | |||
| Seen doctor for possible infertility | 1,374 | 172 | 12.5 | 215 | 24 | 11.2 | 0.57 | 1,214 | 163 | 13.4 | 0.49 | |||
| >12 months to conception | 1,052 | 125 | 11.9 | 146 | 15 | 10.3 | 0.57 | 897 | 107 | 11.9 | 0.98 | |||
| Parity ≥3 | 1,364 | 49 | 3.6 | 225 | 23 | 10.2 | <0.001 | 1,237 | 61 | 4.9 | 0.09 | |||
| Gestational weight gain, kg | 1,285 | 12.5 (4.4) | 220 | 12.1 (4.9) | 0.25 | 1,168 | 12.49 (4.6) | 0.84 | ||||||
| Gestational age, weeks | 1,399 | 39.6 (1.6) | 259 | 39.5 (1.9) | 0.09 | 1,300 | 39.5 (1.8) | 0.11 | ||||||
| Birth weight, g | 1,287 | 3,393 (477) | 234 | 3,362 (571) | 0.38 | 1,194 | 3,385 (504) | 0.69 | ||||||
| Size for gestational age | 1,287 | 234 | 0.35 | 1,194 | 0.52 | |||||||||
| Small | 145 | 11.3 | 32 | 13.7 | 124 | 10.4 | ||||||||
| Appropriate | 1,048 | 81.4 | 181 | 77.4 | 993 | 83.2 | ||||||||
| Large | 94 | 7.3 | 21 | 9.0 | 77 | 6.5 | ||||||||
Abbreviations: AMH, anti-Müllerian hormone; BMI, body mass index; SD, standard deviation.
a Compared with included participants.
b Values expressed as median (interquartile range). P values for these measurements were obtained by comparing logged values.
c Weight (kg)/height (m)2.
d Defined as Tanner stage 5 on breast development or pubic hair.
Table 2.
Associations of Maternal and Paternal Exposures With Offspring Anti-Müllerian Hormone Level at 15 Years of Age, The Avon Longitudinal Study of Parents and Children, Avon, United Kingdom, 1991–2008
| Covariate and Modela | No. Measured | Maternal |
Paternal |
||||
|---|---|---|---|---|---|---|---|
| Ratio of Geometric Meansb | 95% CI | P Value | Ratio of Geometric Meansb | 95% CI | P Value | ||
| Age (per 5 years) | 1,049 | ||||||
| Model 1 | 1.04 | 0.99, 1.09 | 0.12 | 1.01 | 0.98, 1.05 | 0.45 | |
| Model 2 | 1.03 | 0.98, 1.08 | 0.22 | 1.01 | 0.97, 1.05 | 0.63 | |
| Model 3 | 1.03 | 0.98, 1.09 | 0.18 | 1.01 | 0.97, 1.05 | 0.64 | |
| Smoking before pregnancy (yes vs. no) | 1,144 | ||||||
| Model 1 | 0.95 | 0.86, 1.06 | 0.36 | 0.87 | 0.79, 0.96 | 0.005 | |
| Model 2 | 0.96 | 0.87, 1.06 | 0.44 | 0.87 | 0.79, 0.96 | 0.007 | |
| Model 3c | 0.96 | 0.87, 1.07 | 0.45 | 0.88 | 0.80, 0.97 | 0.01 | |
| Smoking during pregnancy (yes vs. no) | 1,066 | ||||||
| Model 1 | 1.00 | 0.87, 1.14 | 1.00 | 0.89 | 0.81, 0.98 | 0.01 | |
| Model 2 | 1.00 | 0.87, 1.15 | 0.98 | 0.88 | 0.80, 0.97 | 0.01 | |
| Model 3c | 0.99 | 0.87, 1.14 | 0.94 | 0.89 | 0.80, 0.98 | 0.02 | |
| Body mass indexd | 1,023 | ||||||
| Model 1 | 0.99 | 0.98, 1.01 | 0.35 | 1.00 | 0.98, 1.01 | 0.51 | |
| Model 2 | 1.00 | 0.98, 1.01 | 0.45 | 1.00 | 0.98, 1.01 | 0.55 | |
| Model 3 | 0.99 | 0.98, 1.01 | 0.26 | 1.00 | 0.98, 1.01 | 0.46 | |
| Alcohol intake per category | 1,147 | ||||||
| Model 1 | 1.03 | 0.98, 1.09 | 0.27 | 1.01 | 0.96, 1.06 | 0.70 | |
| Model 2 | 1.02 | 0.97, 1.08 | 0.44 | 1.00 | 0.96, 1.05 | 0.84 | |
| Model 3 | 1.03 | 0.97, 1.08 | 0.38 | 1.01 | 0.97, 1.06 | 0.60 | |
Abbreviation: CI, confidence interval.
a Model 1 was unadjusted. Model 2 was adjusted for maternal age and household social class. Model 3 was adjusted for maternal age, household social class, gestational age, offspring pubertal stage, and body mass index.
b A ratio of geometric means is interpreted as relative % differences with a null value of 1.
c This version of model 3 was adjusted for maternal age, household social class, gestational age, offspring pubertal stage, body mass index, and cotinine level.
d Weight (kg)/height (m)2.
Table 3.
Mutually Adjusted Associations of Maternal and Paternal Exposures With Offspring Anti-Müllerian Hormone Level at 15 Years of Age, The Avon Longitudinal Study of Parents and Children, Avon, United Kingdom, 1991–2008a
| Covariate | Maternal |
Paternal |
P Value for Differencec | ||||
|---|---|---|---|---|---|---|---|
| Ratio of Geometric Meansb | 95% CI | P Value | Ratio of Geometric Meansb | 95% CI | P Value | ||
| Age (per 5 years) | 1.03 | 0.98, 1.08 | 0.30 | 1.00 | 0.98, 1.03 | 0.89 | 0.45 |
| Smoking before pregnancy (yes vs. no)d | 1.00 | 0.89, 1.12 | 0.99 | 0.88 | 0.79, 0.98 | 0.02 | 0.14 |
| Smoking in pregnancy (yes vs. no)d | 1.06 | 0.92, 1.23 | 0.70 | 0.87 | 0.79, 1.02 | 0.008 | 0.06 |
| Body mass indexe | 0.99 | 0.98, 1.01 | 0.29 | 1.00 | 0.98, 1.01 | 0.54 | 0.77 |
| Alcohol intake per category | 1.02 | 0.97, 1.08 | 0.54 | 1.01 | 0.96, 1.06 | 0.90 | 0.74 |
Abbreviation: CI, confidence interval.
a Results from a model that included maternal age, household social class, gestational age, offspring pubertal stage, body mass index, and both maternal and paternal exposures.
b A ratio of geometric means is interpreted as relative % differences with a null value of 1.
c Difference between maternal and paternal estimates.
d For smoking exposures, offspring cotinine level is also included in the model.
e Weight (kg)/height (m)2.
Table 4.
Associations of Maternal Fertility and Pregnancy Characteristics With Offspring Anti-Müllerian Hormone Level at 15 Years of Age, The Avon Longitudinal Study of Parents and Children, Avon, United Kingdom, 1991–2008a
| Characteristic | No. of Subjects | Ratio of Geometric Meansb | 95% CI | P Value |
|---|---|---|---|---|
| Maternal menstrual cycles in the year before the index pregnancy | ||||
| Model 1 | ||||
| Regular | 1,094 | 1.00 | Referent | |
| Irregular | 257 | 1.15 | 1.05, 1.26 | 0.003 |
| None | 25 | 1.27 | 0.97, 1.66 | 0.08 |
| Model 2 | ||||
| Regular | 1.00 | Referent | ||
| Irregular | 1.15 | 1.05, 1.27 | 0.002 | |
| None | 1.29 | 0.99, 1.69 | 0.06 | |
| Model 3 | ||||
| Regular | 1.00 | Referent | ||
| Irregular | 1.15 | 1.05, 1.26 | 0.003 | |
| None | 1.30 | 1.00, 1.71 | 0.05 | |
| Mother seen a doctor for possible infertilityc (yes vs. no) | 1,038 | |||
| Model 1 | 1.00 | 0.89, 1.13 | 0.97 | |
| Model 2 | 0.99 | 0.88, 1.12 | 0.89 | |
| Model 3 | 0.99 | 0.88, 1.11 | 0.86 | |
| Time to conception, monthsc | ||||
| Model 1 | ||||
| <6 | 788 | 1.00 | Referent | |
| 6–12 | 138 | 0.97 | 0.86, 1.10 | 0.67 |
| >12 | 122 | 0.97 | 0.85, 1.10 | 0.62 |
| Model 2 | ||||
| <6 | 1.00 | Referent | ||
| 6–12 | 0.97 | 0.86, 1.10 | 0.65 | |
| >12 | 0.96 | 0.84, 1.09 | 0.51 | |
| Model 3 | ||||
| <6 | 1.00 | Referent | ||
| 6–12 | 0.97 | 0.86, 1.10 | 0.64 | |
| >12 | 0.96 | 0.84, 1.09 | 0.50 | |
| Gestational weight gain (top fifth compared to bottom four-fifths of the distribution) | ||||
| Model 1 | 1,285 | 0.83 | 0.76, 0.92 | <0.001 |
| Model 2 | 0.84 | 0.76, 0.92 | <0.001 | |
| Model 3 | 0.84 | 0.76, 0.93 | <0.001 | |
| Parity | 1,365 | |||
| Model 1 | 1.03 | 0.98, 1.07 | 0.27 | |
| Model 2 | 1.01 | 0.96, 1.06 | 0.64 | |
| Model 3 | 1.02 | 0.97, 1.07 | 0.52 | |
| Birth weight (per 500 g) | ||||
| Model 1 | 1,287 | 1.00 | 0.96, 1.04 | 0.83 |
| Model 2 | 1.00 | 0.96, 1.04 | 0.88 | |
| Model 3 | 1.00 | 0.95, 1.04 | 0.90 | |
| Size for gestational age | ||||
| Model 1 | ||||
| Small | 145 | 0.99 | 0.87, 1.11 | 0.81 |
| Average | 1,048 | 1.00 | Referent | |
| Large | 94 | 0.93 | 0.81, 1.08 | 0.34 |
| Model 2 | ||||
| Small | 0.99 | 0.88, 1.11 | 0.83 | |
| Average | 1.00 | Referent | ||
| Large | 0.93 | 0.81, 1.08 | 0.35 | |
| Model 3 | ||||
| Small | 0.97 | 0.86, 1.10 | 0.67 | |
| Average | 1.00 | Referent | ||
| Large | 0.92 | 0.80, 1.07 | 0.29 |
Abbreviation: CI, confidence interval.
a Model 1 was unadjusted. Model 2 was adjusted for maternal age and household social class, Model 3 was adjusted for maternal age, household social class, gestational age, offspring pubertal stage, and body mass index.
b A ratio of geometric means is interpreted as relative % differences with a null value of 1.
c Planned pregnancies only.
RESULTS
AMH values were measured in 1,751 female adolescents, of whom 1,399 (80%) contributed to at least 1 exposure–outcome association (see Figure 1 and Tables 1–4). The median AMH level was 3.67 ng/mL (interquartile range, 2.46–5.57). Table 1 shows the characteristics of included participants compared with 1) those of participants excluded because of missing exposure or confounder data (but for whom AMH measurements were available) and 2) all female adolescents who attended the 14–16–year clinic. Participants for whom we had AMH measurements but who were excluded from analyses because missing exposure or covariate data were slightly older and heavier and had higher cotinine levels on average than those included in analyses. Excluded parents were younger, more likely to smoke before and during pregnancy, and more likely to abstain from alcohol consumption. Differences were fewer and smaller in magnitude when comparing participants included in analyses with those who attended the 14–16–year clinic for whom data was missing on AMH, exposures, or covariates. Web Table 1 (available at http://aje.oxfordjournals.org/) shows a comparison of the included participants and the rest of the original cohort. As expected, included participants were older, were from a higher social class, had a lower mean BMI, and were less likely to smoke and to have 3 or more children than were the reminder of the cohort. They were also less likely to not consume any alcohol, had heavier babies, and had a longer mean gestation.
Within the limited age range during which AMH was measured, offspring age was not associated with AMH levels (per 6-months older age, ratio of geometric mean = 1.03, 95% confidence interval: 0.97, 1.09). The majority of participants were Tanner stage 4 (n = 582; 41.6%) or 5 (n = 741; 53%). Levels of AMH increased across Tanner stages (stage ≤3: median, 2.8 ng/mL, interquartile range, 2.0–4.7; stage 4: median, 3.5 ng/mL, interquartile range, 2.3–5.2; stage 5: median, 3.9 ng/mL, inter quartile range, 2.3–5.9).
Associations of prenatal maternal and paternal characteristics with offspring AMH levels are reported in Table 2. There was no evidence of an association between maternal smoking before or during pregnancy and offspring AMH in any of the models. In contrast, there were inverse associations between paternal smoking both before and during pregnancy and offspring AMH levels that persisted in all models (models 1–3). Associations of maternal and paternal smoking in frequency categories in each trimester are reported in the Web Table 2 and are consistent with the results presented in Table 2. Namely, the frequency of paternal smoking in trimesters 1 and 2 was inversely associated with offspring AMH levels, whereas no association was noted for maternal smoking before pregnancy or in each of the 3 trimesters. There was no evidence of associations of either maternal or paternal age, BMI, or alcohol intake with offspring AMH levels.
There was no strong statistical evidence of differences between any of the maternal and paternal estimates except with regard to parental smoking during pregnancy and offspring AMH levels (for difference between maternal and paternal estimates, P = 0.06). Maternal and paternal mutually adjusted estimates and results of the Wald tests for a difference between estimates are provided in Table 3.
Table 4 shows associations of fertility and pregnancy characteristics with offspring AMH levels. Mothers who had no or irregular menstrual cycles in the year preceding pregnancy had offspring with higher AMH levels than did those with regular cycles, with point estimates being larger for women who reported no periods compared with those who reported irregular periods in all models. Visiting a doctor to investigate potential infertility, time to conception, and parity were not associated with offspring AMH levels. There was evidence that being in the top fifth of the GWG distribution was associated with lower offspring AMH levels in all models (Table 4). There was no evidence of associations of birth weight or size for gestational age with AMH levels.
Results were not substantially changed when we excluded participants in the top 20% of the AMH distribution as a proxy measure for the presence of polycystic ovary syndrome (PCOS), although confidence intervals were wider (results available from authors on request). Results (also available from authors on request) were unchanged if pubertal stage was replaced with age at menarche.
DISCUSSION
In the present study, paternal (but not maternal) smoking before and during pregnancy and high GWG were associated with lower AMH levels in female adolescent offspring. No or irregular maternal menstrual cycles prior to conception were associated with higher offspring AMH levels. Other characteristics, including parental age, BMI, and alcohol intake in pregnancy, maternal parity, and time to conception were not associated with offspring AMH levels.
The lack of a strong association between maternal smoking and offspring AMH level is striking given the detrimental impact of a woman's own smoking on her reproductive health (26). However, our findings are consistent with those from a recent analysis of 527 adult women (mean age, 32.7 years) in which prenatal exposure to maternal smoking had no influence on serum AMH levels. Stereological analysis of neonatal ovarian tissue would provide the definitive answer on the impact of maternal smoking on follicular development; however, this is not ethically feasible. Analysis of AMH levels in cord blood may also not be feasible, as AMH has been shown to be undetectable in a large proportion of cord blood samples (17), although this may reflect the limited sensitivity of the current assay (23). In our cohort, we do not have cord blood measures of AMH.
The mechanism underlying the association of paternal smoking before and during pregnancy with offspring AMH levels remains unclear, but our results are in line with those of a recent publication in which an association between paternal smoking during pregnancy and a shorter reproductive lifespan of their daughters (defined as age at menopause minus age at menarche) was reported (27). Preconceptual paternal smoking has been associated with DNA damage in the cord blood of the offspring whereas maternal passive smoking has not, indicating that the association of paternal smoking was transmitted via the spermatozoal genome (28). In animal models, transient tobacco exposure can inhibit granulosa cell proliferation and promote apoptosis (29, 30). Whether this can be transmitted transgenerationally is at present unknown. It is unlikely that the association of paternal smoking during pregnancy is simply a reflection of a detrimental influence of postnatal smoking, as we might then expect to see an association with maternal smoking as well. Nevertheless, we cannot rule out the possibility that the association between paternal smoking and offspring AMH is a chance finding, particularly in light of the large number of associations for which we tested. Moreover, the magnitude of the association (AMH levels 11%–12% lower in smokers than in nonsmokers) is modest.
Higher AMH levels in pre- and peripubertal daughters of mothers with PCOS have previously been reported (31, 32). Our finding that AMH levels were higher in offspring of women with no menstrual bleeding and irregular cycles, even after adjustment for Tanner stage, is in line with these findings (33). Although we do not have detailed PCOS phenotyping in the mothers, the history of either no or irregular cycles in women who subsequently conceived suggests that this may reflect the relative severity of PCOS rather than a perimenopausal state, particularly as none of these women conceived using donor oocytes. However, there may be other reasons for cycle irregularity, such as breast feeding and the use of various medications. Unfortunately, information on the cause of cycle irregularity was not collected. Our observed lack of association between offspring AMH levels and the parental time to conception or their need to see a doctor regarding infertility is consistent with a recent study in which investigators demonstrated that young women (20–35 years of age) with low AMH levels for their age did not take longer to conceive (19); however, a study in older women (30–44 years of age) found that lower AMH levels were associated with reduced fecundability (34).
We found no association between being born small for gestational age and AMH levels at 15 years of age. This is in line with some (35, 36) but not all previous reports (12). Different results may reflect age at sampling and/or cohort size (37). Moreover, we found no association of birth weight with offspring AMH levels, which was also consistent with previous studies in adults (38).
Finally, we found an association of high GWG with lower offspring AMH levels. Although excessive GWG is associated with increased offspring adiposity during childhood (39), adjustment for offspring BMI did not alter the estimate, which suggests that excess adiposity in the offspring is not responsible for the relationship of maternal GWG with lower AMH levels.
Strengths and limitations
The strengths of the present study are its large sample size, the detailed information on both maternal and paternal characteristics measured in pregnancy, and the information on other pregnancy characteristics. Although this study provides a comprehensive assessment of a wide range of hypothesized plausible prenatal risk factors, we have conducted multiple tests and it is not impossible that some findings may be chance findings. Hence, further replication of our findings is required.
We appreciate that although AMH directly reflects the number of stereologically determined follicles remaining within the primordial pool in adults, this relationship may differ in children and adolescents, particularly in the peripubertal years. However, it has been shown that variation in AMH levels at any given age most likely reflects the variation in the number of primordial follicles and that AMH is unaltered by the onset of menarche (40). We have previously demonstrated that AMH levels throughout life parallel follicular recruitment, which peaks at 14 years of age whereas AMH continues to rise after the age of 14 years, potentially reflecting granulosa cell mass and maturation (14). Further repeated measurements in these adolescents during their adult lives will further clarify these relationships.
The lack of ultrasound and biochemical information to allow diagnosis of PCOS is an additional limitation. PCOS is characterized by elevated AMH levels (41, 42), but higher AMH levels within the normal range particularly at 15 years of age, may also indicate a “healthy” reproductive system. We found no evidence of a nonlinear effect in any of the associations we examined, and results were unchanged when participants with AMH values in the top 20% of the distribution were excluded, which suggests that the top end of the AMH distribution reflects a healthy phenotype. Another potential limitation is selection bias, but although we cannot directly test this, there is no reason to believe that associations of interest would be fundamentally different in excluded participants. In summary, we found that paternal smoking and maternal GWG and irregular periods prior to conception were associated with circulating AMH levels in female adolescent offspring, which suggests that these exposures may influence the development and function of the female ovary.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Medical Research Council Integrative Epidemiology Unit at the University of Bristol, University of Bristol, Bristol, United Kingdom (Abigail Fraser, Emma L. Anderson, Debbie A. Lawlor); School of Medicine, University of Glasgow, Glasgow, United Kingdom (William McNally, Richard Fleming, Scott M. Nelson); British Heart Foundation Glasgow Cardiovascular Research Centre, Faculty of Medicine, University of Glasgow, Glasgow, United Kingdom (Naveed Sattar); and Department of Human Metabolism, University of Sheffield, Sheffield, United Kingdom (Hany Lashen).
The work presented in this paper was funded by grants from Glasgow Centre for Reproductive Medicine Ltd., Wellcome Trust (grant WT087997) and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK077659). Abigail Fraser is funded by a UK Medical Research Council research fellowship (grant 0701594). The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provided core funding support for the Avon Longitudinal Study of Parents and Children. The UK Medical Research Council and the University of Bristol provided core funding for the MRC Integrative Epidemiology Unit at the University of Bristol.
Debbie A. Lawlor and Scott M. Nelson contributed equally to this work.
We thank Anne Currie and Anne Alexander based at Glasgow Royal Infirmary Department of Biochemistry for the excellent technical input to key laboratory measurements. We are grateful to the midwives for their help in recruiting participant and the whole Avon Longitudinal Study of Parents and Children team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
The views expressed in this paper are those of the authors and not necessarily those of any funding body or others whose support is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Morris DH, Jones ME, Schoemaker MJ, et al. Familial concordance for age at natural menopause: results from the Breakthrough Generations Study. Menopause. 2011;18(9):956–961. doi: 10.1097/gme.0b013e31820ed6d2. [DOI] [PubMed] [Google Scholar]
- 3.Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352(9134):1084–1085. doi: 10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- 4.van Asselt KM, Kok HS, Pearson PL, et al. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82(5):1348–1351. doi: 10.1016/j.fertnstert.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83(6):1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 6.de Bruin JP, Bovenhuis H, van Noord PA, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16(9):2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- 7.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5(1):e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strohsnitter WC, Hatch EE, Hyer M, et al. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol. 2008;167(6):727–733. doi: 10.1093/aje/kwm351. [DOI] [PubMed] [Google Scholar]
- 9.Hatch EE, Troisi R, Wise LA, et al. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol. 2006;164(7):682–688. doi: 10.1093/aje/kwj257. [DOI] [PubMed] [Google Scholar]
- 10.Tom SE, Cooper R, Kuh D, et al. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod. 2010;25(3):791–798. doi: 10.1093/humrep/dep451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner AZ, D'Aloisio AA, DeRoo LA, et al. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. 2010;172(2):140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sir-Petermann T, Marquez L, Carcamo M, et al. Effects of birth weight on anti-Müllerian hormone serum concentrations in infant girls. J Clin Endocrinol Metab. 2010;95(2):903–910. doi: 10.1210/jc.2009-1771. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KR, Hodnett GM, Knowlton N, et al. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Fleming R, Kelsey TW, Anderson RA, et al. Interpreting human follicular recruitment and antimullerian hormone concentrations throughout life. Fertil Steril. 2012;98(5):1097–1102. doi: 10.1016/j.fertnstert.2012.07.1114. [DOI] [PubMed] [Google Scholar]
- 15.Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 17.Hagen CP, Aksglaede L, Sorensen K, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003–5010. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey TW, Anderson RA, Wright P, et al. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18(2):79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 19.Hagen CP, Vestergaard S, Juul A, et al. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril. 2012;98(6):1602–1608. doi: 10.1016/j.fertnstert.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 21.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace AM, Faye SA, Fleming R, et al. A multicentre evaluation of the new Beckman Coulter anti-Müllerian hormone immunoassay (AMH Gen II) Ann Clin Biochem. 2011;48(pt 4):370–373. doi: 10.1258/acb.2011.010172. [DOI] [PubMed] [Google Scholar]
- 24.Fraser A, Tilling K, MacDonald-Wallis C, et al. Associations of gestational weight gain with mothers' BMI, waist circumference and blood pressure measured 16 years post-pregnancy: the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93(6):1285–1292. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteilh C, Kieszak S, Flanders WD, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25(1):75–87. doi: 10.1111/j.1365-3016.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 26.Dechanet C, Anahory T, Mathieu Daude JC, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011;17(1):76–95. doi: 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda M, Fukuda K, Shimizu T, et al. Paternal smoking habits affect the reproductive life span of daughters. Fertil Steril. 2011;95(8):2542–2544. doi: 10.1016/j.fertnstert.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 28.Laubenthal J, Zlobinskaya O, Poterlowicz K, et al. Cigarette smoke-induced transgenerational alterations in genome stability in cord blood of human F1 offspring. FASEB J. 2012;26(10):3946–3956. doi: 10.1096/fj.11-201194. [DOI] [PubMed] [Google Scholar]
- 29.Paixão LL, Gaspar-Reis RP, Gonzalez GP, et al. Cigarette smoke impairs granulosa cell proliferation and oocyte growth after exposure cessation in young Swiss mice: an experimental study. J Ovarian Res. 2012;5(1):25. doi: 10.1186/1757-2215-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordel R, Laschke MW, Menger MD, et al. Nicotine does not affect vascularization but inhibits growth of freely transplanted ovarian follicles by inducing granulosa cell apoptosis. Hum Reprod. 2006;21(3):610–617. doi: 10.1093/humrep/dei393. [DOI] [PubMed] [Google Scholar]
- 31.Crisosto N, Codner E, Maliqueo M, et al. Anti-Müllerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(7):2739–2743. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- 32.Sir-Petermann T, Codner E, Maliqueo M, et al. Increased anti-Müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(8):3105–3109. doi: 10.1210/jc.2005-2693. [DOI] [PubMed] [Google Scholar]
- 33.Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 34.Steiner AZ, Herring AH, Kesner JS, et al. Antimüllerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lem AJ, Boonstra VH, Renes JS, et al. Anti-Müllerian hormone in short girls born small for gestational age and the effect of growth hormone treatment. Hum Reprod. 2011;26(4):898–903. doi: 10.1093/humrep/deq391. [DOI] [PubMed] [Google Scholar]
- 36.Sadrzadeh-Broer S, Kuijper EA, Van Weissenbruch MM, et al. Ovarian reserve in young women with low birth weight and normal puberty: a pilot case-control study. Gynecol Endocrinol. 2011;27(9):641–644. doi: 10.3109/09513590.2010.508544. [DOI] [PubMed] [Google Scholar]
- 37.Sir-Petermann T, Hitchsfeld C, Codner E, et al. Gonadal function in low birth weight infants: a pilot study. J Pediatr Endocrinol Metab. 2007;20(3):405–414. doi: 10.1515/jpem.2007.20.3.405. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhof GF, Leunissen RWJ, Willemsen RH, et al. Influence of preterm birth and small birth size on serum anti-Müllerian hormone levels in young adult women. Eur J Endocrinol. 2010;163(6):937–944. doi: 10.1530/EJE-10-0528. [DOI] [PubMed] [Google Scholar]
- 39.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagen CP, Aksglaede L, Sorensen K, et al. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27(3):861–866. doi: 10.1093/humrep/der435. [DOI] [PubMed] [Google Scholar]
- 41.Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 42.Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


