Abstract
Subjects from the Epidemiologic Catchment Area Program, interviewed during 1979–1983, were linked to data in the National Death Index through 2007 to estimate the association of mental and behavioral disorders with death. There were more than 25 years of follow-up for 15,440 individuals, with 6,924 deaths amounting to 307,881 person-years of observation. Data were analyzed by using age as the time scale and parametric approaches to quantify the years of life lost due to disorders. Alcohol, drug use, and antisocial personality disorders were associated with increased risk of death, but there was no strong association with mood and anxiety disorders. Results of high- and low-quality matches with the National Death Index were similar. The 3 behavioral disorders were associated with 5–15 years of life lost, estimated along the life course via the generalized gamma model. Regression tree analyses showed that risk of death was associated with alcohol use disorders in nonblacks and with drug disorders in blacks. Phobia interacted with alcohol use disorders in nonblack women, and obsessive-compulsive disorder interacted with drug use disorders in black men. Both of these anxiety disorders were associated with lower risk of death early in life and higher risk of death later in life.
Keywords: anxiety disorders, generalized gamma distribution, mental disorder, mood disorders, mortality, personality disorders, proportional hazards model, substance-related disorders
The risk of death associated with mental disorders has been studied for more than a century (1, 2), mostly for severe disorders in institutionalized populations (3–7). The relationship to death is part of the basic descriptive epidemiologic information about mental and behavioral disorders, aiding clinicians and patients who desire information about natural history, informing estimates of disease burden, yielding potential insights into etiology, and prioritizing efforts at prevention. These relationships are less well-documented for common anxiety and mood disorders or disorders involving the use of alcohol or illegal drugs, because only a minority of individuals with these disorders receives treatment in residential settings (8, 9), where most of the research on mortality risk has been conducted. The enhanced specificity of criteria for diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III) of the American Psychiatric Association, as well as improvements in survey technology, facilitated a shift in methodology to samples drawn probabilistically from household-residing populations by using interviews that generated diagnoses similar to those used in psychiatric practice and consistent with the DSM-III, in the National Institute of Mental Health's Epidemiologic Catchment Area (ECA) program (10, 11). Sampling and recruitment for the ECA surveys, conducted in 1979–1983, were combined with follow-up through 2007 to provide more than 300,000 person-years of observation.
In studies conducted so far, the common mental and behavioral disorders are associated with increased risk of death. A recent review of death linked to major depressive disorder (12) included 14 prospective, population-based studies in which the median relative risk was 1.7. A study published after that review followed 78,282 women for 6 years and found a relative risk of 1.7 for depression (in women without diabetes) (13). There have been 7 studies of alcohol use disorders, with a median relative risk of 1.8. There have been no prior population-based studies of mortality risk and drug use disorders (DSM-III drug abuse and/or drug dependence) outside the ECA program. The research for other specific disorders includes 4 studies of panic disorder (3 were analyses of ECA data), 1 study of obsessive-compulsive disorder (from the ECA program), and no studies of social or simple phobic disorders. This knowledge gap promoted our collaboration to estimate the reduction of years of life associated with 11 categories of specific common mental and behavioral disorders by linking ECA data to the National Death Index (NDI).
MATERIALS AND METHODS
Sample
The methods of the ECA program have been described (14). This analysis combines 4 ECA sites (New Haven, Connecticut; Baltimore, Maryland; St. Louis, Missouri; and Durham, North Carolina), with a total sample size of 15,440 subjects drawn from the household-residing stratum of the population. There were 6,924 observed deaths during the follow-up period. A fifth site, in Los Angeles, California, was not included because the data permitting linkage to the NDI had been discarded. Sample weights are available that adjust the results to the population of the United States according to age, race, and sex in the 1980 US Census (15), but these weights are not used in this analysis because we have no information about vital status except for those actually interviewed.
Measures
Assessment of mental disorders was conducted by using the National Institute of Mental Health Diagnostic Interview Schedule (16, 17), which generates diagnoses according to the DSM-III (18). The Diagnostic Interview Schedule is designed for use by interviewers without clinical training, making population-based surveys such as the ECA feasible. We focused on lifetime prevalence of the 11 most common disorders. Demographic variables that may be important to the risk of death include age, race, and sex, as well as education, marital status, and a measure of socioeconomic position based on occupational prestige (19, 20).
Ascertaining vital status
The NDI provided linkage of individuals to vital statistics data from the 50 states. The following identifiers (where available) were linked to the NDI: last name, first name, sex, race, date of birth, social security number, father's surname, and last state of residence. The NDI provided up to 50 potential matches of deaths to each submitted person. Other sources of information about vital status included the Social Security Death Index and information obtained during the process of recruiting subjects for follow-up surveys.
From these sources we estimate that 6,924 of the 15,440 subjects who participated in baseline interviews were deceased by the end of 2007 (Table 1). Because there are many situations in which the matching is less than perfect, an ordinal scale ranking the quality of information about vital status from these various sources was created, allowing us to study the sensitivity of the results to the quality of the match (Table 1). For 1,771 subjects whose deaths were recorded in searches prior to this current project (21, 22), the scale could not be applied. The scale was applied to the remaining 5,153 subjects presumed to be dead (Table 1).
Table 1.
Frequency and Percent of Rating Criteria for Matching Subjects From 4 Epidemiologic Catchment Area Programa Sites With the National Death Index, 1979–1983 Through 2007
| Match Quality | New Haven, Connecticut |
Baltimore, Maryland |
St. Louis. Missouri |
Durham, North Carolina |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Definiteb | 0 | 0.0 | 940 | 73.6 | 80 | 8.2 | 0 | 0.0 | 1,020 | 19.8 |
| Near definitec | 0 | 0.0 | 52 | 4.1 | 51 | 5.3 | 0 | 0.0 | 104 | 2.0 |
| Very probabled | 1,112 | 79.0 | 175 | 13.7 | 560 | 57.6 | 1,087 | 72.7 | 2,933 | 56.9 |
| Probablee | 138 | 9.8 | 63 | 4.9 | 85 | 8.7 | 162 | 10.8 | 448 | 8.7 |
| Likelyf | 32 | 2.3 | 19 | 1.5 | 36 | 3.7 | 68 | 4.6 | 155 | 3.0 |
| Possibleg | 45 | 3.2 | 3 | 0.2 | 21 | 2.2 | 55 | 3.7 | 124 | 2.4 |
| Potentialh | 81 | 5.8 | 26 | 2.0 | 139 | 14.3 | 123 | 8.2 | 369 | 7.2 |
| Total rated | 1,408 | 100.0 | 1,278 | 100.0 | 972 | 100.0 | 1,495 | 100.0 | 5,153 | 100.0 |
| Not rated | 1,409 | 362 | 0 | 0 | 1,771 | |||||
| Percent of total | 50.0 | 22.1 | 0.0 | 0.0 | 25.6 | |||||
| Total deceased | 2,817 | 1,640 | 972 | 1,495 | 6,924 | |||||
a National Institute of Mental Health's Epidemiologic Catchment Area Program (Eaton, et al. Public Health Rep. 1981;96(4):319–324).
b All submitted identifying characteristics, including the social security number, matched perfectly.
c All characteristics matched except 1 digit in the social security number.
d The social security number was not submitted, but all submitted fields matched.
e All submitted fields matched except race (n = 53), or race was not submitted (n = 6), or 1 or more letters after the first letter of the first name did not match (n = 156), or 1 field in the date of birth did not match.
f In the absence of a good National Death Index match, there was a strong match to the Social Security Death Index, including name, date of birth, and, often, state of death.
g The day of birth and the social security number were not submitted, but all other fields matched, and the name was not 1 of the 1,000 most common.
h Presumed to be deceased, but the match is less convincing.
Analysis
The analyses reported below used the Cox proportional hazards procedure to quantify the relative hazard of each mental disorder adjusted for 6 sociodemographic characteristics, as well as for the presence of 1 or more additional disorders (i.e., comorbidities). To quantify reductions in absolute years of life due to mental disorders, we used the generalized gamma (GG) distribution (23). The GG method fits a curve to the survival distribution and estimates 3 parameters, β, σ, and κ, corresponding to location (e.g., median), scale (e.g., ratio of third to first quartiles), and shape (e.g., form of the hazard), respectively. The pth percentile of the GG t(p; β, σ, κ) can be expressed in terms of the 3 parameters by the equation t(p; β, σ, κ) = eβ[t(p; 0, 1, κ)]σ, which determines the percentiles of the distribution (i.e., the pth percentile of the standard (β = 0 and σ = 1) GG with shape κ elevated to the power σ and multiplied by the antilog of β yields the pth percentile of GG(β, σ, κ)). Once the shape is fixed by a value for κ, the ratio of 2 percentiles (e.g., third to first quartiles) determines the value of the scale parameter σ, and the median determines the value of the location parameter β. The lognormal distribution corresponds to the case of κ = 0 and, in this case, the location parameter β corresponds to the logarithm of the median. It often occurs that results from proportional hazards models agree closely with those of Weibull regression, which is the case of a GG for κ = 1 (23). The analyses based on the GG distribution do not require the assumption of proportionality for the hazards and have the advantage of describing the nature of the hazard function, as well as measures of the extension or contraction of the survival times due to a beneficial or harmful exposure (23). Software to fit GG models is available (e.g., streg in Stata software (StataCorp LP, College Station, Texas), lifereg in SAS software (SAS Institute, Inc., Cary, North Carolina), R (R Foundation for Statistical Computing, Vienna, Austria), and S-PLUS (Insightful Corporation, Seattle, Washington) as offered at www.statepi.jhsph.edu/software/generalgamma). The goodness of fit of the GG models was assessed by comparing the nonparametric Kaplan-Meier curves to the curves estimated by the GG models.
The time scale for survival analysis was age, allowing control over this strong predictor of death. Because there were only 23 deaths (out of 6,924) in subjects under 30 years of age, we used years after age 30 as the time scale for the analysis, and these early deaths were excluded from the analysis, yielding an analytical sample of 15,417 individuals. The 23 excluded individuals were 74% male and 48% white and included 11 individuals who had any mental or behavioral disorder. Those who were younger than 30 years at baseline contributed to the analysis with years after age 30 entered into the analysis at time 0 (i.e., no late entry). Subjects alive on December 31, 2007, or whose apparent ages were greater than 105 years were censored.
The multivariable analysis included use of the GG model to construct regression trees to maximize the ability to detect interactions (especially comorbidities of mental and behavioral disorders). For each race- and sex-specific stratum, binary recursive partitioning methodology (24, 25) was used for the presence/absence of each of the 11 behavioral and mental disorders. At a given node, for each of the disorders eligible to determine a split, we fit 2 GG models (1 for those with and 1 for those without a given disorder) and determined the significance of the difference in survival between the 2 groups by the likelihood ratio test. The variable that yielded the highest likelihood ratio statistic was then used as the splitter to define subsequent child nodes. This process continued until the likelihood ratio statistic was no longer significant at the α level of 0.05 under a χ2 distribution with 3 degrees of freedom, and/or a child node contained fewer than 25 individuals. Once the final nodes for each race- and sex-specific stratum were determined, we compared the percentiles of the GG model describing the survival of the reference group (i.e., free of behavioral and mental disorders) with those of each of the other nodes corresponding to a particular profile of disorders. Statistical significance was determined by using 1,000 bootstrap replications to estimate 95% confidence intervals for the differences in the first and last deciles and the median age or life expectancy for each node relative to the reference node.
In this study, it was assumed that all individuals died by age 105 years (i.e., even if not matched in the NDI). To consider these as interval-censored observations (26) and to develop the regression trees, we programmed the maximum likelihood methods by using PROC NLMIXED in SAS software (SAS Institute, Inc.). The studies reported here were approved by the Johns Hopkins Bloomberg School of Public Health's institutional review board.
RESULTS
Sixty percent of the baseline sample was women, and 27% was black. The nonblack subjects (73%) included a small proportion (3.5%) of Asians, Hispanics, and Native Americans. At the New Haven and Baltimore sites, more than one-third of the total sample (combined) was age 65 years or older because of oversampling in that age group. Table 2 provides the number of individuals, the total person-years, and the death rates per 1,000 person-years in 3 strata of age at enrollment (18–44, 45–64, and ≥65 years).
Table 2.
Sociodemographic Characteristics, Person-years, and Mortality Rate Stratified by Age Group From 4 Epidemiologic Catchment Area Programa Sites, 1979–1983 Through 2007
| 18–44 Years of Age |
45–64 Years of Age |
≥65 Years of Age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No.b | Person- years | Mortality Rate, % | No.b | Person- years | Mortality Rate, % | No.b | Person-years | Mortality Rate, % | |
| Total | 6,683 | 170,186 | 3.6 | 3,439 | 70,570 | 24.5 | 5,318 | 67,125 | 68.2 |
| Site | |||||||||
| New Haven, Connecticut | 1,655 | 43,692 | 2.7 | 803 | 17,436 | 22.1 | 2,576 | 31,602 | 73.2 |
| Baltimore, Maryland | 1,716 | 43,383 | 5.3 | 842 | 15,941 | 33.4 | 923 | 10,091 | 87.0 |
| St. Louis, Missouri | 1,705 | 43,580 | 3.5 | 721 | 15,222 | 22.2 | 578 | 7,413 | 64.8 |
| Durham, North Carolina | 1,607 | 39,531 | 2.9 | 1,073 | 21,971 | 21.4 | 1,241 | 18,019 | 50.6 |
| Sex | |||||||||
| Male | 2,794 | 70,587 | 4.2 | 1,355 | 26,007 | 31.3 | 1,988 | 22,398 | 79.0 |
| Female | 3,889 | 99,599 | 3.2 | 2,084 | 44,564 | 20.5 | 3,330 | 44,727 | 62.9 |
| Race/ethnicity | |||||||||
| Nonblack | 4,382 | 113,018 | 2.8 | 2,519 | 52,382 | 24.0 | 4,338 | 53,286 | 72.2 |
| Black | 2,294 | 56,986 | 5.3 | 915 | 18,069 | 26.1 | 957 | 13,608 | 52.6 |
| Educational level | |||||||||
| Some/no elementary | 40 | 988 | 7.1 | 162 | 2,929 | 33.8 | 539 | 6,312 | 69.2 |
| Elementary graduate | 50 | 1,184 | 10.1 | 216 | 3,864 | 32.1 | 512 | 6,185 | 69.4 |
| Some high school | 1,251 | 31,227 | 5.6 | 1,185 | 23,029 | 29.9 | 2,276 | 28,588 | 69.5 |
| High school graduate | 2,549 | 64,827 | 3.6 | 1,087 | 23,154 | 21.9 | 1,023 | 13,378 | 66.2 |
| Some college | 1,452 | 37,171 | 3.3 | 379 | 8,316 | 19.2 | 467 | 6,226 | 64.7 |
| College graduate | 1,319 | 34,245 | 1.9 | 391 | 8,887 | 15.9 | 439 | 5,806 | 66.8 |
| Marital status | |||||||||
| Married | 3,159 | 80,981 | 3.1 | 2,021 | 42,653 | 22.7 | 2,155 | 28,480 | 65.2 |
| Widowed | 70 | 1,726 | 6.4 | 566 | 10,859 | 28.8 | 2,428 | 29,375 | 71.4 |
| Divorced/separated | 1,187 | 29,675 | 5.3 | 608 | 12,386 | 24.5 | 369 | 4,759 | 64.7 |
| Never married | 2,261 | 57,667 | 3.4 | 241 | 4,620 | 30.3 | 354 | 4,451 | 69.2 |
| Occupational status percentilec | |||||||||
| 0.0%–25.0% | 1,233 | 31,127 | 4.3 | 852 | 16,367 | 29.0 | 1,456 | 19,018 | 63.0 |
| 25.1%–50.0% | 2,406 | 60,964 | 4.1 | 1,189 | 24,463 | 24.7 | 1,666 | 20,896 | 70.5 |
| 50.1%–75.0% | 1,454 | 37,187 | 3.2 | 709 | 14,956 | 22.4 | 1,017 | 12,423 | 72.6 |
| 75.1%–100.0% | 1,140 | 29,439 | 2.2 | 474 | 10,423 | 19.9 | 577 | 7,532 | 67.3 |
| Missing | 450 | 11,469 | 4.1 | 215 | 4,361 | 24.1 | 602 | 7,256 | 68.8 |
a National Institute of Mental Health's Epidemiologic Catchment Area Program (Eaton, et al. Public Health Rep. 1981;96(4):319–324).
b Because of missing data, the figures in some categories do not sum to the total for each age category.
c According to the Nam-Powers occupational status index described in Nam C, et al. Soc Forces. 1968;47(2):158–170.
The death rates in the 3 age groups were radically different (3.6, 24.5, and 68.2 per 1,000 person-years for age groups 18–44, 45–64, and ≥65 years, respectively). This dictated the need to describe the death rates for the rest of the variables in Table 2, stratifying by age, as well as providing logic for using age as the time scale in the analysis. Death rates among women were consistently lower than those among men in each age group (Table 2). In contrast, young blacks (18–44 years of age) had twice the risk of dying compared with nonblacks (i.e., 5.3 and 2.8, respectively), but the directionality reversed among those older than 65 years, when blacks had three-fourths the rate of nonblacks (i.e., 52.6 and 72.2, respectively). This change in relative risk over the lifespan would be masked by an analysis assuming proportional hazards, contributing to the logic of the GG approach. Education had a strong protective effect among those less than 44 years of age, which attenuated among those between 45 and 64 years of age and essentially vanished among those older than 65 years. Widowed, divorced, or separated individuals younger than 45 years had close to double the risk of dying compared with those who were married, but otherwise there was no strong association with marital status. Those in the lower quartile of occupational prestige had a higher risk of death in the young and middle age groups, but not among those over age 65 years.
Estimates of the lifetime prevalence of the mental disorders shown in Table 3 are consistent with early publications from the ECA program (27). Phobic disorder was the most common (15.2%), and alcohol use disorder was the second most common (10.3%). Nearly a third of the sample (31.0%) had at least 1 of the 11 specific disorders. Even the rarest specific disorder (panic, 1.3%) provided more than 4,700 person-years of risk.
Table 3.
Lifetime Prevalence of 11 Mental Disorders at Baseline, Frequency of Death, Person-years, and Adjusted Proportional Hazards From 4 Epidemiologic Catchment Area Programa Sites, 1979–1983 Through 2007
| Mental Disorder | Total |
Deceased |
Person-years | Adjusted Proportional HRb | 95% CI | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Panic disorder | 205 | 1.3 | 49 | 23.9 | 4,742 | 1.01 | 0.76, 1.36 |
| Any phobic disorder | 2,348 | 15.2 | 912 | 38.8% | 49,050 | 1.00 | 0.92, 1.08 |
| Agoraphobia | 969 | 6.3 | 390 | 40.3% | 20,191 | 1.02 | 0.92, 1.14 |
| Social phobia | 324 | 2.1 | 105 | 32.4% | 7,035 | 1.08 | 0.89, 1.32 |
| Simple phobia | 1,966 | 12.7 | 776 | 39.5% | 40,810 | 1.02 | 0.94, 1.10 |
| Obsessive-compulsive disorder | 389 | 2.5 | 113 | 29.1% | 8,548 | 0.78 | 0.63, 0.95 |
| Major depressive disorder | 731 | 4.7 | 164 | 22.4% | 17,133 | 0.86 | 0.72, 1.03 |
| Dysthymic disorder | 465 | 3.0 | 162 | 34.8% | 10,119 | 0.84 | 0.71, 1.00 |
| Drug dependence/abuse | 603 | 3.9 | 81 | 13.4% | 15,100 | 1.44 | 1.13, 1.84 |
| Alcohol dependence/abuse | 1,590 | 10.3 | 642 | 40.4% | 32,882 | 1.33 | 1.21, 1.47 |
| Antisocial personality disorder | 272 | 1.8 | 88 | 32.4% | 6,037 | 2.03 | 1.61, 2.57 |
| Cognitive impairment | 380 | 2.5 | 269 | 70.8% | 5,057 | 1.02 | 0.89, 1.16 |
| Any of the above disorders | 4,779 | 31.0 | 1,829 | 38.3% | 99,351 | 1.09 | 1.03, 1.16 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a National Institute of Mental Health's Epidemiologic Catchment Area Program (Eaton, et al. Public Health Rep. 1981;96(4):319–324).
b Proportional hazards are adjusted for all variables in Table 2, as well as for comorbidity, indicated by a dichotomous variable taking the value of 0 for no other disorder and 1 for the presence of 1 or more additional disorders (data not shown). The model for any disorder was not adjusted for comorbidity. The time scale was years of age after 30. Data on diagnosis were missing for 579 individuals who were too ill or cognitively impaired to respond to questions or whose interviews were cut short for various reasons.
The following 3 behavioral disorders were associated with a significantly higher risk of death: DSM-III drug use disorder (44% increased risk of death); alcohol use disorder (33% higher risk of death); and antisocial personality disorder (double the risk of death) (Table 3). Obsessive-compulsive disorder was associated with a 22% reduced risk of death. Risk of death was higher if the individual reported more than 1 disorder (not shown).
An analysis of the sensitivity of the results to the quality of the match was conducted by running the proportional hazards models again, eliminating observations in which the match was probable, likely, possible, and potential, changing the number of deaths from 6,924 to 5,828 (data not shown). Matches obtained in earlier submissions to the NDI were included in this analysis, assuming that the majority of these would be in the “very probable” category. The results were similar, not changing the significance levels of any of the mental and behavioral disorders and changing the proportional hazards only trivially (not shown).
The GG model is not constrained by the assumption of proportional hazards and is statistically more efficient, yielding significant differences at the 5% level in all but 3 of the 13 comparisons shown in Table 4. Only 3 disorders (panic disorder, major depressive disorder, and dysthymia) did not indicate differences at the level of P < 0.05. The median and 90th percentiles for persons meeting diagnostic criteria at baseline for panic, agoraphobic, simple phobic, obsessive-compulsive, major depressive, or dysthymic disorder were only slightly different than for persons without those disorders. As with the proportional hazards model, the 3 behavioral disorders (drugs, alcohol, and antisocial personality) were associated with the largest reduction in years of life. The 10th, 50th, and 90th percentiles were approximately 10 years earlier for those with antisocial personality disorder compared with those without this disorder and approximately 7 years earlier for those with alcohol or drug use disorder.
Table 4.
Generalized Gamma Models of Mental and Behavioral Disorders Using Years of Age After 30 as the Time Scale, With Selected Percentiles From 4 Epidemiologic Catchment Area Programa Sites, 1979–1983 Through 2007
| Mental Disorder | Total No. | No. of Deaths | β (Location) | σ (Scale) | κ (Shape) | Age at Death, years |
|||
|---|---|---|---|---|---|---|---|---|---|
| 10th Percentile | 50th Percentile | 90th Percentile | P Value | ||||||
| Panic disorder | |||||||||
| No | 14,701 | 6,474 | 4.051 | 0.202 | 1.808 | 57.2 | 79.2 | 94.8 | |
| Yes | 205 | 49 | 4.089 | 0.192 | 2.259 | 54.3 | 79.0 | 95.6 | 0.39 |
| Phobic disorder | |||||||||
| No | 12,573 | 5,620 | 4.056 | 0.197 | 1.848 | 57.4 | 79.5 | 94.8 | |
| Yes | 2,346 | 910 | 4.046 | 0.214 | 1.803 | 55.9 | 78.6 | 95.0 | 0.03 |
| Agoraphobia | |||||||||
| No | 13,969 | 6,150 | 4.051 | 0.201 | 1.800 | 57.4 | 79.3 | 94.8 | |
| Yes | 968 | 389 | 4.084 | 0.196 | 2.198 | 54.4 | 78.9 | 95.5 | 0.05 |
| Social phobia | |||||||||
| No | 9,687 | 3,714 | 4.061 | 0.201 | 1.888 | 56.7 | 79.3 | 95.2 | |
| Yes | 324 | 105 | 4.018 | 0.261 | 2.007 | 48.9 | 74.2 | 94.1 | <0.0001 |
| Simple phobia | |||||||||
| No | 12,966 | 5,761 | 4.057 | 0.197 | 1.851 | 57.4 | 79.5 | 94.9 | |
| Yes | 1,965 | 775 | 4.039 | 0.219 | 1.779 | 55.6 | 78.2 | 94.8 | 0.01 |
| Obsessive-compulsive | |||||||||
| No | 14,473 | 6,385 | 4.051 | 0.201 | 1.806 | 57.3 | 79.3 | 94.8 | |
| Yes | 388 | 112 | 4.138 | 0.169 | 2.586 | 55.2 | 80.9 | 97.3 | 0.03 |
| Major depressive | |||||||||
| No | 14,179 | 6,359 | 4.050 | 0.203 | 1.809 | 57.0 | 79.2 | 94.8 | |
| Yes | 731 | 164 | 4.081 | 0.177 | 1.996 | 58.7 | 80.8 | 95.3 | 0.07 |
| Dysthymia | |||||||||
| No | 14,443 | 6,361 | 4.050 | 0.202 | 1.809 | 57.1 | 79.2 | 94.7 | |
| Yes | 464 | 161 | 4.119 | 0.167 | 2.350 | 57.3 | 81.3 | 96.5 | 0.17 |
| Drug dependence/ abuse | |||||||||
| No | 14,253 | 6,427 | 4.058 | 0.196 | 1.843 | 57.6 | 79.6 | 94.9 | |
| Yes | 598 | 76 | 3.946 | 0.266 | 1.725 | 50.2 | 72.7 | 91.0 | <0.0001 |
| Alcohol dependence/ abuse | |||||||||
| No | 13,274 | 5,864 | 4.064 | 0.192 | 1.830 | 58.3 | 80.2 | 95.2 | |
| Yes | 1,584 | 636 | 3.923 | 0.263 | 1.571 | 51.6 | 72.8 | 90.1 | <0.0001 |
| Antisocial personality | |||||||||
| No | 14,544 | 6,398 | 4.057 | 0.197 | 1.831 | 57.6 | 79.6 | 94.9 | |
| Yes | 270 | 86 | 3.818 | 0.284 | 1.554 | 48.4 | 68.1 | 85.0 | <0.0001 |
| Cognitive impairment | |||||||||
| No | 14,682 | 6,385 | 4.051 | 0.200 | 1.814 | 57.3 | 79.3 | 94.7 | |
| Yes | 379 | 268 | 4.037 | 0.281 | 2.933 | 40.0 | 67.5 | 92.5 | <0.0001 |
| None of the above | 10,333 | 4,851 | 4.066 | 0.187 | 1.835 | 58.9 | 80.4 | 95.1 | |
| Any of the above | 4,768 | 1,818 | 4.020 | 0.229 | 1.776 | 54.2 | 77.0 | 94.0 | <0.0001 |
a National Institute of Mental Health's Epidemiologic Catchment Area Program (Eaton, et al. Public Health Rep. 1981;96(4):319–324).
By starting with the full sample to develop the regression tree, we found that the most significant variable was sex (women living longer); within each category of sex, the most significant variable was race (prior to the 70th percentile, nonblacks living longer). Figure 1 depicts the Kaplan Meier and GG curves for the sex/race strata that defined the first 4 nodes of the regression tree. The fit of the GG model to the empirical survival data was good for all groups except black men, for whom there is a suggestion of bimodality with a small subset dying at a young age (∼35 years). Nonblack women (curves in purple) had the best survival, and black men (curves in blue) had the worst, with the other 2 groups (nonblack men in red; black women in green) in the middle. The curves that cross in Figure 1 show that the assumption of proportional hazards was violated for both sex and race. In the GG model, the relative hazards of black to nonblack were substantially higher at younger ages with downward trends crossing the null value of 1 at 79.5 years of age among women and 83.5 years of age among men. Thus, as in Table 2, the association of black race with death was prominent at low percentiles, but the top 20%–30% of each racial group died at similar old ages. The association of sex with death was prominent toward middle-level percentiles (40%–80%).
Figure 1.
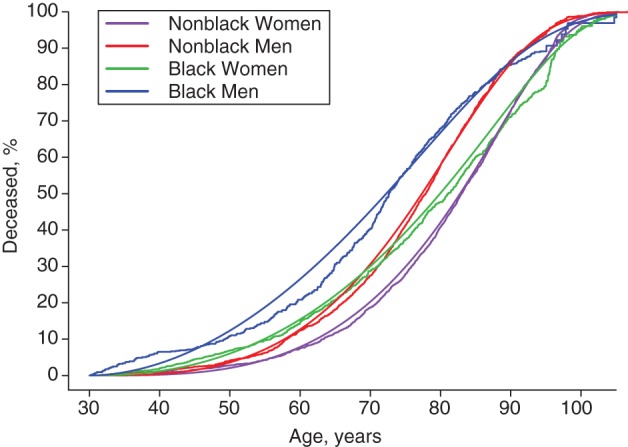
Survival curves for 4 sex/race strata in 4 sites of the Epidemiologic Catchment Area Study, 1979–1983 through 2007. Nonparametric Kaplan-Meier curves as step functions and smoothed curves for the generalized gamma (GG) distribution for cumulative proportion deceased in g groups according to nonblack/black race and female/male sex. The estimated parameters of GG models were: GG(4.10, 0.16, 1.86) for nonblack women, GG(3.99, 0.21, 1.53) for nonblack men, GG(4.11, 0.19, 2.23) for black women, and GG(3.97, 0.28, 1.87) for black men.
The regression tree identified 11 strata in final nodes with significantly distinct mortality trajectories (Table 5). Among nonblacks, alcohol use defined the first split of the regression trees for women and men. The second split among nonblack women was the protective effect of simple phobia, but only among alcohol users. In turn, the second split among nonblack men was the presence of antisocial personality, which elevated risk irrespective of alcohol use. Among blacks, drug use defined the first split of the regression trees for women and men. No other variable determined a second split among black women, but for black men, obsessive-compulsive disorder was protective for non–drug users.
Table 5.
Characteristics of 11 Categories of Respondents With Significant Relationships to Risk of Death, Proportion Deceased, and Percentile for Age at Death From 4 Epidemiologic Catchment Area Programa Sites, 1979–1983 Through 2007
| Sex and Mental or Behavioral Disorder by Race | No. of Deaths | Total No. | Age at Death, years |
||
|---|---|---|---|---|---|
| 10th Percentile | 50th Percentile | 90th Percentile | |||
| Nonblack | |||||
| Women | 2,950 | 6,308 | |||
| No alcohol use disorderb | 2,891 | 6,090 | 62.8 | 83.0 | 96.1 |
| Alcohol use disorder | 59 | 218 | |||
| No simple phobia disorder | 44 | 166 | 54.5 | 78.0 | 92.5 |
| Simple phobia disorder | 15 | 52 | 62.9c | 76.1 | 82.4 |
| Men | 2,127 | 4,476 | |||
| No alcohol use disorder | 1,772 | 3,534 | |||
| No antisocial personality disorderb | 1,761 | 3,546 | 60.4 | 78.7 | 92.5 |
| Alcohol use disorder | 355 | 892 | |||
| No antisocial personality disorder | 312 | 771 | 54.4 | 73.7 | 89.5 |
| Antisocial personality disorderd | 54 | 159 | 47.8 | 67.9 | 81.0 |
| Black | |||||
| Women | 841 | 2,646 | |||
| No drug use disorderb | 825 | 2,553 | 55.4 | 80.2 | 97.0 |
| Drug use disorder | 16 | 93 | 48.0 | 71.3 | 87.3 |
| Men | 556 | 1,373 | |||
| No drug use disorder | 533 | 1,296 | |||
| No obsessive compulsive disorderb | 522 | 1,268 | 49.1 | 73.0 | 92.5 |
| Obsessive compulsive disorder | 11 | 28 | 54.1c | 60.7c | 79.3c |
| Drug use disorder | 23 | 77 | 40.5 | 64.8 | 86.6 |
a National Institute of Mental Health's Epidemiologic Catchment Area Program (Eaton, et al. Public Health Rep. 1981;96(4):319–324).
b Reference category.
c Not significantly different from the race-by-sex reference category (see Figures 2 and 3 for confidence intervals).
d The group diagnosed with antisocial personality disorder contains individuals from both the alcohol use disorder group and the no alcohol use disorder group.
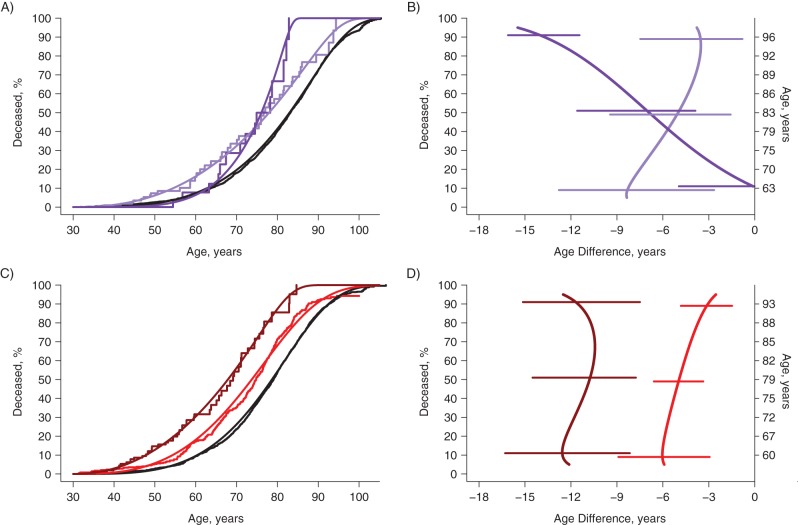
Within each of the sex/race strata, the group with the lowest mortality risk served as the reference category (curves colored black in Figures 2A, 2C, 3A, and 3C). For nonblack women, the presence of an alcohol use disorder added risk, but simple phobia was protective for those with an alcohol use disorder (Figure 2A) until approximately age 70 years, at which point a history of simple phobia was associated with higher risk of death (dark purple curve; see also the 10th and 90th percentiles for simple phobia in Table 4). From Figure 2B, it can be seen that alcohol use in the absence of phobia (light purple curve) significantly reduced survival of the earliest 10% to die by 8 years (from age 63 years in the unexposed group), decreasing to 5 years for the median and 3.5 years for those dying in the latest 10%. In contrast, those with alcohol and simple phobia disorders (dark purple curve) were not different early, but the reduction in years of life for the top 10% was a dramatic and significant reduction of 14 years relative to age 96 years, when 90% of the unexposed were estimated to die.
Figure 2.
Survival curves and estimates of years of life lost due to alcohol, phobia, and antisocial personality disorders, among nonblack women and men in 4 sites of the Epidemiologic Catchment Area Study, 1979–1983 through 2007. Kaplan-Meier and generalized gamma survival curves for nonblack women and men, showing the estimated effects of alcohol use disorder and simple phobia and alcohol use disorder and antisocial personality disorder. B and D show the years of life lost compared with individuals in the general population who do not have a history of the disorders (age at death in those without disorders is displayed on right-hand axes) with 95% confidence intervals from bootstrapping procedures represented by horizontal bars. For women (A and B), black lines denote no alcohol use disorder (2,891 of 6,090), light purple lines denote alcohol use disorder and no simple phobia disorder (44 of 166), and dark purple lines denote alcohol use and simple phobia disorders (15 of 52). For men (C and D), black lines denote no antisocial personality and no alcohol use disorders (1,761 of 3,546), red lines denote alcohol use disorder and no antisocial personality disorder (312 of 771), and burgundy lines denote antisocial personality disorder (54 of 159).
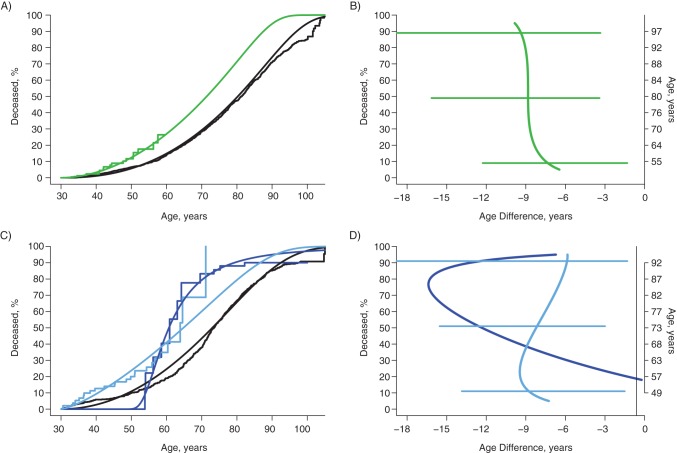
Figure 3.
Survival curves and estimates of years of life lost due to drug use disorder and obsessive-compulsive disorder in black women and men in 4 sites of the Epidemiologic Catchment Area Study, 1979–1983 through 2007. Kaplan-Meier and generalized gamma survival curves for black women and men, showing the estimated effects of drug use disorder in black women and the combination of drug use disorder and obsessive-compulsive disorder in black men. B and D show the years of life lost compared with individuals in the general population who do not have a history of the disorders (age at death in those without disorders displayed on right-hand axes), with the 95% confidence intervals from bootstrapping procedures represented by horizontal bars. The confidence intervals for the 28 individuals with no drug use disorder or obsessive-compulsive disorder (D) were so large as to be uninformative and are not shown. For women (A and B), black lines denote no drug use disorder (825 of 2,553) and green lines denote drug use disorder (16 of 93). For men (C and D), black lines denote no obsessive-compulsive disorder and no drug use disorder (522 of 1,268), dark blue lines denote obsessive-compulsive disorder and no drug use disorder (11 of 28), and light blue lines denote drug use disorder (23 of 77).
For nonblack men, the presence of antisocial personality was so strongly associated with death that the distinction between those with and without alcohol disorders was trivial, yielding 3 important final nodes in the group of nonblack men (Figure 2C). Alcohol disorder was associated with a lower age at death by approximately 5 years, and this was slightly greater for the first 10% (6 years) relative to the top 10% (4 years), as depicted in the light red curve in Figure 2D. Among nonblack individuals, the associations of death with alcohol alone in both women and men were similar (compare the light purple curve in Figure 2B with the light red curve in Figure 2D). Antisocial personality disorder among nonblack men was associated with a lower age at death by approximately 12 years, and the effect was constant across the life course (dark red curve in Figure 2D).
Drug use disorder raised the risk of death for black women (Figure 3A) and shortened life by approximately 9 years, consistently and significantly over the course of life (Figure 3B). For black men, the presence of drug use disorder at baseline had the most important association with risk of death, lowering the age at death by approximately 9 years for the first 10%–30% and decreasing to 6 years for the top 10% (light blue curves Figure 3C and 3D). Among black men who did not have a drug disorder at baseline, obsessive-compulsive disorder was associated with more than 10 years’ reduction in years of life for the 50th–90th percentiles (Figure 3D). This association had wide confidence limits because of the small number of subjects (n = 28, with 11 deaths) in this category (confidence interval not shown).
DISCUSSION
Alcohol use and antisocial personality disorders were strongly associated with death in nonblacks and with drug use disorder in blacks. The risk of death associated with mood, anxiety, and other psychiatric disorders was low, and there was evidence that obsessive-compulsive disorder was protective, consistent with a prior study of the protective effect of trait anxiety (28). The association of death with the 3 behavioral disorders is consistent with those reported in the existing literature on alcohol use disorders and adds weight to the literature on personality disorders (for which there are only 2 existing population-based studies (29, 30)) and drug use disorders (for which there are 2 prior ECA studies (22, 31)).
The combination of the GG model with regression tree analyses yielded new results on simple phobia and obsessive-compulsive disorder. Simple phobia might be associated with lower risk of death in nonblack women who have a history of alcohol use disorders because greater levels of anxiety might contribute to avoidance of injury and enhanced health-protective behaviors. Likewise, obsessive-compulsive disorder might be protective in black men who have no history of drug use disorders. However, it is not clear why both of these disorders are associated with added risk after the age of 55 or 60 years. This result might be caused by sampling variation and the limited number of subjects in these groups, or it could be caused by comorbid physical disorders that develop during this part of the life course.
The results on major depressive disorder are not consistent with those in the literature. The inconsistency is puzzling because major depressive disorder has been associated with fatal self-directed violence (32), onset of type 2 diabetes (33), heart attack (34), stroke (35), and dementia (36). It could be that earlier studies revealed an association because they did not adjust for the sociodemographic characteristics included in Table 2. However, an unadjusted model with these data (not shown) also estimated no increased risk of death associated with major depressive disorder. It could be that the influence of depressive disorder on death has been exercised earlier in the life history of individuals not included in the sample because they have died. However, an analysis that included 305 individuals who had never met criteria for major depressive disorder at baseline and who experienced an episode meeting criteria for the first time in their lives during the first year of follow-up showed little difference in results (odds ratio = 1.15, 95% confidence interval: 1.02, 1.30). The age span of the follow-up may explain the discrepancy. In the systematic review noted above (12), 6 of the 14 studies were of samples with a minimum age of 60 years or older (37–42); in another study, the minimum age was 40 years, and the average age was 67 years (21); and in the recent analysis from the Nurses’ Health Study, the minimum age was 54 years (13). Thus, depression in the elderly may take a different form (43) associated with physical decline. However, in an analysis of these data stratified by age at baseline, there was no increase in the risk of death associated with depressive disorder in those aged 65 years or older. It could be that the measure of depression in the ECA is not as sensitive as in earlier studies. To address this possibility, we conducted analyses that estimated the association of a diagnosis of depressive disorder with death at either the baseline wave or the 1-year follow-up wave, with no appreciable change in the pattern of results. It could be that the Diagnostic Interview Schedule taps a less severe form of depressive disorder than has been assessed in prior literature. To address this possibility, we created a measure of depressive disorder in which subjects reported that they had told their doctors about the problem, with no appreciable change in results (not shown). It could be that the lifetime form of prevalence used in these analyses fails to include early episodes more distant in time from the interview, and that these forgotten episodes are more severe; or, perhaps the lifetime form includes early episodes that are mild and dilute the effect. But the pattern of results of analyses with the 1-year form of prevalence was no different than for lifetime prevalence (data not shown). It could be that the risk of death is more likely to appear in a study of the elderly in which the follow-up is not as long as in this study, because episodes more distant in time are less likely to be recalled by the elderly, and more recent episodes are of the form more highly associated with death (44, 45). Indeed, it could be that the somatic symptoms of chronic fatal diseases (e.g., problems with fatigue, sleep, appetite, movement, or concentration) overlap with those of depressive disorder, and that individuals who recognize themselves to be in physical decline are sad, anhedonic, and/or thinking of death. Of the population-based studies reviewed (13, 39, 41, 42, 46–51), only the Stirling County Study (52) and the Nurses’ Health Study (13) had follow-up periods longer than 10 years. When we analyzed these data by time since baseline assessment, we saw a slightly heightened risk for depression in the first 9 years of follow-up and a reduced risk in the next 15 years of follow-up (data not shown). Therefore, the most credible explanation for the entire sum of results is that depressive disorder itself is not strongly associated with high risk of death, but rather is concomitant with rapidly declining health and may even be protective in the long term. Future analyses of these data focused on specific causes of death may yield insight into this issue.
The strengths of this analysis include the large number of person-years of observation, the population-based sample, the sensitivity analyses that suggest that imprecise matching has not threatened the results, and the use of GG and regression tree models that generate enhanced precision, as well as informative analysis of comorbidity. The parametric approach based on the GG model provided excellent goodness of fit, did not restrict the analysis to proportional hazards, and facilitated quantification of reduction of life expectancies due to deleterious exposures at all percentiles of the distribution of ages at death for different groups (Figures 2B, 2D, 3B, and 3D). Weaknesses include the relatively small number of subjects in some of the groups defined by the regression tree procedure and the limitation to common mental and behavioral disorders, excluding important disorders with documented effects on mortality risk, such as schizophrenia and bipolar disorder. The regression tree analysis has the advantage of being flexible and directly incorporating effect modification (e.g., among nonblack individuals, alcohol is a primary risk factor for death, but among blacks, it is drug use); however, to arrive at the final tree, many comparisons are estimated. As a result, the nominal significance level may be higher than 5%, and it is important to describe the magnitude of the differences in years of life lost as indicated in the tables and figures herein, as well as the level of statistical significance.
ACKNOWLEDGMENTS
Author affiliations: Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (William W. Eaton, Kimberly Roth, George Rebok); Department of Psychiatry, Westchester Division, Weill Cornell Medical College, White Plains, New York (Martha Bruce); Department of Epidemiology, College of Public Health and Health Professions and College of Medicine, University of Florida, Gainesville, Forida (Linda Cottler); Department of Psychiatry, School of Medicine, Duke University, Durham, North Carolina (LiTzy Wu); Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Gerald Nestadt, O. Joseph Bienvenu); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Dan Ford); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Rosa Crum, Alvaro Muñoz); and Department of Epidemiology, Michigan State University, East Lansing, Michigan (James C. Anthony).
This work was supported by the National Institute on Drug Abuse (grant DA026652).
We are grateful to Preben Bo Mortensen for helpful comments.
Conflict of interest: none declared.
REFERENCES
- 1.Editor. Mortality in lunatic asylums. Am J Insanity. 1848;4:253–258. [Google Scholar]
- 2.Hanley AJ, Meigs JB, Williams K, et al. Re: “(Mis)use of factor analysis in the study of insulin resistance syndrome” [letter] Am J Epidemiol. 2005;161(12):1182–1184. doi: 10.1093/aje/kwi153. [DOI] [PubMed] [Google Scholar]
- 3.Malzberg B. Rates of discharge and rates of mortality among first admissions to the New York Civil State Hospitals. III. Ment Hyg. 1953;37(4):619–654. [PubMed] [Google Scholar]
- 4.Malzberg B. Rates of discharge and rates of mortality among first admissions to the New York Civil State Hospitals. II. Ment Hyg. 1952;36(4):618–638. [PubMed] [Google Scholar]
- 5.Malzberg B. Rates of discharge and rates of mortality among first admissions to the New York Civil State Hospitals. Ment Hyg. 1952;36(1):104–120. [PubMed] [Google Scholar]
- 6.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999–2006. BMJ. 2011;343:d5422. doi: 10.1136/bmj.d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojtabai R, Eaton WW, Maulik PK. Faculty, Students and Fellows of the Department of Mental Health, Bloomberg School of Public Health. Pathways to care: need, attitudes, barriers. In: Eaton WW, editor. Public Mental Health. New York, NY: Oxford University Press; 2012. pp. 415–456. [Google Scholar]
- 9.Shapiro S, Skinner EA, Kessler LG, et al. Utilization of health and mental health services. Three Epidemiologic Catchment Area sites. Arch Gen Psychiatry. 1984;41(10):971–978. doi: 10.1001/archpsyc.1984.01790210053007. [DOI] [PubMed] [Google Scholar]
- 10.Eaton WW, Regier DA, Locke BZ, et al. The Epidemiologic Catchment Area Program of the National Institute of Mental Health. Public Health Rep. 1981;96(4):319–325. [PMC free article] [PubMed] [Google Scholar]
- 11.Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area (ECA) Program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934–941. doi: 10.1001/archpsyc.1984.01790210016003. [DOI] [PubMed] [Google Scholar]
- 12.Eaton WW, Alexandre PI, Kessler RC, et al. the Faculty Students, and Fellows of the Department of Mental Health, Bloomberg School of Public Health. The population dynamics of mental disorders. In: Eaton WW, editor. Public Mental Health. New York, NY: Oxford University Press; 2012. pp. 125–150. [Google Scholar]
- 13.Pan A, Lucas M, Sun Q, et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68(1):42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton WW, Kessler LG. Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, FL: Academic Press, Inc; 1985. [Google Scholar]
- 15.Holzer CE, Spitznagel E, Jordan KB, et al. Sampling the household population. In: Eaton WW, Kessler LG, editors. Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, FL: Academic Press, Inc; 1985. pp. 23–48. [Google Scholar]
- 16.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 17.Robins LN, Helzer JE, Orvaschel H, et al. The Diagnostic Interview Schedule. In: Eaton WW, Kessler LG, editors. Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, FL: Academic Press, Inc; 1985. pp. 143–170. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual. 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 19.Nam C, LaRocque J, Powers M, et al. Changes in the relative status level of workers in the United States, 1950–1960. Soc Forces. 1968;47(2):158–170. [Google Scholar]
- 20.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–122. doi: 10.1093/oxfordjournals.epirev.a036030. [DOI] [PubMed] [Google Scholar]
- 21.Bruce ML, Leaf PJ, Rozal GP, et al. Psychiatric status and 9-year mortality data in the New Haven Epidemiologic Catchment Area Study. Am J Psychiatry. 1994;151(5):716–721. doi: 10.1176/ajp.151.5.716. [DOI] [PubMed] [Google Scholar]
- 22.Kouzis A, Eaton WW, Leaf PJ. Psychopathology and mortality in the general population. Soc Psychiatry Psychiatr Epidemiol. 1995;30(4):165–170. doi: 10.1007/BF00790655. [DOI] [PubMed] [Google Scholar]
- 23.Cox C, Chu H, Schneider MF, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 24.Breiman L, Friedman JH, Olshen RA, et al. Classification and Regression Trees. Monterey, CA: Wadsworth, Inc; 1984. [Google Scholar]
- 25.Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York, NY: Springer; 1999. [Google Scholar]
- 26.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome–infected and human immunodeficiency syndrome–negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robins LN, Regier DA. Psychiatric Disorders in America—The Epidemiologic Catchment Area Study. New York, NY: The Free Press; 1991. [Google Scholar]
- 28.Lee WE, Wadsworth ME, Hotopf M. The protective role of trait anxiety: a longitudinal cohort study. Psychol Med. 2006;36(3):345–351. doi: 10.1017/S0033291705006847. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf R, Bijl RV, Smit F, et al. Psychiatric and sociodemographic predictors of attrition in a longitudinal study: the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Am J Epidemiol. 2000;152(11):1039–1047. doi: 10.1093/aje/152.11.1039. [DOI] [PubMed] [Google Scholar]
- 30.Meller I, Fichter MM, Schroppel H. Mortality risk in the octo- and nonagenerians: longitudinal results of an epidemiological follow-up community study. Eur Arch Psychiatry Clin Neurosci. 1999;249(4):180–189. doi: 10.1007/s004060050085. [DOI] [PubMed] [Google Scholar]
- 31.Eaton WW, Kalaydjian A, Scharfstein DO, et al. Prevalence and incidence of depressive disorder: the Baltimore ECA follow-up, 1981–2004. Acta Psychiatr Scand. 2007;116(3):182–188. doi: 10.1111/j.1600-0447.2007.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2890. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 35.Ramasubbu R, Patten SB. Effect of depression on stroke morbidity and mortality. Can J Psychiatry. 2003;48(4):250–257. doi: 10.1177/070674370304800409. [DOI] [PubMed] [Google Scholar]
- 36.Jorm A. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 37.Pulska T, Pahkala K, Laippala P, et al. Six-year survival of depressed elderly Finns: a community study. Int J Geriatr Psychiatry. 1997;12(9):942–950. [PubMed] [Google Scholar]
- 38.Penninx B, Guralnik J, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 39.Gallo JJ, Bogner HR, Morales KH, et al. Depression, cardiovascular disease, diabetes, and two-year mortality among older, primary-care patients. Am J Geriatr Psychiatry. 2005;13(9):748–755. doi: 10.1176/appi.ajgp.13.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saz P, Dewey ME. Depression, depressive symptoms and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16(6):622–630. doi: 10.1002/gps.396. [DOI] [PubMed] [Google Scholar]
- 41.Davidson IA, Dewey ME, Copeland JRM. The relationship between mortality and mental disorder: evidence from the Liverpool longitudinal study. Int J Geriatr Psychiatry. 1988;3(2):95–98. [Google Scholar]
- 42.Henderson AS, Korten AE, Jacomb PA, et al. The course of depression in the elderly: a longitudinal community-based study in Australia. Psychol Med. 1997;27(1):119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- 43.Gallo J, Rabins P. Depression without sadness: alternative presentations of depression in late life. Am Fam Physician. 1999;60(3):820–826. [PubMed] [Google Scholar]
- 44.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365(9475):1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 45.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Joukamaa M, Heliovaara M, Knekt P, et al. Mental disorders and cause-specific mortality. Br J Psychiatry. 2001;179:498–502. doi: 10.1192/bjp.179.6.498. [DOI] [PubMed] [Google Scholar]
- 47.Mogga S, Prince M, Alem A, et al. Outcome of major depression in Ethiopia: population-based study. Br J Psychiatry. 2006;189:241–246. doi: 10.1192/bjp.bp.105.013417. [DOI] [PubMed] [Google Scholar]
- 48.Murphy JM, Burke JD, Jr, Monson RR, et al. Mortality associated with depression: a forty-year perspective from the Stirling County Study. Soc Psychiatry Psychiatr Epidemiol. 2008;43(8):594–601. doi: 10.1007/s00127-008-0323-3. [DOI] [PubMed] [Google Scholar]
- 49.Penninx BW, Geerlings SW, Deeg DJ, et al. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56(10):889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 50.Pulska T, Pahkala K, Laippala P, et al. Major depression as a predictor of premature deaths in elderly people in Finland: a community study. Acta Psychiatr Scand. 1998;97(6):408–411. doi: 10.1111/j.1600-0447.1998.tb10023.x. [DOI] [PubMed] [Google Scholar]
- 51.Saz P, Launer LJ, Dia JL, et al. Mortality and mental disorders in a Spanish elderly population. Int J Geriatr Psychiatry. 1999;14(12):1031–1038. doi: 10.1002/(sici)1099-1166(199912)14:12<1031::aid-gps59>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 52.Murphy E, Smith R, Lindesay J, et al. Increased mortality rates in late-life depression. Br J Psychiatry. 1988;152:347–353. doi: 10.1192/bjp.152.3.347. [DOI] [PubMed] [Google Scholar]