Abstract
The functional capacity of the adaptive immune system is dependent on the size and the diversity of the T cell population. In states of lymphopenia, T cells are driven to proliferate to restore the T cell population size. However, different T cell clones proliferate at different rates, and some T cells experience burst-like expansion termed spontaneous lymphopenia-induced proliferation (LIP). These T cells are likely receiving stimulation from cognate antigens and are most responsible for inflammatory pathology that can emerge in lymphopenic states. Foxp3+ regulatory T cells (Tregs) selectively inhibit spontaneous LIP, which may contribute to their ability to prevent lymphopenia-associated autoimmunity. We hypothesized that another potential negative consequence of unrestrained spontaneous LIP is constriction of the total T cell repertoire. We demonstrate that absence of Foxp3+ regulatory T cells (Tregs) during the period of immune reconstitution results in development of TCR repertoire ‘holes’ and loss of antigen-specific responsiveness to infectious microorganisms. In contrast, presence of Tregs during the period of immune reconstitution preserves optimal TCR diversity and foreign antigen responsiveness. This finding contrasts with the generally accepted immunosuppressive role of Tregs, and provides another example of Treg activity that actually enhances immune function.
Introduction
Maintenance of peripheral T cell homeostasis is a critical feature of the adaptive immune system (1, 2). Strict control of T cell homeostasis ensures adequate size of the T cell population, T cell receptor (TCR) diversity, responsiveness to foreign antigens, and self-tolerance. If a state of peripheral T cell deficiency is created, the residual T cells are driven to undergo proliferation by a process termed lymphopenia-induced proliferation (LIP) to reconstitute the optimal T cell numbers (3). LIP can be observed in a number of physiological and pathological situations (4). Lymphopenia is physiologic during the prenatal and neonatal periods, and LIP may contribute to generation of sufficient numbers of T cells with a memory phenotype (3). In addition, as the thymus involutes with increasing age, LIP assumes greater importance in maintaining T cell population size (5). Transient lymphopenia can accompany virtually any viral infection, and some viral infections, e.g., HIV, lead to chronic and progressive lymphopenia. In addition, there are multiple iatrogenic causes of lymphopenia, including cytodepletion by radiation, chemotherapy, and depleting antibodies. Notably, lymphopenia can be a trigger to autoimmunity, which is at least in part explained by T cell resistance to tolerance induction during LIP (6, 7). Furthermore, as individual T cell clones expand at different rates during LIP, the emerging T cell population risks further loss of TCR diversity and diminished immune fitness. However, although lymphopenic states occur multiple times during lifetimes of all individuals, autoimmunity and immune deficiency do not manifest in most people. Therefore, there must be mechanisms that help to maintain optimal immunologic tolerance and immune fitness during immune reconstitution.
Regulatory CD4+CD25+Foxp3+ T cells (Tregs) constitute 5–15% of peripheral CD4 T cells in healthy adult mice and humans (8), and are critical in the maintenance of immunologic tolerance and peripheral T cell homeostasis. These cells are able to suppress a range of immunologic responses in vitro and in vivo. Their discovery was aided by a number of animal models of autoimmunity associated with lymphopenia, e.g., neonatal thymectomy (9), colitis induced by adoptive transfer into SCID mice (10), and thyroiditis in the rat (11). However, early studies failed to demonstrate their ability to suppress LIP (12). Since then at least two major forms of LIP were recognized – homeostatic and spontaneous (13). Homeostatic LIP is driven primarily by cytokines such as IL-7 and IL-15, and is generally slow and steady. Spontaneous LIP is burst-like, likely driven by cognate antigen stimulation, and is the most likely source of pathogenic T cells specific for self- and commensal flora antigens. Importantly, the ultimate size of the T cell population is independent of the size of the input T cell population and relative proportion of homeostatic and spontaneous forms of LIP (14). We have shown previously that while Tregs do not suppress homeostatic LIP, they do suppress the spontaneous form of LIP (15, 16). This likely contributes to their protective function against development of autoimmunity during LIP. Since expansion of T cells by spontaneous LIP is dependent on higher affinity TCR stimulation, this T cell population is likely oligoclonal. However, it is possible that T cells undergoing either form of LIP, spontaneous and homeostatic, also compete for some of the same common resources, e.g., cytokines. Therefore, excessive spontaneous LIP may adversely affect the remainder of the T cell population resulting in constriction of the TCR repertoire during immune reconstitution. This reasoning led us to speculate that selective suppression of spontaneous LIP by Tregs could benefit the remainder of the T cell population by allowing them greater access to those cytokine resources important for their survival and homeostatic LIP. Furthermore, we hypothesized that the presence of Tregs during immune reconstitution by LIP should lead to preservation of greater diversity of TCR repertoire, and consequently, better immune fitness. This is an important issue with significant clinical implications in various situations that involve T cell depletion, e.g., AIDS or intentional T cell depletion in treatment of organ rejection, autoimmunity, or cancer.
Here, we characterized the TCR repertoire in T cell populations expanded by LIP in the presence or absence of Tregs following adoptive transfer into recombinase activating gene-deficient (RAG−/−) or TCR-α−/− recipients. Using TCR Vβ CDR3 spectratyping, we demonstrated significantly enhanced preservation of structural TCR diversity in the presence of Tregs during LIP. The reduction of TCR diversity in absence of Tregs resulted in development of functional ‘holes’ in the repertoire that were revealed by enumerating antigen-specific T cells following infection with Listeria monocytogenes. Thus, markedly greater numbers of Listeria-specific responding T cells were found if Tregs were present than if they were absent during LIP. These findings suggest that Tregs preserve TCR diversity and immune responsiveness during immune reconstitution from lymphopenia. This positive effect of Tregs on immunity contrasts with their generally accepted immunosuppressive function.
Materials and methods
Mice
C57BL6 (B6) and CD45.1 congenic mice were purchased from the National Cancer Institute (Frederick, MD). Some B6 mice used were wild-type littermates to IL-10−/− originally obtained from The Jackson Laboratory (Bar Harbor, ME) and CTLA-4+/− mice, which were a generous gift from Dr. J. Allison (Memorial Sloan-Kettering Cancer Center, New York, NY), respectively. Recombinase gene 1-deficient (RAG-1−/−), T-cell receptor alpha chain-deficient (TCRα−/−), and Thy1.1 congenic on the B6 background were obtained from Jackson Labs. B6 RAG-1−/− were bred onto the CD45.1 congenic background in our facility. Thy1.1 BALB/c mice were originally provided by Dr. L. Turka (University of Pennsylvania, Philadelphia, PA). and were crossed with BALB/c RAG-2−/− in our facility. All mice used were generally 4–20 weeks of age. All animals were maintained in a specific pathogen-free facility in microisolator cages with filtered air according to the National Institutes of Health guidelines.
Cell Isolation, labeling, adoptive transfer, and antibody-mediated in vivo depletion
Donor T cells were collected from secondary lymphoid tissues (axillary, brachial, cervical, mesenteric, and inguinal lymph nodes, and spleen). CD44low CD4, CD8, and bulk (both CD4 and CD8) T cells were purified in two stages. First, CD8 or CD4 T cells were prepared by negative selection against CD8 or CD4, MHC class II, CD11b, B220, CD25, and in some cases CD103 (all Abs labeled with FITC) using anti-FITC BioMag particles (Polysciences, PA). Second, CD44high cells were depleted (B6 only) using magnetic microbeads (Miltenyi Biotec, CA), as previously described (17). Briefly, the purified T cells were suspended in labeling buffer (2% FBS in PBS), incubated with 0.004 µg anti-CD44-FITC (eBioscience, CA) per 106 cells for 20 minutes, washed, and labeled with anti-FITC magnetic microbeads. The negative fraction was collected following Miltenyi Biotec magnetic column separation. CD25+ T cells were prepared using positive selection with anti-CD25 biotinylated mAb, PC61, and streptavidin-labeled magnetic microbeads (Miltenyi). An example FACS profile showing the purity and Foxp3 expression of cells in this prep was published previously (16). Purified cells were transferred into Rag−/− or TCRα−/− hosts via intravenous (i.v.) tail injection. In some cases cells were labeled with 7µM CFSE or SNARF-1 prior to transfer. Regulatory T cells were depleted in vivo using a single i.p. injection of 400–450 µg anti-Thy1.1 mAb (clone 1A14) (18).
TCR Vβ and Jβ spectratyping
Regulatory and conventional T cells collected from secondary lymphoid tissues were MACS sorted by positive selection for Thy1.1, as described above. Total RNA was extracted from positive (Thy1.1+/Treg) and negative (Thy1.1-/Tconv) fractions with an RNeasy Mini Kit (Qiagen, CA), and first-strand cDNA was generated from total RNA with Oligo(dT)20 and SuperScript III reverse transcriptase (Invitrogen, CA). Each cDNA sample was checked for integrity and for detectable TCR Cβ chain using β actin (5’: GTG GGC CGC TCT AGG CAC CAA; 3’: CTC TTT GAT GTC ACG CAC GAT TTC) and Cβ-specific primers (5’ Cβ1A; 3’ Cβ3C) under PCR amplification conditions described below (19). The CDR3 size distribution PCR assay was performed in duplicate for each individual sample as described (20, 21). Briefly, each TCR Vβ was amplified with Vβ8.1, 8.3 and/or Vβ10-specific sense primers and a Cβ antisense primer (22). For generation of Vβ spectratypes, the Cβ antisense primer was labeled with 6-fluorescein phosphoramidite (6-FAM) on the 5’ end. One µl of the previous synthesis, corresponding to the reverse transcription of 0.05-1ug of total RNA was used in the amplification. cDNA was added to tubes already containing a mixture of Taq Master Mix (Qiagen), DNAse/RNAse-free water, Vβ-specific and Cβ-specific primers (10–20µM) and amplification was performed as follows: thirty cycles of 95°C for 1 min/52°C for 1 min/72°C for 1 min, followed by 5 min at 72°C. A second, nested amplification for 12 specific Jβ sequences (Jβ1.1, Jβ1.2, Jβ1.3, Jβ1.4, Jβ1.5, Jβ1.6, Jβ2.1, Jβ2.2, Jβ2.3, Jβ2.4, Jβ2.5, Jβ2.7) was performed using one µl of Vβ-specific product (22). Cycling conditions remained the same, with the exceptions of Master Mix containing 5’ 6-FAM – labeled Jβ-specific sense primers and twenty cycles of amplification. The size distribution of each fluorescent PCR product was determined by electrophoresis on an Applied Biosystems 3130xl Genetic Analyzer (Foster City, CA), and data were analyzed using Genoprofiler 2.1 (23, 24).
TCR Spectratyping Complexity Scores
To assess each individual sample diversity profile as normal or limited, we used a variation of a previously reported complexity scoring system (25); that is, a complexity score = (sum of all the peak areas/sum of the major peak areas) × (number of the major peaks). Major peaks were defined as those on the spectratype histogram with an area at least 10% of the sum of all the peak areas.
Bacterial infections and peptide:MHC tetramer-based enrichment protocol
Wild-type B6 mice were immunized intravenously with approximately 1×107 CFU of a ΔActA strain of L. monocytogenes transformed by a plasmid containing ovalbumin and an I-Ab-specific mutant epitope of I-Eα (‘2W1S’) (26). Bacteria were grown in LB media with 24 µg/ml of chloramphenicol to an absorbance at 600 nm of about 0.1. The actual number of bacteria injected was confirmed by dilution and growth on LB agar plates containing chloramphenicol. Kb-OVA and I-Ab-2W1S tetramers, respectively, were produced in the laboratories of Drs. Stephen Jameson and Marc Jenkins, respectively, as previously described (27, 28). Lymph nodes and spleens were harvested together and processed for Kb-OVA and I-Ab-2W1S double-tetramer enrichment according to the protocol established by Moon et al. (28, 29).
FACS analysis
Mice were sacrificed by CO2 asphyxiation before spleen and lymph nodes were removed. All secondary lymphoid tissues were disrupted by mashing with a syringe. In some cases, complete media (10% FBS) was supplemented with 10 µg/mL brefeldin A (Sigma, MO) to prevent lymphokine secretion. Fixation and intracellular staining for cytokines was done following 2–3 hours in vivo challenge with approximately 5×107 CFU LM-2W1S-OVA. All antibodies used for cell surface staining, negative selection, and tetramer enrichment were purchased from eBioscience, with the exceptions of anti-B220 and anti-CD11c (Biolegend, CA), anti-CD8α (Invitrogen), and anti-CD4 single-stain controls (BD Biosciences, CA). Specific T cell subsets were identified using fluorochrome-labeled antibodies against a panel of TCR Vβ receptors (15 in total), CD3ε, CD4, CD8, CD44, and congenic markers such as anti-Thy1.1/Thy1.2 and anti-CD45.1/CD45.2. Anti-IFN-γ antibodies were purchased from eBioscience. Staining for Foxp3 was done using an eBioscience kit and instructions provided by the manufacturer. Absolute numbers of T cells were calculated using PKH reference beads (Sigma-Aldrich). All flow cytometry data was acquired on a FACSCalibur, an LSR, or an LSRII (BD Immunocytometry Systems, CA) and analyzed with FlowJo software (Tree Star, OR).
Statistical analysis
All error bars represent the standard error of the mean (SEM), unless noted otherwise. For data in Figures 2, 3, and 5, a two-tailed, unpaired Student’s t test was used for assessment of the differences between groups. Prism (Graphpad Software, La Jolla, CA) was used for graphs and statistical analysis. Differences were considered to be significant when the p value was less than 0.05. Twelve primers were used in the two-factor ANOVA and Bonferroni correction was applied for multiple hypothesis testing; the cutoff for statistical significance in that analysis was 0.05/12 = 0.004.
FIGURE 2.
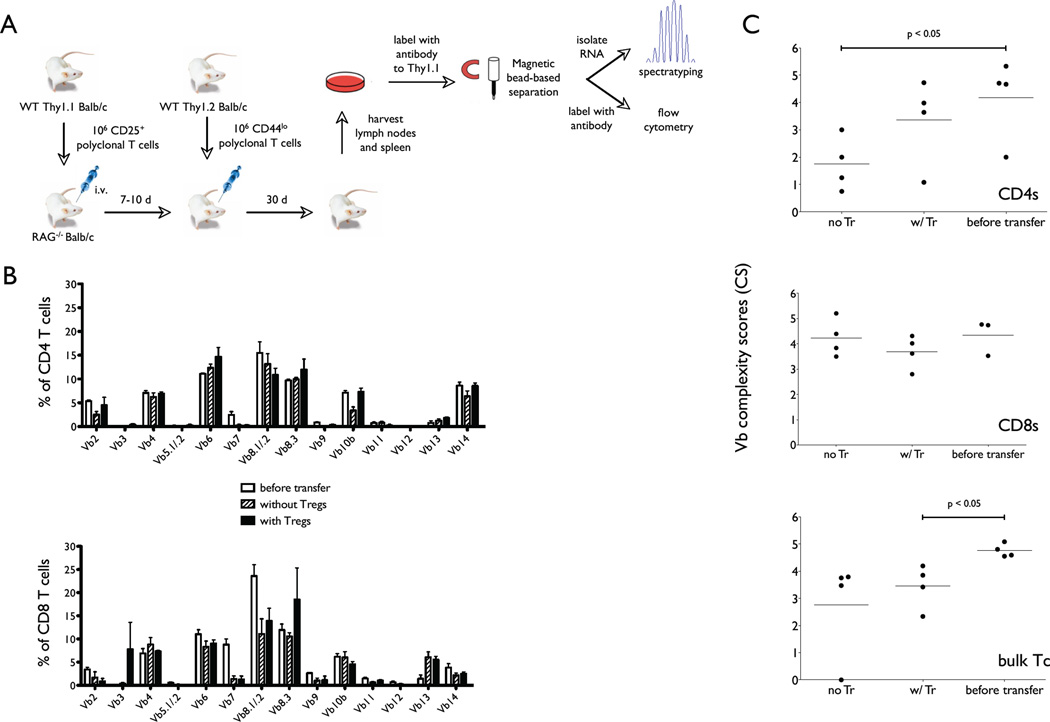
Assessing T cell diversity at the level of TCR Vβ chain usage after completion of LIP. A, The experiment protocol for assessment of structural T cell diversity post-LIP in the presence or absence of Tregs involves two separate measurements from each sample: TCR Vβ surface staining for FACS, and RNA isolation and spectratyping. B, RAG−/− T cell recipients were sacrificed and lymphoid tissue samples were processed on Day 30 after adoptive transfer. Half of each processed sample was treated to cell surface staining with antibodies specific to T cell markers (CD4, CD8, Thy1.2) and FACS analysis. The panels demonstrate %CD4 T cells (top) and %CD8 T cells (bottom) expressing individual Vβ chains in the input T cell populations (clear bars), and post-LIP T cell populations that emerged in the absence of Tregs (striped bars) or presence of Tregs (black bars). C, The remaining half of each sample was processed for RNA preparation and TCR Vβ spectratyping. The same conditions, i.e., Tregs absent in the recipient mice (no Tr), present (w/Tr), and the input T cell population (before transfer) were tested. Each graphed data bar or point represents an average of 3–4 animals combined from 2 independent experiments. Complexity scores were calculated from spectratyping of Vβ 10 and 8.1 (experiment #1), and from Vβ 10 and 8.3 (experiment #2). The top panel shows complexity scores of the CD4 T cell population; the middle panel shows the complexity scores of the CD8 T cell population, and the bottom panel shows the complexity scores of total, unseparated T cell population (bulk Tc).
FIGURE 3.
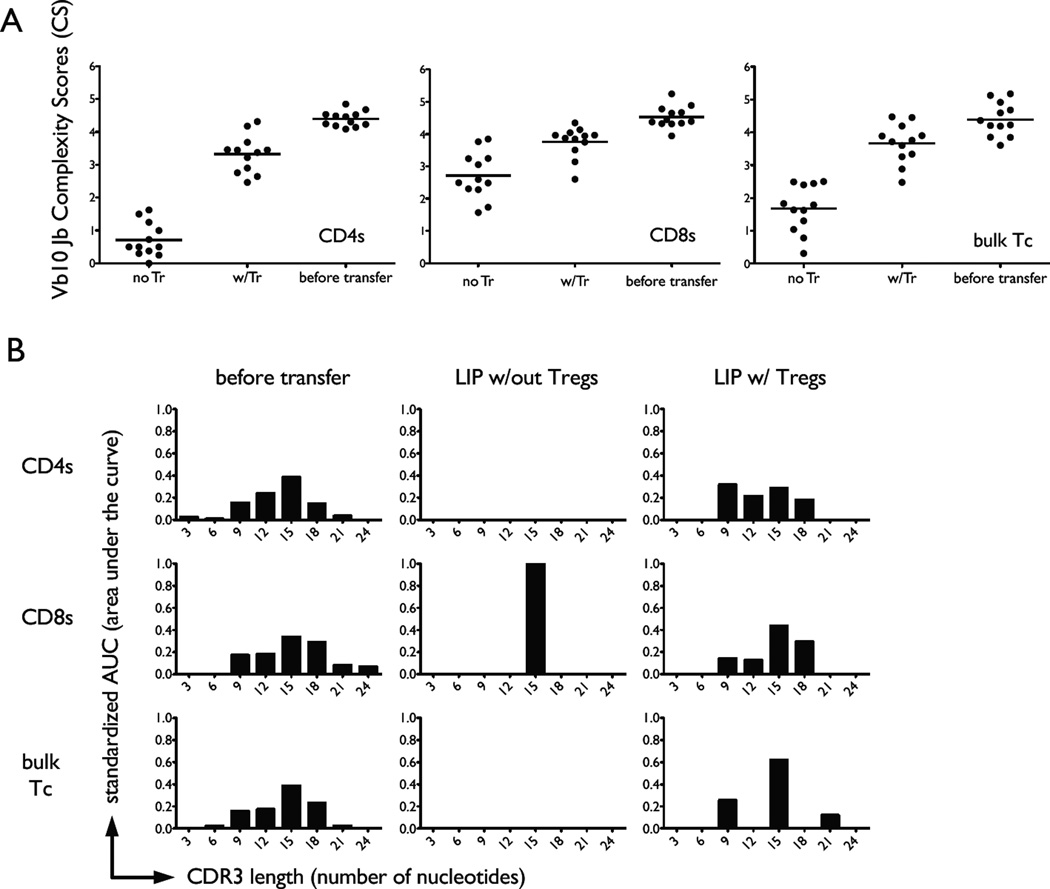
Tregs prevent constriction of the T cell repertoire during LIP. A, RNA samples from CD4 T cells (left panel), CD8 T cells (middle panel) and unseparated T cells (bulk Tc; right panel) from the indicated experimental groups were PCR amplified for Vβ10 expression and spectratyped for CDR3 diversity by further PCR amplification for different Jβ segments. Each dot represents the complexity score for each individual Jβ chain from two separate experiments. A mean value representing data from all 12 chains under each treatment condition (post-LIP in the absence (no Tr) or presence (w/Tr) of Tregs and input T cell populations labeled as ‘before transfer’) was used to determine statistical significance. Statistical significance (p < 0.05) between indicated groups is marked with a horizontal bar.
FIGURE 5.
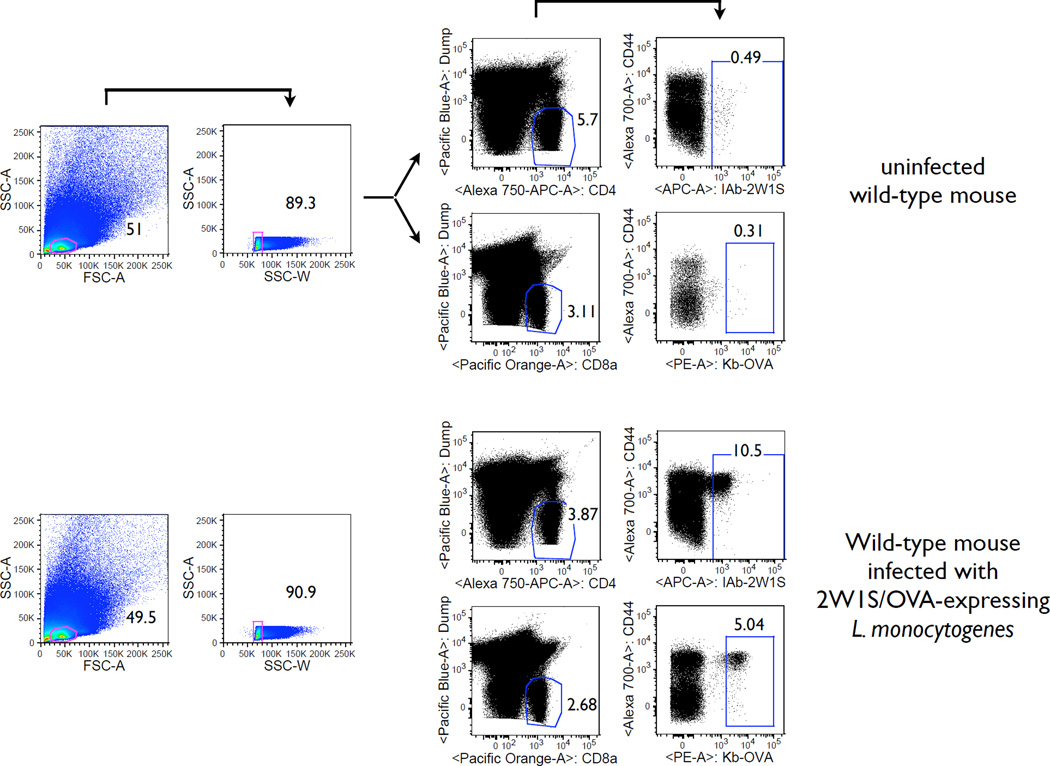
Antigen-specific T cells are enumerated in the secondary lymphoid tissues following L. monocytogenes infection. The antigen-specific T cells were enriched using tetramer-bound magnetic beads. The gating strategy is shown in the Figure. First, a tight lymphocyte gate is imposed that excludes doublet events (SSC-W). Next, non-T cells are excluded by using a ‘dump’ channel. Finally, antigen-specific T cells are gated using fluorochrome-bound tetramers. As expected, antigen-specific T cells are rare before the infection and mostly CD44low. After the infection their numbers increase dramatically, and they become CD44high.
Results
T cell adoptive transfer system for studies of the TCR repertoire during LIP
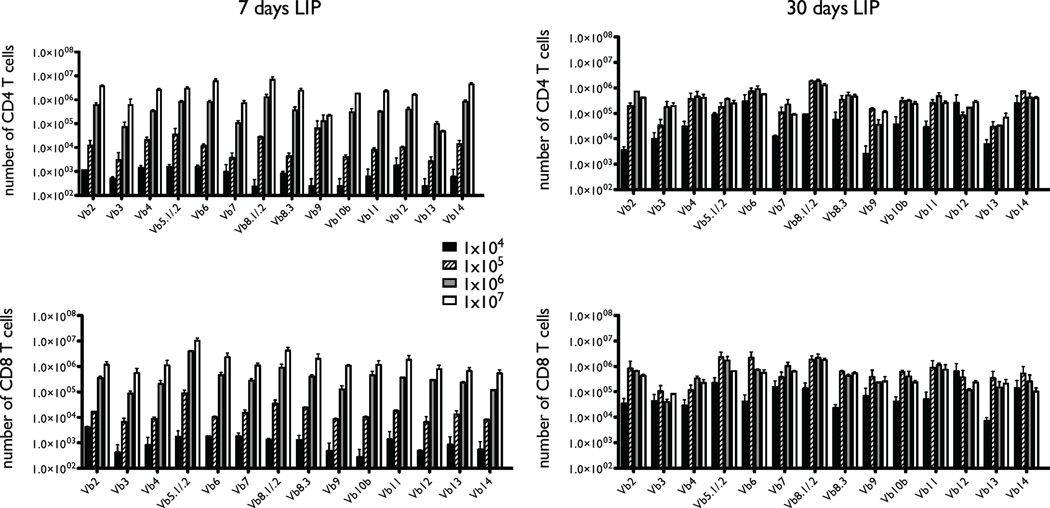
Tregs are well established in their ability to prevent and treat immunopathology arising in the course of immune reconstitution from lymphopenia by suppression of emergence and reactivity of pathogenic T cells. Here, we set out to test the idea that another function of Tregs during immune reconstitution is preservation of maximal immune fitness by maintaining optimal structural TCR diversity of the residual T cell population. In order to establish LIP we used the RAG−/− adoptive transfer model. First, we titrated numbers of bulk (CD4 and CD8), CD44loCD25− T cells transferred on day 0 and measured the number of T cells expressing each TCR Vβ chain on days 7 and 30. Similarly to what has been reported previously (14) we found that the ultimate number of T cells in the lymphoid periphery is independent of the initial input cell number (Fig. 1). Specifically, the T cell population size at 30 days was the same for inputs of 105 - 107 T cells, and it was still rising following adoptive transfer of 104 T cells. We decided to exclude 104-105 T cell transfers from future experiments because any potential TCR repertoire that could emerge from these would by definition be severely limited. Furthermore, adoptive transfers of very low numbers of T cells is associated with development of more pronounced immunopathology (30, 31). The distribution of different Vβ usage by the emerging T cell populations appeared indistinguishable between 106 and 107 T cell transfers (data not shown). However, we excluded 107 T cell transfers because these filled the T cell niche within a mere 7 days, largely preserving the TCR complexity of the input T cell repertoire (14). We chose adoptive transfer of 106 T cells as optimal in allowing an experimental window into TCR repertoire changes that might emerge during LIP. Notably, this number of input cells is generally not optimal for induction of immunopathology such as colitis; fewer T cells are more efficient presumably because of greater availability of resources for pathogenic T cell clones (31). We observed only development of mild dermatitis in the course of these experiments and no clinical or histopathologic colitis.
FIGURE 1.
T cell reconstitution in RAG−/− mice following adoptive transfer. Varying numbers of naïve (CD25CD44lo) T cells (104-107) taken from wild-type C57BL/6 mice were transferred into C57BL/6 RAG-1−/− recipients on Day 0 (two recipients per condition). Major lymph nodes (cervical, brachial, axillary, inguinal, and mesenteric) and spleens were harvested on Days 7 and 30, stained for T cell markers (CD4 and CD8) and a panel of antibodies reactive to 14 mouse TCR Vβ chains, and analyzed by FACS. The y-axes indicate the absolute numbers of recovered T cells expressing individual Vβ chains ± variance.
In order to test the effects of Tregs on the responder T cell population during LIP, we constructed the following experimental system (Fig. 2A). First, 106 CD25+Tregs are purified from Thy1.1 BALB/c mice and transferred into RAG−/− recipients and allowed to expand for 7–10 days, after which 106 Thy1.2 CD44loCD25− responder T cells are adoptively transferred. The lymphoid tissues are harvested for T cell population analysis 30 days after the responder T cell transfer. We used this protocol previously to show that Tregs selectively suppress only the spontaneous form of LIP by the responder T cells (16). In agreement with previous reports (14), the population size of Foxp3- T cells within secondary lymphoid tissues at 30 days is the same regardless of the presence or absence of the separate Treg adoptive transfer (data not shown).
As anticipated, the TCR Vβ usage of the reconstituted population was similar to the initial population regardless of the presence or absence of Tregs in the host (Fig. 2B). Although simple and robust, flow cytometric measurements of TCR Vβ usage are a relatively insensitive measure of TCR diversity, in that they do not allow determination of clonal dominance or restriction within populations of cells expressing the same Vβ chain. In order to probe the TCR repertoire further, we generated spectratypes at the level of a particular Vβ chain, e.g., Vβ8.1, Vβ8.3, and Vβ10 shown in Figure 1C. This assay did suggest that CD4 T cells undergoing LIP in absence of Tregs do experience contraction in TCR diversity compared to that measured before LIP or after LIP in the presence of Tregs. However, we could not detect decreased TCR diversity when CD8 T cells or un-fractionated naïve (CD25-CD44lo) responder T cells were allowed to undergo LIP under the same conditions.
Presence of Tregs during immune reconstitution preserves structural diversity of the responder T cell population
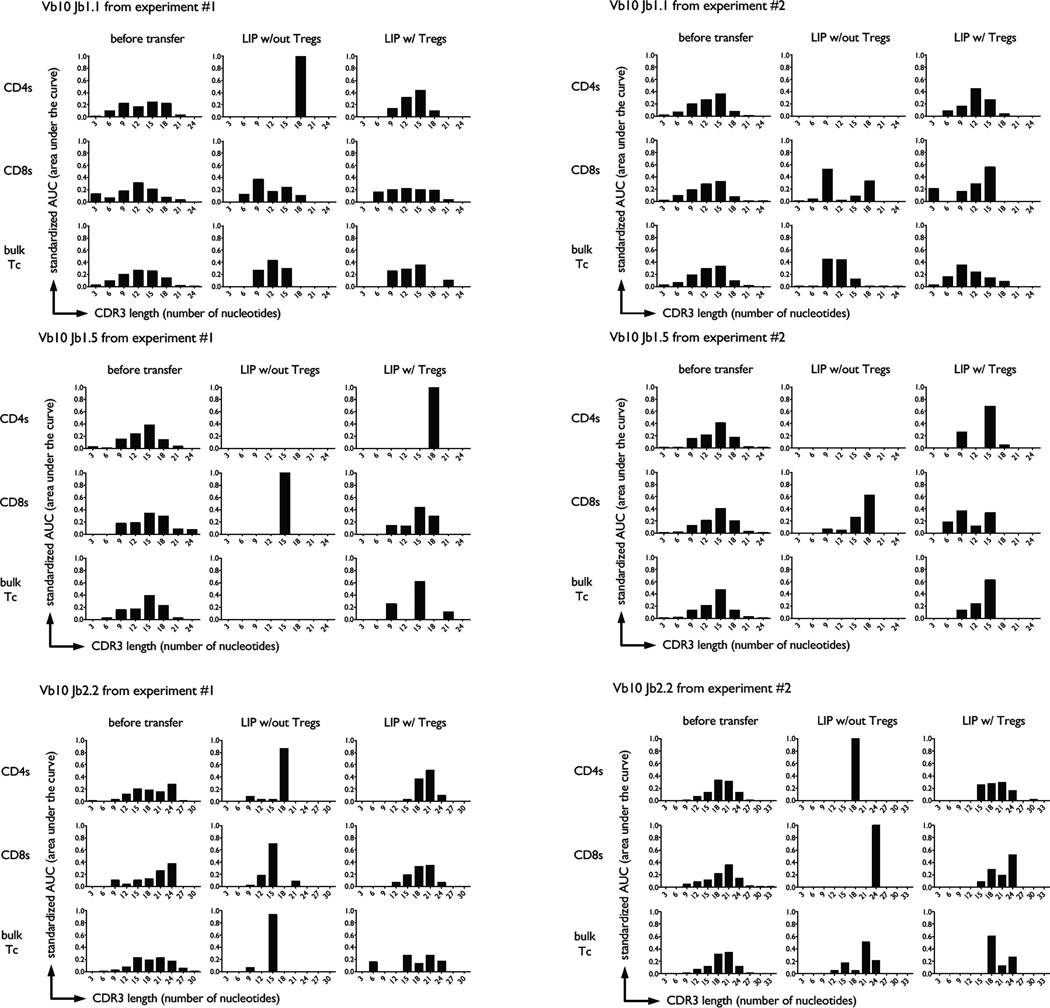
Although Vβ spectratyping does reflect some level of structural TCR diversity within a T cell population, it lacks sensitivity because it is not focused on the hypervariable regions of the TCR. In contrast, TCR Vβ-Jβ spectratyping is based on measurement of complementarity-determining region 3 (CDR3) size distribution within a population of T cells, and provides a more accurate reflection of its clonality. We performed TCR Vβ10-Jβ spectratyping of naive responder T cells that had undergone LIP in the presence or absence of Tregs. We measured spectratypes of CD4 T cells, CD8 T cells, and unfractionated T cells (bulk) under these conditions. In all cases the diversity of the TCR repertoire, at least as implied by CDR3 length size distribution, became severely constricted if Tregs were absent during LIP as measured by the VβJβ complexity scores and tested with a two-sample t-test (Fig. 3). The same result was seen upon additional analysis with a two-factor ANOVA applied to each primer and using the Bonferroni procedure to correct for multiple hypothesis testing. Visual inspection of spectratyping histograms from several TCR Vβ families also allowed us to see actual ‘holes’ in the reconstituted repertoire, as defined by specific Jβ chain representation below our spectratyping limit of detection (Fig. 4). These ‘holes’ appeared only if Tregs were absent during LIP. In contrast, relative preservation of TCR complexity was noted if Tregs were present during LIP (Fig. 4).
FIGURE 4.
Repertoire ‘holes’ develop during LIP in absence of Tregs. Three different T cell populations were adoptively transferred into individual RAG−/− mice: CD4 T cells (CD8-CD44loCD25-), CD8 T cells (CD4-CD44loCD25-), or bulk T cells (CD44loCD25-). Representative CDR3 length bar graph histograms from for individual Vβ10Jβ TCR segments are shown in this figure. Panels A and B represent different experiments. Histograms from the same individual animal are shown per experiment and the specific experimental condition.
Presence of Tregs during immune reconstitution prevents emergence of repertoire ‘holes’ that can limit antigen-specific responsiveness to microbial pathogens
The value of a diverse, polyclonal T cell repertoire is best appreciated in the context of infection by a microbial pathogen where pathogen-specific T cells are required for protection. Therefore, we wished to test the biological significance of LIP-associated TCR repertoire constriction in a model of infectious disease such as infection with an intracellular pathogen Listeria monocytogenes. Specifically, we used the infection model with the attenuated L. monocytogenes engineered to express two nominal antigens, the 2W1S peptide along with a truncated portion of ovalbumin containing the 257–264 epitope (ActA− LM-2W1S-OVA) (26). The infection with this recombinant strain of L. monocytogenes allows tracking of antigen-specific CD4 and CD8 T cell responses using magnetic bead-based MHC tetramer enrichment and multiparameter flow cytometry within the endogenous T cell repertoire (26, 28, 29). Briefly, magnetic enrichment ensures that all the epitope-specific T cells within the collected lymphoid tissues of the animal can be concentrated into a single sample and analyzed in entirety on a flow cytometer. The technique is illustrated in Figure 5 where antigen-specific CD4 and CD8 are enumerated before and after infection of wild-type C57BL/6 mice with ActA− LM-2W1S-OVA.
Extensive TCR sequence analysis by Casrouge and colleagues demonstrated that in steady-state a mouse spleen contains about 2 × 106 distinct naïve αβ TCR clones (32). Each T cell clone might be able to recognize more than one antigen, and it has been estimated that lymphoid tissues of a mouse contain 50–500 distinct T cell clones in the naïve T cell repertoire able to recognize a specific peptide/MHC epitope (33). Work published by Moon et al. using tetramer-based enrichment of epitope-specific T cells estimated the number of IAb-2W1S-specific CD4+ cells in a naïve C57BL/6 mouse to be ≥200 (28). Using the same basic methodology, the naïve (precursor) frequency of Kb-OVA-specific CD8+ cells was found by Obar et al. to be about 100 cells per mouse (34). One would, therefore, expect that adoptive transfer of 1 × 106 naïve donor T cells into RAG−/− or TCR-α−/− recipients should introduce approximately 2–4 T cells with specificity for Kb-OVA and IAb-2W1S, respectively. Our hypothesis, however, predicts that the fate of these naïve precursor cells during LIP may be different depending on the presence or absence of Tregs. Specifically, we thought that since these T cells have foreign antigen specificity, they would be less likely to undergo spontaneous LIP, and would, in fact, benefit from suppression of spontaneous LIP by Tregs.
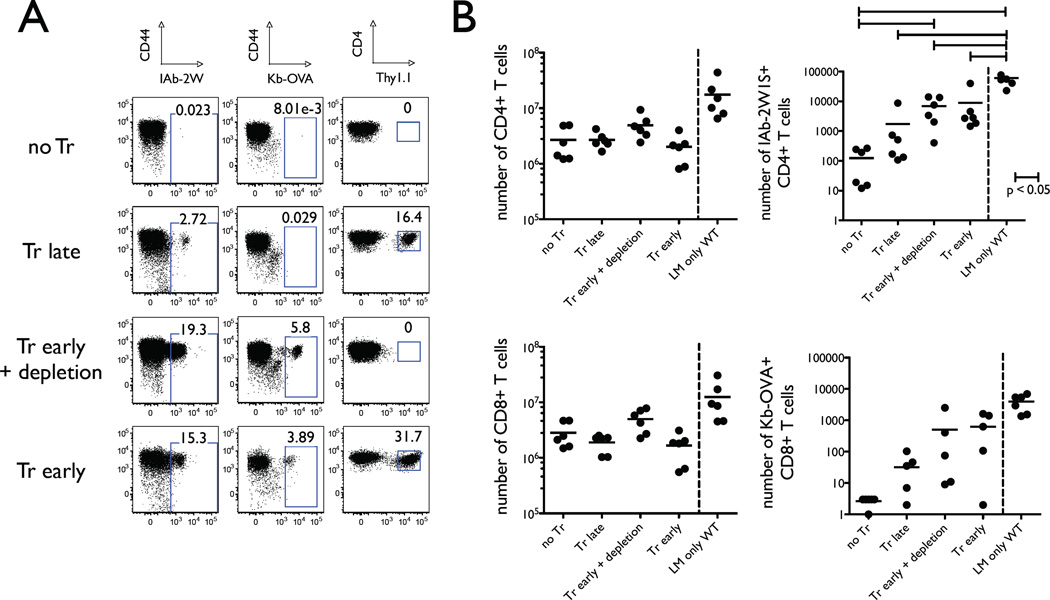
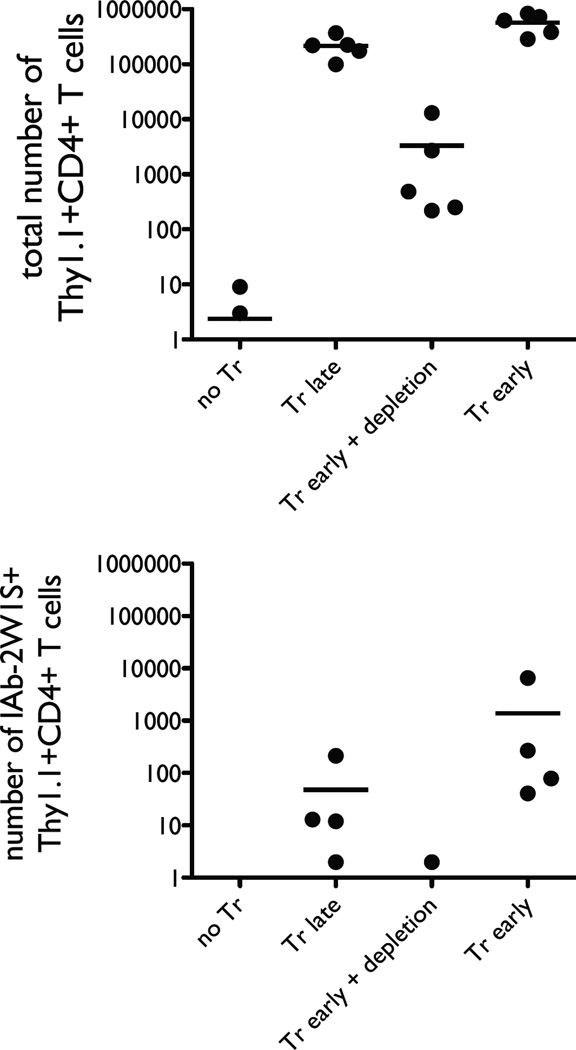
In order to test the hypothesis, we reconstituted RAG−/− or TCRα−/− mice by adoptive transfer of Thy1.2 CD25-CD44low T cells. Some of the recipient animals were pre-transferred with Thy1.1 CD25+ Tregs, and some were not. After 30 days of reconstitution the mice were infected with ActA− LM-2W1S-OVA. The effect of the variable we wanted to test was presence or absence of Tregs during LIP. Obviously, presence or absence of Tregs during the infection could also alter the T cell response. Therefore, we added two additional experimental groups. Some of the mice that received Tregs before the adoptive transfer of naïve responder T cells were treated with depleting anti-Thy1.1 antibody several days before the infection was introduced. In this group transferred Tregs were present only during immune reconstitution, but not the infection. Another group of mice was adoptively transferred Tregs only after immune reconstitution was complete so that Tregs would be absent during LIP, but present during the infection. In summary, the four groups of T cell reconstituted RAG−/− that were challenged with L. monocytogenes differed by time of presence of Tregs: 1) no Tregs transferred (no Tr), 2) Tregs absent during LIP, but present during infection (Tr late), 3) Tregs present during LIP, but absent during infection (Tr early + depletion), and 4) Tregs present during LIP and during infection (Tr early) (Fig. 6A).
FIGURE 6.
Presence of Tregs during LIP preserves capacity of the T cell population to respond to foreign antigens. RAG−/− mice were adoptively transferred with naïve Thy1.2 T cells on day 0 and infected with the attenuated L. monocytogenes bacteria expressing 2W1S and OVA peptides on day 30. The lymphoid tissues were harvested for analysis on day 40. The four experimental groups differed by the timing and presence of adoptively transferred Thy1.1 Tregs. These four groups were the following: 1) No Tr, no Tregs were transferred; 2) Tr late, Tregs were transferred on day 28; 3) Tr early + depletion, Tregs were transferred on day −7 and depleted with anti-Thy1.1 mAb on day 28; 4) Tr early, Tregs were transferred on day −7. A, Representative flow cytometry dots plots show percentages of antigen-specific CD4 T cells (CD44highIAb2W1Stetramer+) and CD8 T cells (CD44highKbOVAtetramer+) in the left and middle panels, respectively. The right panels demonstrate Thy1.1 staining which marks adoptively transferred Tregs. The dot plots show samples from one representative experiment in which two animals per group were taken to enumerate the antigen-specific T cells by magnetic bead enrichment and FACS analysis. B, Enumeration of antigen-specific CD4 T cells (top panels) and CD8 T cells (bottom panels) from all individual experiments are shown in these graphs. The left panels show the total non-Treg CD4 (top) and CD8 (bottom) T cell numbers from the reconstituted RAG−/− animals and wild-type mice infected with the attenuated L. monocytogenes. The right panels show the numbers of antigen-specific CD4 (top) and CD8 (bottom) T cells. Statistical significance (p < 0.05) between indicated groups is marked with a horizontal bar.
We found that the total numbers of conventional T cells in the lymphoid tissues after LIP and L. monocytogenes infection were identical (Figure 6B, left panels). However, the percentages (Figure 6A) and absolute numbers of IAb-2W1S-specific CD4 T cells and Kb-OVA-specific CD8 T cells (Figure 6B, right panels) revealed that presence of Tregs during LIP significantly enhanced expansion of antigen-specific T cells. In fact, the percentage of antigen-specific T cells in reconstituted RAG−/− mice that had Tregs present during LIP was similar to those seen in wild-type animals, although the absolute numbers were somewhat lower reflective of the fact that reconstituted RAG−/− animals lack the truly naïve T cell compartment. Depletion of Tregs by anti-Thy1.1 mAb after completion of LIP did not impact expansion of Listeria-specific responder T cells during the infection. In contrast, RAG−/− mice allowed to reconstitute in absence of Tregs had very few Listeria-specific T cells, and there was no significant difference seen between animals that never received Tregs or received Tregs after LIP was already completed. Virtually all measured pathogen-specific T cells derived from the naïve responder T cell population and not the Thy1.1+ Tregs, as documented by tetramer staining (Fig. 7). This observation is consistent with the results reported previously where infection by the same attenuated strain of Listeria used in our experiments was not seen to cause expansion of Listeria-specific Foxp3+ T cells (26). Furthermore, the conversion rate of naïve CD4 T cells into Foxp3+ T cells was minimal (Table 1), and the Treg compartment was dominated by adoptively transferred Thy1.1 T cells.
FIGURE 7.
Few Tregs are specific for IAb-2W1S. Adoptively transferred Tregs were identified by congenic marker Thy1.1 in the indicated experimental groups of reconstituted RAG−/− mice infected with the attenuated L. monocytogenes (top panel). IAb-2W1S-specific T cells were identified using magnetic bead enrichment and tetramer staining for flow cytometry.
Table 1.
| LIP sample |
% Thy1.1+ that are Foxp3+ |
Thy1.1+Foxp3+ T cell number |
% Thy1.2+ that are Foxp3+ |
Thy1.2+Foxp3+ T cell number |
% Foxp3+ of total CD4 T cells |
|---|---|---|---|---|---|
| Tr early | 56.4 +/− 6.0 | 247526 +/− 106369 | 1.9 +/− 0.1 | 19666 +/− 13161 | 18.5 +/− 3.0 |
| Tr late | 61.5 +/− 9.6 | 93409 +/− 37418 | 0.7 +/− 0.4 | 12463 +/− 2280 | 4.6 +/− 0.4 |
| no Tr | 1.6 +/− 0.1 | 50595 +/− 43626 | 1.6 +/− 0.1 | ||
| Tr early + depletion | 25.5 +/− 4.4 | 358 +/− 325 | 5.3 +/− 2.8 | 247743 +/− 239088 | 5.2 +/− 2.8 |
| wild-type sample |
% Foxp3+ | Foxp3+ T cell number |
|---|---|---|
| LM only | 6.9 +/− 2.5 | 637252 +/− 329660 |
| no Tx | 10.0 +/− 4.0 | 1357831 +/− 939317 |
Presence of Tregs during immune reconstitution preserves functional responsiveness of the T cell population
Ideally, we would have liked to correlate the adaptive immune response to Listeria with clinical outcomes such as animal survival or bacterial counts. However, the adaptive immune response is dispensable for clearance of the primary infection by the attenuated organism (data not shown) (26).
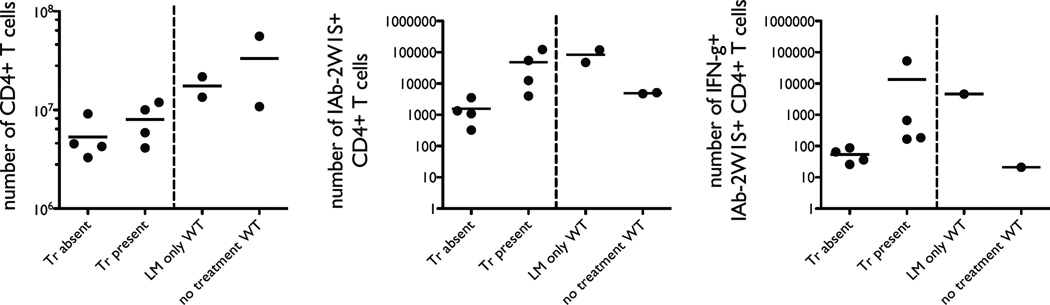
Nevertheless, production of interferon-γ by adaptive and innate immune systems contributes to clearance of intracellular pathogens such as L. monocytogenes (35–37). Therefore, it would be reasonable to presume that numbers of Listeria-specific CD4 T cells that can produce IFN-γ relate to the immune fitness of an individual animal challenged with such infection. In order to examine this question, we repeated some experiments described above using TCRα−/− recipients. Once again, animals were reconstituted with conventional naïve T cells in the presence or absence of Tregs for 30 days and infected with 107 CFU of LM-2W1S-OVA bacteria. IFN-γ production was stimulated in vivo on day 10 after infection by intravenous injection of 5 × 107 CFU of live LM-2W1S-OVA 2–3 hours prior to harvest of the lymphoid tissues. We found that if Tregs were present during LIP, the reconstituted animals had more antigen-specific CD4 T cells overall, and more antigen-specific CD4 T cells capable of producing IFN-γ(Fig. 8).
FIGURE 8.
The presence of Tregs during LIP allows enrichment of antigen-specific CD4 T cells capable of making IFN-γ following infection. Wild-type mice (right of each panel) or 30-day reconstituted TCRα−/− mice (left of each panel) were infected with the attenuated L. monocytogenes bacteria. IFN-γ production by IAb-2W1S-specific T cells was induced by injection of live L. monocytogenes bacteria 2–3 hours prior to tissue harvest. The left panel shows the number of total CD4 T cells, the middle panel shows the number of IAb-2W1S-specific CD4 T cells, and the right panel shows the number of IAb-2W1S-specific CD4 T cells producing IFN-γ. Each dot represents lymph node and spleen cells from two animals processed together for each of the two independent experiments.
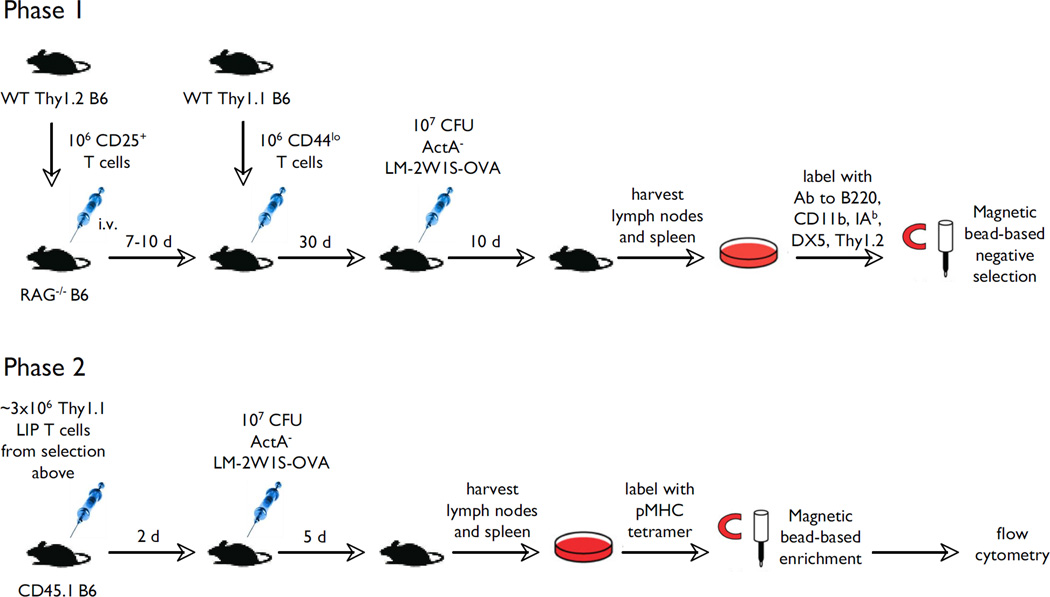
Finally, we tested whether Listeria-specific effector T cells generated within T cell populations that emerged in the course of immune reconstitution in the presence or absence of Tregs were capable of expansion when re-challenged with the pathogen (experiment is outlined in Fig. 9). RAG−/− mice were repopulated with naïve Thy1.1 T cells in the presence or absence of Thy1.2 Tregs. After 30 days of reconstitution all animals were infected with 107 ActA− LM-2W1S-OVA bacteria. 10 days after infection, lymphoid tissues of the animals were harvested and T cells that derived from the conventional T cell inoculum (Thy1.2− T cells in this experiment) were purified by negative selection. New wild-type CD45.1 congenic recipients received 3 × 106 of these purified T cells (Thy1.1+CD45.2+), and half of the animals were challenged with 107 ActA− LM-2W1S-OVA bacteria. Some wild-type recipients did not receive T cells from reconstituted RAG−/− animals and were used simply as wild-type controls. All mice were sacrificed on day 5 after the infection challenge and lymphoid tissues were harvested for analysis. The results were in keeping with the previous results (Fig. 10). If Tregs were present during LIP, we could detect significant numbers of Thy1.1 CD4 T cells, most of which were specific for the 2W1S epitope. If Tregs were absent during LIP the numbers of 2W1S-specific CD4 T cells were indistinguishable from the numbers in the pre-immune repertoire.
FIGURE 9.
Experimental protocol to test antigen-specific memory T cell responses. In phase I of the experiment two groups of RAG−/− mice were adoptively transferred naïve T cells: RAG−/− animals with or without pre-transfer of Thy1.2 Tregs. After 30 days of reconstitution these mice were infected with 107 attenuated L. monocytogenes bacteria. 10 days after infection the phase I animals were sacrificed and their Thy1.1 T cells were re-transferred into wild-type (Thy1.2/CD45.1 RAG+/+) mice, 3 × 106 per recipient. The new recipients were then either infected with 107 attenuated L. monocytogenes bacteria or not. Magnetic bead enrichment and flow cytometric analysis was used to enumerate Thy1.1 (donor) and Thy1.2 (recipient) IAb-2W1S-specific T cells.
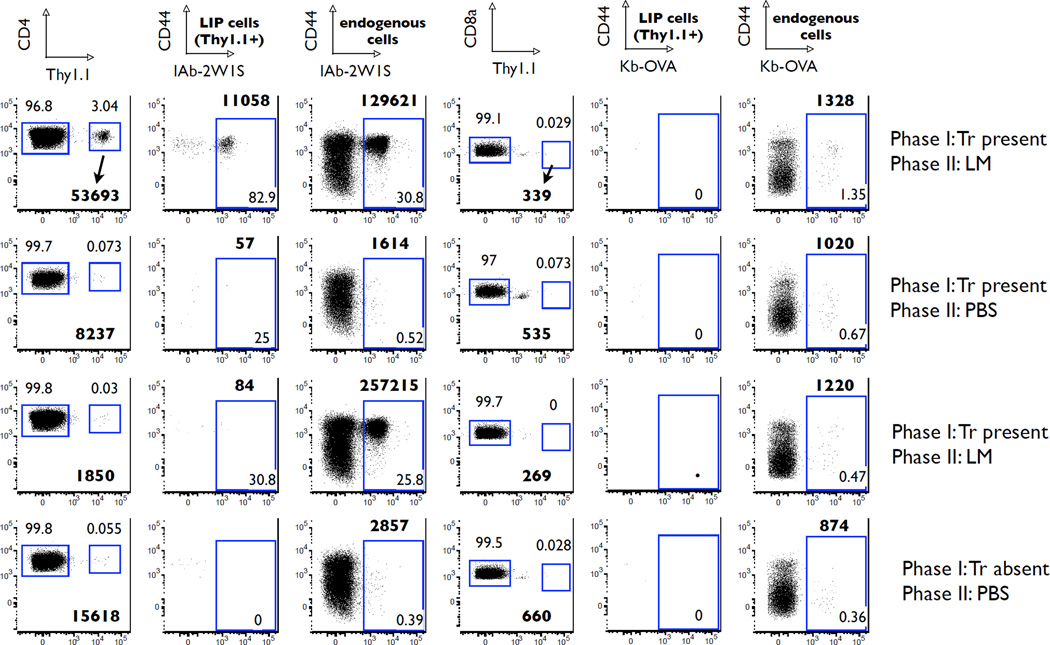
FIGURE 10.
Antigen-specific memory T cells from reconstituted RAG−/− mice are detected only if Tregs were present during reconstitution. The figure shows the flow cytometric data from the experiment described in Figure 9. Thy1.1 T cells originate from the reconstituted RAG−/− animals and are now within the Thy1.2 wild-type secondary recipients. Antigen-specific CD4 T cells are identified with the IAb-2W1S tetramers. Numbers in bold print represent the absolute numbers of indicated cells. The regular print numbers in the lower right corners represent percentages within the indicated gates.
Discussion
Since their discovery and characterization, CD25+Foxp3+ regulatory T cells have primarily been noted for their immunosuppressive functions and a critical role in the maintenance of immunologic tolerance (38, 39). However, in recent years there has been increasing appreciation for more nuanced regulatory functions of these cells, which ultimately enhance the immune response. For example Tregs can inhibit or delay pathogen clearance, which at first glance may seem detrimental to the host. However, in some models, such as leishmaniasis and schistosomiasis, antigen persistence ensured by Tregs contributes to maintenance of immunity to reinfection (40, 41). In ocular HSV-1 infection, Tregs were shown to inhibit immunopathology caused by inflammatory responses that would otherwise severely disable the host (42). In vaginal HSV-2 infection ablation of Tregs was demonstrated to enhance entry and retention of effector immune cells in the draining lymph nodes and reduce their migration into the actual site of infection (vagina), which correlated with increased viral loads and fatality (43). In these examples of infections Tregs may allow losing a ‘battle’ with a pathogen, but at the same time help to win the ‘war’. Of course, the paradigm of ‘war’ does not apply well to most of the activity of the immune system. The majority of the immune effort is concentrated at the mucosal surfaces, where it helps to maintain homeostasis with the commensal microbial flora and aid the physical barrier to exclude pathogen entry. That is one of the main functions of the secreted IgA, for example, which requires transforming growth factor-β (TGF-β) for class switching, one of the cytokines elaborated by Tregs (44).
At the outset of our experiments we were intrigued by the differential ability of Tregs to inhibit spontaneous versus homeostatic forms of LIP (16). This finding suggested that Tregs could play another positive role in the function of the immune system. Specifically, we thought they might optimize the immune fitness of the residual T cell population during its recovery from lymphopenia by shaping its TCR repertoire. Lymphopenia is a common occurrence during lifetimes of individuals and peripheral mechanisms of immune reconstitution assume increasing importance with age as the thymus involutes (45). Our experimental adoptive transfer system models absence of the thymus and allows recovery of the T cell population exclusively by LIP.
We specifically wanted to start out with a minimally restricted potential TCR repertoire in order to both mimic realistic clinical scenarios, e.g., recovery of the T cell population in AIDS patients treated with highly active anti-retroviral therapy (HAART), and provide an experimental window to measure significant differences. A normal mouse contains about 2 × 106 T cell clones within its naïve TCR repertoire (32), and a starting population significantly below that would ensure severely restricted responsiveness to potential foreign antigens. In contrast, a starting population significant above that would limit LIP because there would be little lymphopenia. We employed p-MHC tetramers to probe the responsiveness of T cell populations to two foreign peptides, the SIINFEKL peptide from ovalbumin and 2W1S, expressed by an infectious pathogen, L. monocytogenes. Enumeration of Kb-OVA- and IAb-2W1S-specific T cells in the naïve repertoire reported previously and reproduced in our own work suggested that adoptive transfer of 1 × 106 T cells would result in transfer of about 2–4 T cells per mouse specific for these antigens (28). In fact, it has been shown that a single precursor T cell is capable of massive clonal expansion and functional subset diversification following immunization (46). Therefore, we expected to detect significant antigen-specific T cell expansion in all our experimental conditions in absence of significant distortion of the T cell repertoire during the period of immune reconstitution.
We found that the greatest numbers of responder foreign antigen-specific T cells recovered after infection were seen only in mice that had Tregs during the period of LIP. The simplest explanation for this finding is that some antigen-specific precursors from the naïve T cell inoculum did not survive the immune reconstitution period in absence of Tregs. In fact, in some experiments when Tregs were absent during LIP we could not detect antigen-specific responder T cells at all following infection, and in others they were markedly decreased in numbers. This idea is also supported by results of characterization of the TCR repertoire obtained by TCR Vβ-Jβ spectratyping, which demonstrated that absence of Tregs during immune reconstitution correlated with development of marked loss of structural TCR diversity. One would predict that the diversity of the few recovered antigen-specific responder T cells from populations established in the absence of Tregs would be extremely constricted. In fact, we have done several preliminary experiments using flow cytometric analysis of Vβ chain usage by antigen-specific responders, and these were compatible with that prediction (data not shown). Specifically, the Vβ chain usage of antigen-specific 2W1S-specific responders in mice reconstituted in presence of Tregs was comparable to the repertoire of 2W1S-specific responders in wild-type mice. Whereas, the Vβ chain repertoire of 2W1S-specific responders in mice reconstituted in absence of Tregs appeared distorted. However, because of the small numbers of antigen-specific T cells in these mice and their complete absence in some experiments, the total number of mice necessary to characterize the Vβ usage by these cells was prohibitive. It may be interesting to focus future studies on the characteristics of such residual tetramer-positive T cells that persist within the T cell population established by LIP in absence of Tregs. We might anticipate, for example, that these cells have greater affinity for self-antigens that provide them with more tonic survival signals. Such cells might have relatively low affinity for foreign antigens that the organism may encounter.
Our results indicate that the beneficial role of Tregs on the antigen-specific T cell response is exerted during the period of LIP. Tregs adoptively transferred after completion of immune reconstitution had no beneficial effect. Furthermore, if Tregs were depleted following immune reconstitution, the beneficial effect persisted. It should be noted that Tregs were depleted using an antibody directed against a congenic marker, Thy1.1. While that was very effective in depleting Thy1.1+ T cells, early presence of Tregs also appeared to encourage some emergence of Foxp3+ T cells derived from the naive responder T cell population (Table 1). This might represent an example of ‘infectious tolerance’, and is consistent with the idea that factors produced by Tregs are somehow needed for de novo peripheral Treg induction (47). However, the effect was relatively small and the numbers of induced Tregs in this group was considerably smaller than that produced by late adoptive transfer of Tregs. In both cases Tregs appearing after completion of LIP did not enhance the diversity or immune fitness of the reconstituted responder T cell population.
The mechanisms of how Tregs may shape the TCR repertoire during LIP remain unclear at this time. However, a reasonable speculative model can be constructed based on current knowledge of the specific signaling requirements of different T cell subpopulations, i.e., Tregs and T cells undergoing spontaneous versus homeostatic forms of LIP. Although these three T cell subpopulations all require or benefit from TCR stimulation, inhibition of spontaneous LIP by Tregs cannot be explained by competition for TCR signaling (16). However, Tregs and T cells undergoing spontaneous LIP benefit from B7 costimulation (3, 15, 16, 48, 49). In contrast, B7 co-stimulatory signals are not needed for the homeostatic form of LIP, which is in turn driven mainly by mere tonic TCR signals and cytokines such as IL-7 (50). Thus, it is possible that Tregs, which are known to express high levels of CTLA-4, interfere with B7 costimulation of the responder T cells by direct competition or negative signaling into the APCs leading to B7 downregulation (51). In fact, Sojka et al. have recently reported that CTLA-4-deficient Tregs cannot inhibit the spontaneous form of LIP, while they remain highly suppressive in vitro (52). Similarly, in our own experiments we noted that residual spontaneous LIP experienced by CD28-deficient responder T cells cannot be suppressed in absence of B7 expression on host APCs (data not shown). It is reasonable to speculate that T cells undergoing both forms of LIP do utilize some common resources, e.g., IL-7, which may not be consumed significantly by Tregs (53). Therefore, suppression of the T cells undergoing spontaneous LIP would promote survival and homeostatic proliferation of all the other T cells by allowing greater access to limiting amounts of IL-7. It is also possible that Tregs and at least some subsets of T cells undergoing spontaneous LIP may compete for additional common resources, e.g., IL-2 and IL-15, which are known to be important for both Tregs and memory CD8 T cells (54, 55), and can drive spontaneous LIP of CD8 T cells (56). Of course, additional mechanisms are formally possible. It is conceivable that Tregs may somehow selectively identify T cells undergoing spontaneous LIP and suppress directly via cell-to-cell contact mechanisms leading to arrest in their cell cycle progression or induction of apoptosis. In addition, we cannot exclude the less likely possibility that Tregs may help to promote survival and homeostatic LIP of T cells that are not recruited into spontaneous LIP by facilitating production of some positive factors by APCs and other immune cells. Further mechanistic dissection of Treg mediated control of the T cell repertoire development during immune reconstitution is certainly warranted.
We do not know at this time whether Tregs are uniquely capable of preserving the functionality of the T cell population during immune reconstitution. We hypothesized that Tregs might have this function because of their ability to selectively inhibit spontaneous LIP, while sparing T cells undergoing homeostatic LIP. In previous work we showed that bulk CD25- T cell competitors composed of both memory and naïve T cells could not inhibit spontaneous LIP efficiently (15), or even enhanced spontaneous LIP of CD8 T cell responders (16). However, we have not tested other more narrowly defined T cell subsets. It is now recognized that Tregs are not all uniform and stable in their phenotype (57, 58). For example, a small population of Foxp3+ T cells loses Foxp3 expression following adoptive transfer and expansion in lymphopenic hosts (59). These ex-Foxp3+ T cells may come to represent about 50% of the transferred population in the new hosts, which is consistent with our results (Table I), and may in fact be pathogenic themselves (60). It may be interesting for mechanistic investigations to compare stable Foxp3+ T cells and ex-Foxp3+ T cells in their ability suppress spontaneous LIP and regulate diversity of the TCR repertoire.
The results presented here have potential immediate clinical implications. Massive peripheral immune reconstitution occurs in a number of settings, including treatment of AIDS with highly active anti-retroviral therapy (HAART) and organ transplantation following cytoablative treatment. These interventions can be associated with potentially fatal inflammatory conditions such as the immune reconstitution inflammatory syndrome (IRIS) and graft-versus-host disease (GVHD), which are also associated with immunodeficiency caused by insufficient numbers of antigen-specific T cells and poor T cell function (61, 62). Interestingly, there is at least some evidence that CD4 Tregs are preferentially spared in HIV infection (63). This may be one of the reasons why the risk of IRIS is inversely related to pre-treatment CD4 counts (64). In addition, delaying the initiation of HAART and lower pre-treatment CD4 T cell counts result in poor functional immune restoration despite normalization of CD4 T cell numbers (65). Our results suggest that presence of sufficient numbers of Tregs during immune reconstitution should not only minimize the risk of triggering autoimmunity and immunopathology caused by opportunistic infections, but should also optimize future functional capacity of the restored immune system in its responsiveness to infectious challenges. In fact, there are already attempts underway to introduce human Treg cell infusion as an adjunct therapy following bone marrow transplantation (66). Of course, multiple practical challenges still need to be overcome before acceptance of adoptive Treg transfers in the clinics. However, it is also possible that administration of CTLA-4-Ig, which is already in clinical use, during the period of immune reconstitution may have similar beneficial effects on immune restoration.
Acknowledgements
We are grateful to Drs. Erik Peterson, and Daniel Mueller for critical review of the manuscript.
Footnotes
This work was supported in part by the NIH Grant RO1 DK061961 (AK) and Institutional Immunology Training Grant 5T32AI007313-20 (CJW).
References
- 1.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 4.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunology letters. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, Cellular, and Antigen Requirements for Development of Age-Associated T Cell Clonal Expansions In Vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 6.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends in Immunology. 2005;26:5. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual review of immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 9.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 11.Penhale WJ, Farmer A, McKenna RP, Irvine WJ. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973;15:225–236. [PMC free article] [PubMed] [Google Scholar]
- 12.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 13.Min B, Paul WE. Endogenous proliferation: Burst-like CD4 T cell proliferation in lymphopenic settings. Seminars in immunology. 2005;17:201–207. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagen KA, Moses CT, Drasler EF, Podetz-Pedersen KM, Jameson SC, Khoruts A. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J Immunol. 2004;173:3909–3915. doi: 10.4049/jimmunol.173.6.3909. [DOI] [PubMed] [Google Scholar]
- 16.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+CD25+Foxp3+ T Cells Selectively Inhibit the Spontaneous Form of Lymphopenia-Induced Proliferation of Naive T Cells. J Immunol. 2008;180:7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]
- 17.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 18.Mayerova D, Wang L, Bursch LS, Hogquist KA. Conditioning of Langerhans cells induced by a primary CD8 T cell response to self-antigen in vivo. J Immunol. 2006;176:4658–4665. doi: 10.4049/jimmunol.176.8.4658. [DOI] [PubMed] [Google Scholar]
- 19.Soloff RS, Wang TG, Lybarger L, Dempsey D, Chervenak R. Transcription of the TCR-beta locus initiates in adult murine bone marrow. J Immunol. 1995;154:3888–3901. [PubMed] [Google Scholar]
- 20.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madakamutil LT, Maricic I, Sercarz EE, Kumar V. Immunodominance in the TCR repertoire of a [corrected] TCR peptide-specific CD4+ Treg population that controls experimental autoimmune encephalomyelitis. J Immunol. 2008;180:4577–4585. doi: 10.4049/jimmunol.180.7.4577. [DOI] [PubMed] [Google Scholar]
- 22.Gascoigne NR, Chien Y, Becker DM, Kavaler J, Davis MM. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984;310:387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- 23.You FM, Luo MC, Gu YQ, Lazo GR, Deal K, Dvorak J, Anderson OD. GenoProfiler: batch processing of high-throughput capillary fingerprinting data. Bioinformatics (Oxford, England) 2007;23:240–242. doi: 10.1093/bioinformatics/btl494. [DOI] [PubMed] [Google Scholar]
- 24.You FMaLMC. GenoProfiler User's Manual. 2003 [Google Scholar]
- 25.Bomberger C, Singh-Jairam M, Rodey G, Guerriero A, Yeager AM, Fleming WH, Holland HK, Waller EK. Lymphoid reconstitution after autologous PBSC transplantation with FACS-sorted CD34+ hematopoietic progenitors. Blood. 1998;91:2588–2600. [PubMed] [Google Scholar]
- 26.Ertelt JM, Rowe JH, Johanns TM, Lai JC, McLachlan JB, Way SS. Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection. J Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- 28.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci U S A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 33.Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- 34.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 36.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol. 2005;175:1751–1757. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messingham KA, Badovinac VP, Jabbari A, Harty JT. A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes. J Immunol. 2007;179:2457–2466. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 39.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 40.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 41.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 42.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 43.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 45.Hakim FT, Gress RE. Thymic involution: implications for self-tolerance. Methods Mol Biol. 2007;380:377–390. doi: 10.1007/978-1-59745-395-0_24. [DOI] [PubMed] [Google Scholar]
- 46.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Cobbold SP, Adams E, Graca L, Daley S, Yates S, Paterson A, Robertson NJ, Nolan KF, Fairchild PJ, Waldmann H. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 48.Gudmundsdottir H, Turka LA. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J Immunol. 2001;167:3699–3707. doi: 10.4049/jimmunol.167.7.3699. [DOI] [PubMed] [Google Scholar]
- 49.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 50.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 51.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 52.Sojka DK, Hughson A, Fowell DJ. CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur J Immunol. 2009;39:1544–1551. doi: 10.1002/eji.200838603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourgeois C, Stockinger B. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J Immunol. 2006;177:4558–4566. doi: 10.4049/jimmunol.177.7.4558. [DOI] [PubMed] [Google Scholar]
- 54.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 55.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 56.Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hossain MS, Roback JD, Pollack BP, Jaye DL, Langston A, Waller EK. Chronic GvHD decreases antiviral immune responses in allogeneic BMT. Blood. 2007;109:4548–4556. doi: 10.1182/blood-2006-04-017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, Bertrand Y, Fasth A, Porta F, Cant A, Espanol T, Muller S, Veys P, Vossen J, Fischer A. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91:3646–3653. [PubMed] [Google Scholar]
- 63.Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, James I, French MA. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. Aids. 2007;21:1525–1534. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 64.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. Aids. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 65.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, Valdez H. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. Aids. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 66.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]