Abstract
Purpose.
To compare choroidal thickness (CT) measurements in early AMD between patients with and without reticular pseudodrusen (RPD) using spectral-domain optical coherence tomography (SD-OCT).
Methods.
This cross-sectional study examined 84 age- and sex-matched AMD patients (40 RPD [63 eyes], 44 non-RPD [75 eyes]). Fundus photographs and scanning laser ophthalmoscopy images were graded to identify RPD and non-RPD groups by three retinal specialists (MO, SY, SB) who were masked to corresponding SD-OCTs. CT at the fovea and 2400 to 3000 μm superior and inferior to the fovea was measured on SD-OCT by a grader (AG) and reviewed by a retinal specialist (SB). Only images with a clear posterior choroidal margin were analyzed (six eyes excluded due to poor image quality), and enhanced depth imaging SD-OCT was used when available (20 of 138 eyes). Greatest retinal thickness (RT) on horizontal foveal SD-OCT was also recorded.
Results.
Mean CTs in the superior, foveal, and inferior macula in RPD (191.3 μm ± 57.9 SD, 176.3 μm ± 60.5 SD, 179.7 μm ± 56.24 SD) were significantly less than that of non-RPD (228.0 μm ± 66.1 SD, 216.5 μm ± 70.3 SD, 224.4 μm ± 71.9 SD; P = 0.0010, P = 0.0005, P = 0.0001, respectively), as was greatest RT (P = 0.0301).
Conclusions.
CT was thinner throughout the macula in the RPD group as compared with the non-RPD group. The current analysis supports an association between RPD and a thinned choroidal layer and is consistent with a choroidal etiology of RPD. CT may be integral to understanding RPD, and may be helpful in stratifying AMD progression risk.
Keywords: reticular pseudodrusen, spectral-domain optical coherence tomography, choroidal layer, early age-related macular degeneration, retinal ischemia
In the setting of early age-related macular degeneration, reticular pseudodrusen (RPD) were found to be associated with decreased choroidal thickness (CT) throughout the macula. Findings support a choroidal etiology of RPD and suggest that CT may be helpful in stratifying AMD progression risk.
Introduction
Age-related macular degeneration is the leading cause of vision loss in developing and developed countries,1 and accounts for more than 54% of all blindness in the United States.2 Age-related macular degeneration is a disease characterized by extracellular material, collectively described as drusen, which accumulates under the RPE.
Reticular pseudodrusen (RPD) are found in the fundus of some patients with AMD and were initially characterized as a peculiar yellow pattern occurring in the outer macula best visible under blue light in photographs of patients with AMD.3 RPD have been associated with a higher likelihood of developing late AMD in the forms of choroidal neovascularization (CNV)4–9 and geographic atrophy (GA).10 However, there is no clear agreement about the prevalence of RPD, the relationship between RPD and AMD,11 or the underlying etiology of RPD. Theories proposed for the etiology of RPD include abnormal choroidal perfusion, subretinal drusenoid deposits (SDD), or a combination of both.
RPD have been associated with choroidal vascular abnormalities. A histopathologic study of one RPD eye found that there was a loss of the inner and middle layers of the choroid, subsequently leading to fibrous replacement of choroidal stroma.4 Additionally, reticular patterns found on red free images and confocal scanning laser ophthalmoscopy (cSLO) imaging including infrared (IR) and autofluorescence (AF) imaging have consistently colocalized to the intravascular choroidal stroma seen on spectral domain-optical coherence tomography (SD-OCT) scans.12
Reticular pseudodrusen have also been associated with the accumulation of SDD,13 which are described as material that lies above, rather than under, the RPE and are visible on SD-OCT.14 These deposits are described as the loss of the outer segment (OS)/RPE interface and inner segment (IS)/RPE interface seen on SD-OCT.15 However, SDD and disruptions of the IS and OS on SD-OCT have inconsistently colocalized with RPD.12–14,16
Querques et al.15 recently proposed that in RPD eyes, choroidal fibrosis is the primary insult, leading to the derangement of the RPE, and ultimately SDD.
In normal eyes, the choroidal layer has been found to be thickest in the subfoveal region, where visual acuity and the concentration of cone photoreceptors are highest, with progressive thinning outward in both the horizontal and vertical axes.17 If RPD represent alterations in choroidal flow, one might expect a difference in choroidal thickness (CT) between early AMD eyes with RPD and those without RPD. This study compares measurements of CT in the superior, inferior, and foveal macula in early AMD between patients with and without RPD. Additionally, we examine quantitative RPD area as a fraction of the macula and its relationship to subfoveal choroidal thickness (SFCT).
Methods
Study Population
This study was a subset of the Columbia Macular Genetics Study, had approval through the Columbia University Medical Center Institutional Review Board, and complied with the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. This cross-sectional analysis consisted of a consecutive case series of 149 AMD patients who were seen at a university-based retinal practice and imaged with cSLO between January 1, 2005 and September 1, 2012.
Exclusion criteria included late AMD, which was defined as GA or history of CNV, as well as myopia greater than −6 diopters, central serous chorioretinopathy, or past vitreoretinal surgery. Presence of GA was defined as 500 μm2 or greater total summed area of RPE loss seen on IR or AF imaging, or if atrophy was seen on clinical examination. Eyes were excluded for CNV on the basis of clinical examination and findings on fluorescein angiography and/or OCT.
Each patient's ophthalmologic examination, fundus photography, cSLO imaging, and SD-OCT were all performed within an 18-month time period. A comprehensive chart review was performed to document characteristics for each patient, including age, sex, lens status, best-corrected visual acuity (BCVA), and spherical equivalent refractive error.
Image Acquisition and Analysis
High-resolution digital color fundus photographs were taken with an FF 450plus with VISUPAC camera (Carl Zeiss Meditec, Dublin, CA). AF, IR, and SD-OCT imaging were obtained by cSLO imaging (Heidelberg Spectralis HRA+OCT version 1.7.0.0; Heidelberg Engineering, Heidelberg, Germany). For AF images, the instrument used blue laser light at 488 nm for illumination and a barrier filter at 500 nm. The IR images were obtained at 810 nm.
Infrared, AF, and SD-OCT images were viewed with Heidelberg software (Spectralis Viewing Module 5.4.6.0; Heidelberg Engineering). Age-related macular degeneration classification and RPD status were agreed on by three retinal specialists (MO, SY, SB), and one disputed case was arbitrated by a fourth retinal specialist (SHT); all graders were masked to findings on SD-OCT.
Horizontal SD-OCT sections centered on the fovea were used for SFCT and RT calculations. Choroidal thickness measurements in the superior and inferior macula were made 2400 to 3000 μm directly superior and inferior to the fovea, respectively. The best SD-OCT image with a clear posterior margin of the choroid was chosen for CT analysis. Enhanced depth imaging SD-OCT (EDI SD-OCT) was used when available (20 of 138 eyes). This is a technique that has been previously described, and can allow for better visualization of the choroid.18
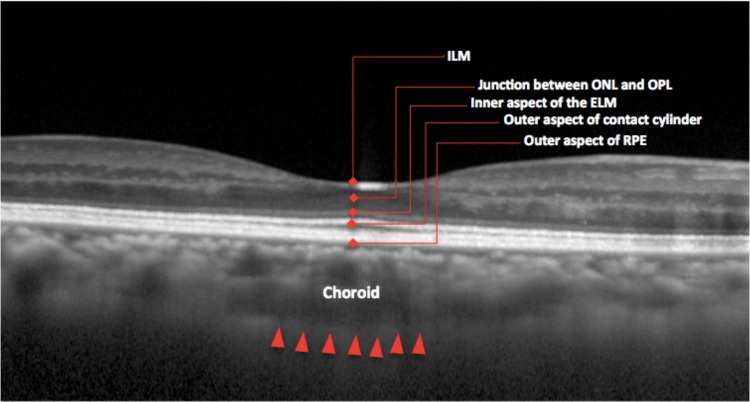
Choroidal thickness was defined as the outer portion of the hyperreflective line corresponding to the RPE up to the inner surface of the sclera,19 and was measured using Heidelberg Eye Explorer interactive software's manual calipers tool (Heidelberg Engineering, Heidelberg, Germany). This is represented in Figure 1. The CT measurements were performed by a grader (AG) and reviewed by a retinal specialist (SB).
Figure 1.
Layers of the retina. Choroidal thickness was defined as the outer portion of the hyperreflective line corresponding to the RPE to the inner surface of the sclera. ILM, internal limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; ELM, external limiting membrane.
Retinal thickness (RT) was measured from the nerve fiber layer to the lower border of the RPE using Heidelberg Eye Explorer's automated segmentation program. Errors in automated segmentation were manually adjusted. Measurements were taken at the thinnest point and thickest point on horizontal sections at the level of the fovea. Eyes with vitreomacular traction were excluded from this arm of the study.
Classification of Disease
All eyes included in the study were classified by a retinal physician as Groups 0b, 1, 2, or 3 by the International Classification and Grading System for Age-Related Macular Degeneration.20 This classification is summarized in Table 1 and denotes early-stage AMD as the presence of few hard, soft distinct, or soft indistinct drusen, and/or RPE hyperpigmentation and/or hypopigmentation. Eyes with GA and/or CNV are classified as late-stage AMD.
Table 1.
Classification of Early and Late AMD by the International Classification and Grading System for Age-Related Macular Degeneration
|
Group |
Description |
| 0 | |
| a | No signs |
| b | Few hard drusen (<63 μm) |
| 1 | |
| a | Soft distinct (>63 μm) |
| b | Pigment irregularities only (no soft drusen) |
| 2 | |
| a | Soft indistinct drusen (>125 μm) |
| b | Soft distinct drusen (>63 μm) and pigment irregularities |
| 3 | Soft indistinct drusen (>125 μm) and pigment irregularities |
| 4 | |
| a | GA |
| b | CNV |
| c | GA and CNV |
Early-stage AMD includes Groups 0b, 1, 2, and 3, whereas late stage is Group 4.
Reticular pseudodrusen were was defined as yellow, interlacing networks seen on fundus photography, groupings of ill-defined, hypoautofluorescent lesions against a background of mildly elevated autofluorescence on AF imaging, and groupings of hyporeflectant lesions against a background of mild hyperreflectance on IR imaging.21 A patient was defined as having RPD if either eye had RPD.
Subfoveal choroidal thickness was categorized according to a study of healthy subjects by Margolis and Spaide22 that established a normal SFCT as being 287 μm ± an SD of 75.7 μm. In the referenced study, the mean age was 50.4 years, and thinning of 15.6 μm was noted with each decade of increasing age. Because our patients, on average, were approximately 2.5 decades older than those in the Margolis study, we adjusted our expected mean SFCT by subtracting 39 μm (2.5 decades × 15.6 μm) from the known mean SFCT; this resulted in an adjusted SFCT of 248 μm ± SD of 75.7 μm.
We created a classification system for SFCT: normal (248 μm ± 1 SD, or 172.3–323.7 μm), thin (96.6 μm–172.3 μm), very thin (2 SD less than 248 μm, or ≤96.6 μm), thick (323.7 μm–399.4 μm), and very thick (2 SD more than 248 μm, or ≥399.4 μm).
The entire image was used for classification into RPD and non-RPD groups; evidence of RPD anywhere in the macula led to a diagnosis of RPD. However, within the RPD group, the area of reticular pattern seen in the IR or AF image was graded using the Early Treatment Diabetic Retinopathy Study (ETDRS) grid so as to standardize quantitative measurements of RPD between eyes to reduce bias. When the ETDRS group is centered on the macula, the area within the grid is divided into nine subdivisions: one central, four inner, and four outer.23 Area of RPD was defined as the fraction of the grid that had presence of RPD. A subdivision of the grid was considered positive for RPD if the pattern was present in at least two disc diameters of the outer subdivisions or occupied at least half the area of the central or inner subdivisions. This metric was used because the outer subdivisions of the ETDRS grid have an overall larger area.
Descriptive statistics were used to analyze the data, and the chi-squared test for independence was used to analyze categorical variables.
Results
Patient Demographics and Clinical Characteristics
A total of 84 subjects were included in the analysis. Of the original group of 149 subjects, 65 were excluded for the following reasons: diagnosis of late AMD in both eyes (31), incomplete charts and imaging (21), and high myopia (1). Subjects older than 85 years were excluded so as to age-match the two groups (nine RPD, one non-RPD). Additionally, four subjects had one eye excluded due to poor image quality, and one subject had both eyes excluded for this reason. All subjects with eyes excluded due to poor image quality (six eyes total) were from the non-RPD group.
Table 2 summarizes demographics of the studied groups. Of the 84 subjects included in the study, 40 were RPD, and 44 were non-RPD. In the RPD group, 32 (80%) of 40 were females and had a mean age of 76.9 years ± SD of 7.5 years (range: 51–85 years). In the non-RPD group, 32 (73%) of 44 were females, and had a mean age of 73.9 years ± SD of 7.8 years (range: 48 to 85 years). Mean age and prevalence of females in both groups were not significantly different (P = 0.0709 and P = 0.4405; Student's t-test for independent samples and χ2 test, respectively).
Table 2.
Demographics of Studied Groups, RPD and Non-RPD
|
RPD |
Non-RPD |
P
Value |
|
| No. of subjects | 40 | 44 | |
| No. of eyes | 63 | 75 | |
| Median age, y | 76.9 ± 7.5 | 73.9 ± 7.8 | 0.0709 |
| Age range, y | 51–85 | 48–85 | |
| % Females | 80% | 73% | 0.4405 |
| % Pseudophakic | 43% | 37% | 0.2317 |
| BCVA | 0.29 ± 0.24 | 0.18 ± 0.22 | 0.0012 |
| AMD classification | |||
| Group 0a, 0b | 1 | 2 | |
| Group 1 | 1 | 8 | |
| Group 2 | 25 | 26 | |
| Group 3 | 13 | 8 |
The RPD and non-RPD groups had similar distributions with regard to AMD classification by the International Classification and Grading System for ARM.20 Of the RPD subjects, 1 was Group 0b, 1 was Group 1, 25 were Group 2, and 13 were Group 3. Of the non-RPD subjects, 2 were Group 0b, 8 were Group 1, 26 were Group 2, and 8 were Group 3. Sixty-three RPD eyes and 75 non-RPD eyes were included in the analysis.
Best-corrected visual acuity was converted to the logarithm of the minimal angle of resolution scale, with an average of 0.290 ± SD of 0.24 (range, 0–0.875) in the RPD group, and 0.164 ± SD of 0.18 (range, 0–0.602) in the non-RPD group. A significant difference in BCVA was found between the RPD and non-RPD group (P = 0.0012; Student's t-test). Percentage of pseudophakic patients in the RPD group was 43%, and in the non-RPD group was 37%. This was not significantly different (P = 0.2317; Student's t-test for independent samples).
A total of 118 subjects had early AMD in at least one eye; 70 of these, or 59%, had RPD, and 48, or 41%, did not have RPD.
Features Noted on SD-OCT
Mean SFCT in RPD eyes was 176.3 μm ± SD of 60.5 μm, and was significantly less than mean SFCT in non-RPD eyes, which was 216.5 μm ± SD of 70.3 μm (P = 0.0005; Student's t-test for independent samples). Representative images of non-RPD and RPD are shown in Figures 2 and 3.
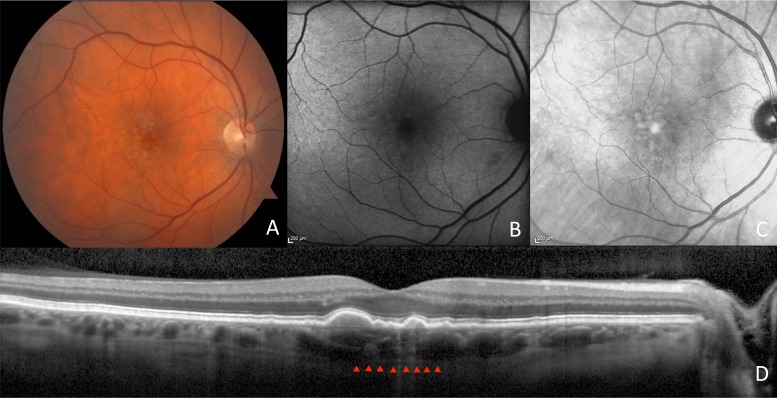
Figure 2.
Images of an AMD patient without RPD. Fundus photography shows an AMD fundus (A). Autofluorescence (B) and IR (C) imaging depict absence of RPD. Enhanced depth imaging SD-OCT (D) shows a normal subfoveal choroidal thickness of 239 μm.
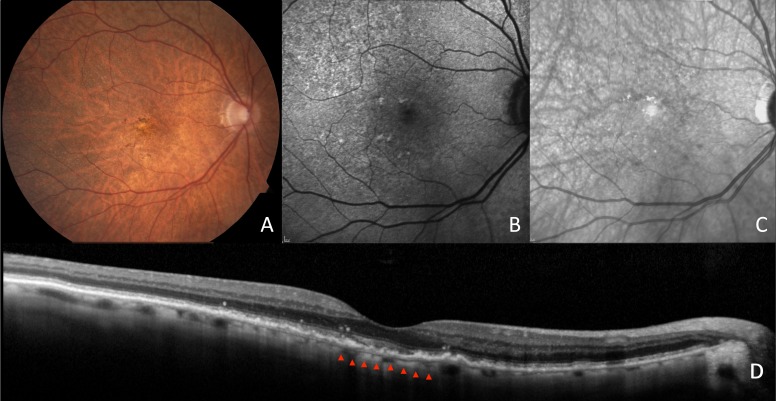
Figure 3.
Images of an AMD patient with RPD. Fundus photography shows a tessellated fundus and yellow, interlacing networks representing RPD (A). Autofluorescence (B) and IR (C) imaging show hyporeflectant and hypoautofluorescent networks consistent with RPD. Spectral-domain optical coherence tomography (D) shows a very thin subfoveal choroidal thickness of 32 μm.
Because all eyes that met inclusion/exclusion criteria were included, we checked for potential bias due to biological correlation of two eyes from the same patient by performing our analysis using just one eye from each patient. We had 23 RPD and 31 non-RPD subjects with both eyes included; in our secondary analysis, we included only left eyes for these patients and any eligible eye for the patients with only one eye included, which totaled 40 RPD eyes and 44 non-RPD eyes. We found that SFCT was significantly different between the two groups, RPD and non-RPD (174.2 μm ± 57.2 vs. 211.2 μm ± 69.9, respectively; P = 0.0098; Student's t-test for independent samples) when including only one eye from each subject.
For the purposes of categorical analysis, only one eye was included from each subject. Of the 40 RPD subjects, 45% had a normal subfoveal choroid, 50% had a thin subfoveal choroid, and 5% had a very thin subfoveal choroid. None of the RPD eyes met criteria for a thick or very thick subfoveal choroid. Of the 44 non-RPD subjects, 64% had a normal subfoveal choroid, 27% had a thin subfoveal choroid, 5% had a very thin subfoveal choroid, 2% had a thick subfoveal choroid, and 2% had a very thick subfoveal choroid. This is represented in Table 3.
Table 3.
Categorization of Subfoveal CT (μm) in the Two Groups, RPD and Non-RPD
|
RPD,
n
(%) |
Non-RPD,
n
(%) |
|
| Very thin, <96.6 μm | 2 (5) | 2 (5) |
| Thin, 96.6–172.3 μm | 20 (50) | 12 (27) |
| Normal, 172.3–323.7 μm | 18 (45) | 28 (64) |
| Thick, 323.7–399.4 μm | 0 (0) | 1 (2) |
| Very thick, >399.4 μm | 0 (0) | 1 (2) |
| Total | 40 | 44 |
Only one eye from each person was included (left eyes were used if both eyes were included in the study).
Mean CT in the superior macula of RPD eyes was 191.3 μm ± 57.9 SD and of non-RPD eyes was 228.0 μm ± 66.1 SD; these were found to be significantly different (P = 0.0010; Student's t-test for independent samples). Mean CT in the inferior macula of RPD eyes was 179.7 μm ± 56.24 SD and of non-RPD eyes was 224.4 μm ± 71.9 SD; these were found to be significantly different (P = 0.0001; Student's t-test for independent samples). Additionally, there was a significant difference in mean CT for each eye taking into account the superior, subfoveal, and inferior fields for the RPD and non-RPD groups (182.9 μm ± 50.7 SD vs. 221.8 μm ± 60.8, respectively; P = 0.0001, Student's t-test for independent samples). These values are listed in Table 4 and representative images of superior and inferior choroids from non-RPD and RPD patients are demonstrated in Figure 4. Differences in CT between the superior and inferior macula within RPD (P = 0.2617; Student's t-test for independent samples), and within non-RPD (P = 0.7522; Student's t-test for independent samples) did not differ.
Table 4.
CT (μm) in the Superior, Subfoveal, and Inferior Fields in the Two Groups, RPD and Non-RPD
|
Choroidal Thickness, μm |
RPD |
Non-RPD |
t-Test |
| Superior | 191.3 ± 57.9 | 228 ± 66.1 | P = 0.0010 |
| Subfoveal | 176.3 ± 60.5 | 216.5 ± 70.3 | P = 0.0005 |
| Inferior | 179.7 ± 56.3 | 224.4 ± 72.0 | P = 0.0001 |
| Mean | 182.9 ± 50.7 | 221.8 ± 60.8 | P = 0.0001 |
Mean CT of all three measurements for each group is also displayed.
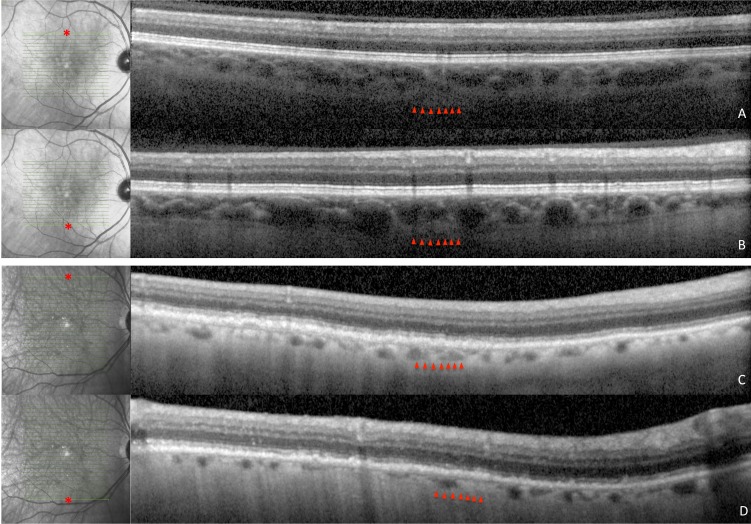
Figure 4.
Superior and inferior spectral domain optical coherence tomography images and corresponding infrared images of patients with AMD with and without RPD. Choroidal thickness in the non-RPD patient is 231 μm superiorly (A) and 242 μm inferiorly (B) and in the RPD patient is 107 μm superiorly (C) and 82 μm inferiorly (D). Point of measurement on macula is denoted by an asterisk in infrared images.
RT measurements nasal and temporal to fovea were taken on horizontal line scans (52 RPD eyes and 67 non-RPD eyes). Eleven RPD eyes and eight non-RPD eyes were excluded from the analysis due to vitreomacular traction. Thickest RT was on average slightly thicker in non-RPD (349.5 μm ± 25.5 SD) than the RPD group (340.1 μm ± 19.7 SD; P = 0.0301; Student's t-test for independent samples). Thinnest RT, which was foveal for nearly all eyes, did not differ between groups (201.9 μm ± 48.5 SD in RPD and 215.9 μm ± 30.9 SD in non-RPD; P = 0.0575; Student's t-test for independent samples).
Area of RPD on cSLO imaging and corresponding SFCT
Area of RPD was not found to be significantly positively or negatively associated with corresponding SFCT (r = −0.0955; Pearson correlation coefficient).
Discussion
Our study is the largest prospective study to date that supports an association between choroidal thinning and presence of RPD12,16,17,19 in the setting of early AMD. Querques et al.17 recently reported on 43 AMD patients (22 RPD eyes without medium/large drusen, 21 non-RPD eyes with early AMD) and showed that, with the exception of the region of the macula 3000 μm superior to the fovea, the choroid was overall thinned in eyes with RPD as compared to early AMD eyes without RPD. Additionally, Switzer et al.16 found an inverse relationship between SFCT and the presence of SDD, which have been associated with RPD.
The subjects included in this analysis were age- and sex-matched, which is important, given the known inverse association between advancing age and CT,19,22 as well as the female preponderance of RPD.10 The demographics of the RPD patients included in our study, with a mean age of 76.9 years and a female majority of 80%,10,21 are in accordance with those of previous studies of RPD patients. A significant difference in BCVA was found between the RPD and non-RPD groups. This finding was expected, as RPD were found to be associated with a thinning choroidal layer.
Our findings show that RPD eyes had overall thinned choroidal layers throughout the macula as compared with non-RPD eyes. However, interestingly, the current analysis does not reveal an association between area of RPD in the macula and corresponding SFCT. This may be explained by the hypothesis of Querques et al.,17 describing an RPD-associated destruction of choroidal vessels, which results in fibrotic replacement of the choroid and leads to a relative increase in thickness in these regions. Thus, although overall quantitative area of RPD likely does not have an effect on SFCT, the predominant region of this pattern may have repercussions on the choroid that lies below.
Thinner choroids appear to be associated with RPD, which in turn is associated with a greater risk for advanced AMD. It is possible that atrophy of this layer leads to photoreceptor death, as the choroid is essential in heat transfer as well as supply and removal of substances.24
Our study found that 59% of those with early AMD had RPD and 41% did not have RPD. This analysis and others12,21 that used cSLO imaging demonstrate that the prevalence of RPD in early AMD may be higher than suggested by studies that used unenhanced fundus photography.10,25,26
The thickest retina, as measured on horizontal line scans nasal and temporal to fovea, was thinner in the RPD group as compared with the non-RPD group. This suggests that atrophy of the choroid may have some correlation with atrophy of the retina in early AMD. It can be theorized that the high oxygen needs of the retina are not be met with a deficient choroidal layer, and this leads to retinal thinning. However, the origin of primary process, retina versus choroid, cannot be ascertained by the current analysis and would require a study on progression of atrophy. Interestingly, the thinnest RT measurement, which in almost all cases was found in the foveal region, was not significantly different between the two groups. This suggests that even in the setting of a thinning choroid, the foveal retina may be preserved until later stages of dry AMD.
There are several limitations to this study. Enhanced depth imaging SD-OCT images were not available for all patients. Although the advent of EDI SD-OCT has made significant contributions in precise imaging of the choroid,18 we believe that our study's results were not significantly affected by using predominantly non-EDI imaging. Only high-quality scans with a clear posterior margin of the choroid were included in the study, and a similar number of EDI scans were used for both groups, restricting the bias that could be induced by this limitation. Additionally, all eyes excluded due to poor image quality were from the non-RPD group. It is possible that intact RPE in this group may have resulted in lower transmission of signal to the choroid, leading to poor image quality. However, the total number of excluded eyes (6 eyes) represents a small fraction of the total included eyes (138 eyes), and likely would not significantly change the outcomes. Furthermore, all 20 eyes with EDI scans also had SD-OCT scans without EDI; a masked grader measured subfoveal choroidal thickness in these scans, and found no significant difference between the measurements with and without EDI (248.7 μm ± 92.1 vs. 252.0 μm ± 94.3, respectively; P = 0.292, paired t-test).
Two eyes were used from each subject, leading to potentially biased results. However, our reanalysis of the data with inclusion of only one eye per subject revealed very similar SFCT values to our original analysis that included both eyes. Furthermore, a significant difference in SFCT was maintained between the two groups, RPD and non-RPD.
Additionally, because eyes are highly correlated, the presence of GA or CNV in the one eye may indicate that the fellow eye also has advanced AMD. We included all eyes that met inclusion/exclusion criteria, and the status of the fellow eye was not considered exclusion criteria. Both groups included a roughly equal number of eyes (17 of 44 in RPD group, 13 of 40 in non-RPD group) with a fellow eye with CNV or GA, further lessening bias.
A potential setback of using the Heidelberg interface is that AF and/or IR images are displayed in the same window as SD-OCT images, which leads to unmasking of the OCT graders. Additionally, Heidelberg Engineering's manual calipers were used to measure SFCT, which may have caused some inaccuracies because of manual measurement. However, in previous studies of SFCT calculations using caliper measurements provided by Heidelberg, a mean intraobserver variability of 23 μm was found, which demonstrates a high level of reproducibility.27
Last, the various forms of assessment for RPD status, which included clinical ophthalmologic exam, fundus photography, and cSLO imaging, were carried out within an 18-month period. It is possible that patients may have developed advanced AMD over the course of this time, although we excluded patients with any clinical or imaging findings consistent with advanced AMD.
Because RPD have been associated with increased risk of advanced AMD, thinned CT may be integral to understanding the RPD process, and may be helpful in stratifying risk of AMD progression. Although we cannot prove causation between the presence of RPD and the thinning of the choroid, we can be certain that these variables, either separately or together, could be important. Atrophy of the choroid and possible subsequent choroidal fibrosis may have significant implications for the diagnosis, treatment, and prognosis of AMD.
Acknowledgments
Supported by a grant from the Doris Duke Charitable Foundation to Columbia University (AG); the Robert L. Burch III Fund Columbia University, New York, New York (SB); and R01EY018213, N09G-302 from New York Stem Cell Science, the Foundation Fighting Blindness, Schneeweiss Stem Cell Fund, the Tistou and Charlotte Kerstan Foundation, Crowley Family Fund, Joel Hoffman Scholarship, Barbara and Donald Jonas Family Fund, and Professor Gertrude Rothschild Stem Cell Foundation (SHT).
Disclosure: A. Garg, Doris Duke Charitable Foundation (F); M. Oll, None; S. Yzer, None; S. Chang, Alcon Laboratories (C); G.R. Barile, PCAsso Diagnostics LLC (I), P; J.C. Merriam, None; S.H. Tsang, None; S. Bearelly, Robert L. Burch III Fund (F)
References
- 1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99: 933–943 [DOI] [PubMed] [Google Scholar]
- 2. Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009; 10: 19–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mimoun G, Soubrane G, Coscas G. Macular drusen [in French]. J Fr Ophtalmol. 1990; 13: 511–530 [PubMed] [Google Scholar]
- 4. Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995; 15: 183–191 [PubMed] [Google Scholar]
- 5. Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007; 91: 354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lois N, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol. 2002; 133: 341–349 [DOI] [PubMed] [Google Scholar]
- 7. Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 5495–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prenner JL, Rosenblatt BJ, Tolentino MJ, et al. Risk factors for choroidal neovascularization and vision loss in the fellow eye study of CNVPT. Retina. 2003; 23: 307–314 [DOI] [PubMed] [Google Scholar]
- 9. Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997; 124: 344–356 [DOI] [PubMed] [Google Scholar]
- 10. Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008; 145: 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011; 95: 979–985 [DOI] [PubMed] [Google Scholar]
- 12. Sohrab MA, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011; 52: 5743–5748 [DOI] [PubMed] [Google Scholar]
- 13. Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010; 117: 1775–1781 [DOI] [PubMed] [Google Scholar]
- 14. Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010; 117: 303–312.e1 [DOI] [PubMed] [Google Scholar]
- 15. Querques G, Querques L, Martinelli D, et al. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 2011; 31: 518–526 [DOI] [PubMed] [Google Scholar]
- 16. Switzer DW Jr, Mendonca LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012; 32: 1265–1271 [DOI] [PubMed] [Google Scholar]
- 17. Querques G, Querques L, Forte R, Massamba N, Coscas F, Souied EH. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012; 53: 1258–1263 [DOI] [PubMed] [Google Scholar]
- 18. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500 [DOI] [PubMed] [Google Scholar]
- 19. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147: 801–810 [DOI] [PubMed] [Google Scholar]
- 20. Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995; 39: 367–374 [DOI] [PubMed] [Google Scholar]
- 21. Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009; 148: 733–743.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815 [DOI] [PubMed] [Google Scholar]
- 23. Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103: 1796–1806 [PubMed] [Google Scholar]
- 24. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000; 41: 3117–3123 [PubMed] [Google Scholar]
- 25. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21 [DOI] [PubMed] [Google Scholar]
- 26. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 2267–2271 [DOI] [PubMed] [Google Scholar]