Abstract
Purpose.
Immunocytochemical and genetic data implicate a significant role for the activation of complement in the pathology of AMD. Individuals homozygous for a Y402H polymorphism in Factor H have elevated levels of membrane attack complex (MAC) in their choroidal blood vessels and RPE relative to individuals homozygous for the wild-type allele. An R95X polymorphism in C9, a protein necessary for the final assembly of MAC, is partially protective against the formation of choroidal neovascularization (CNV) in AMD patients. Aurintricarboxylic Acid (ATA) is a small molecule inhibitor of MAC. Our hypothesis was that attenuation of the formation of MAC on ocular tissues by ATA may protect mice against laser-induced CNV.
Methods.
The ability of ATA to inhibit human complement-mediated cell lysis, inhibit formation of human MAC, and inhibit formation of tubes by endothelial cells was examined in vitro. Subsequently, the Bruch's membrane of adult mice was damaged using an argon laser, followed by intravitreal injection of ATA. One week later, choroidal flat mounts from these mice were stained for the presence of MAC, endothelial cells, and macrophages.
Results.
ATA protects cells from human complement-mediated lysis, attenuates assembly of the MAC, and inhibits tube formation by endothelial cells in vitro. ATA also attenuates CNV, MAC deposition, and macrophage infiltration in a murine model of exudative AMD.
Conclusions.
ATA warrants further study as a potential drug for the treatment of exudative and nonexudative AMD.
Keywords: macular degeneration, choroidal neovascularization, aurintricarboxylic acid, complement, membrane attack complex
Aurintricarboxylic acid (ATA) is a small molecule inhibitor of the membrane attack complex (MAC). In this study, we found that ATA protects mice from laser-induced MAC deposition and choroidal neovascularization.
Introduction
Age-related macular degeneration (AMD) is the most common cause of blindness among the elderly, affecting approximately 1 in 3 people older than 65.1–3 AMD begins with the appearance of lipoproteinaceous deposits between the RPE and Bruch's membrane. This stage of AMD is generally referred to as “dry” or nonexudative AMD that can progress toward a more advanced form known as geographic atrophy, involving degeneration and loss of RPE cells. Approximately 10% of moderate or advanced AMD patients progress toward “wet” or exudative AMD, involving the growth of new blood vessels from the choroid into the subretinal space, that is, choroidal neovascularization (CNV) (see Ref. 4 for review). The growth of these immature and leaky blood vessels results in macular edema and is associated with elevated levels of cytokines, such as VEGF. Inhibitors of VEGF are hence the current standard of care for wet AMD.5,6 However, there are currently no therapies approved by the Food and Drug Administration (FDA) for dry AMD, which accounts for approximately 90% of AMD cases. Moreover, a significant fraction of wet AMD patients do not respond to anti-VEGF agents. Because dry AMD generally precedes wet AMD, any treatment for dry AMD may be efficacious in attenuating the progression to wet AMD.4,7
Although a complex disorder, risk factors for AMD include diet, smoking, environment, and aging. Approximately 50% of AMD patients can be accounted for by a polymorphism in the negative regulator of complement known as Factor H.8–11 Activation of complement terminates with the formation of the membrane attack complex (MAC) on the surface of cells.12 Low levels of MAC deposition are known to lead to cell mitogenesis and cytokine release and high levels of MAC are known to lead to cell lysis.13–15 Individuals who are homozygous for a Y402H polymorphism in Factor H have approximately 70% more MAC in their choroidal blood vessels and RPE relative to individuals who are homozygous for the wild-type allele.16 Prevalence of an R95X polymorphism in C9 in the Japanese population that prevents those individuals from forming MAC is protective against the development of CNV.17 Geographic atrophy patients have reduced levels of CD59 (an inhibitor of MAC) on their RPE cells18 and inhibition of MAC using recombinant CD59 protects mice from laser-induced CNV.19 In addition to MAC deposition, activation of complement produces anaphylatoxins, which are known to mediate chemotaxis of macrophages.20 Macrophage infiltration has been documented in human AMD eyes as well as in laser-induced CNV in mice.21,22 Based on the above observations, a model of AMD pathology is emerging and consistent with the hypothesis that AMD is associated with an imbalance between complement activation and complement inhibition and that MAC deposition may be a key component in the pathology associated with AMD. Although complement is a part of the innate immune system that evolved to attack invading pathogens, complement also plays key roles in various processes and in maintaining tissue homeostasis, including lipid metabolism, tissue regeneration, synapse formation, and angiogenesis. Hence, selection of the appropriate target for attenuation of complement activation needs to be selected carefully.
Previously, we demonstrated that human membrane-anchored or membrane-independent soluble CD59 can protect mice from human or murine MAC deposition and laser-induced CNV.19,23 In those studies, CD59 was delivered via an adenovirus or an adeno-associated virus vector. There are currently at least five anticomplement clinical trials ongoing using antibodies, peptides, or aptamers targeting various parts of the complement pathway, including C3, C5, and Factor D.24 The objective of the current study was to identify and examine the potential efficacy of a small molecule that may inhibit MAC deposition, CNV, and macrophage infiltration following laser-induced disruption of the Bruch's membrane, the most commonly used model of wet AMD.
Aurintricarboxylic acid (ATA) is a 422-Dalton triphenylmethyl dye compound previously shown to inhibit endothelial cell activation and complement activation in an ex vivo model of pig-to-human pulmonary xenotransplantation,25 as well as inhibit MAC formation and improve memory retention in a model of Alzheimer's disease.26 ATA specifically blocks the addition of C9 to the C5b-8 complex, preventing the complete formation of the MAC.26 Based on these and other observations, we examined the potential use of ATA in a murine model of wet AMD.
Materials and Methods
Cell Lines and Reagents
Murine Hepa-1c1c7 hepatoma cells (ATCC, Manassas, VA) were maintained in alpha-MEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Grand Island, NY). Primary human umbilical vein endothelial cells (HUVEC) cells (Invitrogen) were maintained in Medium 200PRF with 2% low serum growth supplement (LSGS; Invitrogen). ATA (Sigma-Aldrich, St. Louis, MO) stock was prepared by mixing 7.5 mg ATA with 1 mL dimethyl sulfoxide (DMSO). This stock was diluted 10-fold with sterile H2O to a final concentration of 0.75 mg/mL ATA in 10% DMSO.
Normal Human Serum-Mediated Cell Lysis
Hepa-1c1c7 cells were plated at 80% confluency in alpha-MEM supplemented with 2% FBS. Three days after plating, cells were washed three times with PBS, treated with 0.25% trypsin, and centrifuged at 1000g for 3 minutes. The pelleted cells were resuspended in 10 mL gelatin veronal buffer with calcium and magnesium (GVB++; Complement Technology, Tyler, TX) and counted using a hemacytometer. A total of 500,000 cells were incubated in either ATA or DMSO in the presence of normal human serum (NHS; Sigma) at a final concentration of 1%. To ensure that lysis was complement-mediated, cells were alternatively incubated with heat-inactivated 1% NHS (HI-NHS). Samples were incubated for 1 hour at 37°C with constant rotation. One hour postincubation, propidium iodide (PI) was added to cells at 2 mg/mL, and uptake was analyzed with FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using CellQuest Pro software (Becton Dickinson).
Deposition of Human MAC on Hepa-1c1c7 Cells
A total of 3.0 × 103 Hepa-1c1c7 cells were plated on poly-D-lysine–coated chamber slides (Becton Dickinson), in alpha-MEM supplemented with 2% FBS. Three days after plating, cells were washed three times with PBS and incubated with a final concentration of 10% NHS or HI-NHS in GVB++ (Complement Technology) in the presence of either 50 μg/mL of ATA in 10% DMSO or 10% DMSO alone, at 37°C for 5 minutes. Cells were washed three times with PBS and fixed with 3.7% formaldehyde (MP Biomedicals, Solon, OH) for 15 minutes. After three washes in PBS, cells were incubated for 2.5 hours at 37°C with mouse monoclonal anti-human C5b-9 (clone aE1; Abcam, Cambridge, MA) and then for 1.5 hours with Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch, West Grove, PA). Cells were imaged using an inverted microscope (Olympus IX51; Olympus, Center Valley, PA), and staining intensity quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
In Vitro Tube Formation by HUVECs
Human umbilical vein endothelial cells were plated at a confluency of 4.5 × 104 cells on 24-well plates that had been precoated with Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen). Low serum growth supplement-supplemented Medium 200PRF (Invitrogen) was supplemented with various concentrations of ATA or DMSO. Subsequently, the cells were incubated for 6 hours at 37°C. Cells were imaged using an inverted microscope (Olympus IX51; Olympus) and data analyzed using ImageJ software (National Institutes of Health).
Animals
All animal studies were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. This study was approved by Tufts University Institutional Animal Care and Use Committee. We used male wild-type C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) between 6 and 8 weeks of age. For intravitreal injection and laser-induced choroidal neovascularization, mice were anesthetized by intraperitoneal injection of 0.1 mg/g body weight of ketamine and 0.01 mg/g body weight xylazine. Pupils were dilated with a drop each of 2.5% phenylephrine HCl and 1% Tropicamide (Bausch and Lomb, Rochester, NY).
Laser-Induced Choroidal Neovascularization
Laser photocoagulation was performed using an argon laser (532 nm) at 100 ms and 150 mW to generate four 75-μm spots in the retina. Laser burns were induced around the optic nerve with the aid of a slit-lamp microscope and a cover-slip held on the cornea as a contact lens to view the posterior of the eye.
Intravitreal Injection
Eyes were injected 2 to 5 minutes after laser. A 32-G needle (Hamilton, Reno, NV) was used in a trans-scleral transchoroidal approach to deliver either 1.5 μg of ATA in 10% DMSO or 10% DMSO vehicle alone, in a total volume of 2 μL.
Immunostaining of RPE/Choroid Flatmounts
Seven days post laser, mice were killed by CO2 asphyxiation and cervical dislocation. Eyes were enucleated and the optic nerve, cornea, lens, iris, and retina removed. The RPE/choroid cups were fixed in 4% paraformaldehyde overnight. Eyecups were stained with either FITC-conjugated Griffonia simplicifolia Lectin (GSL I, Isolectin B4; Vector Labs, Burlingame, CA), rabbit anti-human SC5b-9 (MAC) Neo (IgG) (Complement Technology), or rat monoclonal anti-mouse F4/80 antibody (CI:A3-1; Abcam), followed by CY3-conjugated secondary antibodies where appropriate. The eyecups were dissected for flatmounting and imaged using an Olympus IX51 microscope with appropriate filters, a Retiga 2000R Fast camera (Retiga; Surrey, BC, Canada) and QCapture Pro 5.0 (QImaging, Surrey, BC, Canada) software. GSL I and F4/80 staining was quantified using ImageJ software (National Institutes of Health) to measure the total area of staining. MAC staining was quantified by measuring pixel intensities within the CNV spots.
Results
ATA Protects Hepa-1c1c7 Cells From Human Complement-Mediated Lysis
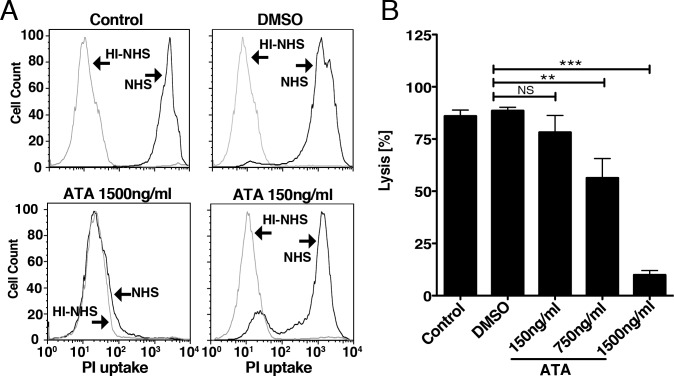
Elevated levels of MAC on membranes lead to cell lysis. Loss of RPE cells is a hallmark of geographic atrophy, an advanced form of dry AMD.7 To examine whether ATA may protect cells from complement-mediated cell lysis, we incubated Hepa-1c1c7 cells in 1% NHS, HI-NHS, or 1% NHS containing DMSO (vehicle) or 1% NHS containing ATA. Percentage of cell lysis was determined by uptake of PI as measured by FACS (Fig. 1A). We found that 86.00% ± 2.85% or 88.58% ± 1.63% of cells were lysed by 1% NHS or 1% NHS containing DMSO, respectively (Fig. 1B). In contrast, only 9.96% ± 2.08% of cells were lysed by 1% NHS containing 1500 ng/mL ATA, leading to approximately 78.62% ± 3.03% (P < 0.0001) ATA-mediated protection from cell lysis relative to DMSO. ATA-mediated protection from cell lysis was dose dependent, as 750 ng/mL and 150 ng/mL ATA conferred approximately 32.20% ± 7.44% (P < 0.002) and 10.34% ± 6.53% (P = 0.14) protection from cell lysis, respectively (Fig. 1B). We conclude that ATA protects Hepa-1c1c7 from complement-mediated cell lysis in a dose-dependent manner.
Figure 1.
ATA protects Hepa-1c1c7 cells from human complement-mediated lysis. (A) Representative FACS histograms of PI uptake by Hepa-1c1c7 cells following incubation in either 1% NHS or 1% HI-NHS in the presence of DMSO (vehicle) or ATA at 150 ng/mL or 1500 ng/ml. (B) Quantification of PI uptake by Hepa-1c1c7 cells following incubation in 1% NHS indicates that ATA protects from cell lysis in a dose dependent manner. Relative to DMSO, 750 ng/mL or 1500 ng/mL ATA confers 32.20% ± 7.44% (P < 0.002) and 78.62% ± 3.03% (P < 0.0001) protection from cell lysis. Total cell lysis observed at 150 ng/mL ATA was not significantly different (10.34% ± 6.53%, P = 0.14) to that observed with DMSO. Bar graph indicates the average of three independent studies (n = 11 for control, n = 8 for DMSO, n = 5 for each concentration of ATA).
ATA Inhibits the Deposition of Human MAC In Vitro
Individuals who are homozygous for the Y402H polymorphism in Factor H have approximately 70% more MAC in their choroid and RPE relative to individuals homozygous for the wild-type allele.16 To examine whether ATA-mediated protection from complement-mediated cell lysis was consistent with reduced levels of MAC on the cell surface, we incubated Hepa-1c1c7 cells in 10% NHS or 10% NHS containing DMSO or ATA for 5 minutes. Subsequently, cells were fixed and stained using a monoclonal antibody that binds the MAC complex (Fig. 2A). Staining of MAC was quantified using ImageJ. We found that whereas there was no significant difference in MAC staining between NHS and NHS containing DMSO (P > 0.5, data not shown), there was an 86.10% ± 0.09% (P < 0.0006) reduction in MAC staining when NHS contained 50 μg/mL ATA (Fig. 2B). Furthermore, unlike the perturbation in cell morphology observed in the presence of NHS or NHS+DMSO, the morphology of cells incubated in NHS+ATA was indistinguishable from that of cells incubated in HI-NHS (data not shown). We conclude that ATA confers protection against the deposition of human MAC on Hepa-1c1c7 cells.
Figure 2.

ATA inhibits the deposition of human membrane attack complex in vitro. (A) Representative micrographs of differential interference contrast (DIC) and fluorescence images of Hepa-1c1c7 cells immunostained with human MAC antibody following incubation in 10% NHS containing either DMSO or 50μg/mL ATA. Nuclei are stained with 4′,6-diamidino-2-phenylindole. (B) Quantification of intensity of MAC staining of Hepa-1c1c7 cells incubated with HI-NHS or NHS containing DMSO or 50 μg/mL ATA. Bar graph represents results of three independent studies (n = 3 for each condition in each experiment). A significant reduction is observed in MAC staining between ATA and the vehicle (DMSO)-incubated cells (86.10% ± 0.09%, P < 0.0006). There is no significant difference observed in MAC staining between ATA and HI-NHS, suggesting a complete inhibition of MAC formation by 50 μg/mL ATA.
ATA Inhibits Tube Formation by Endothelial Cells In Vitro
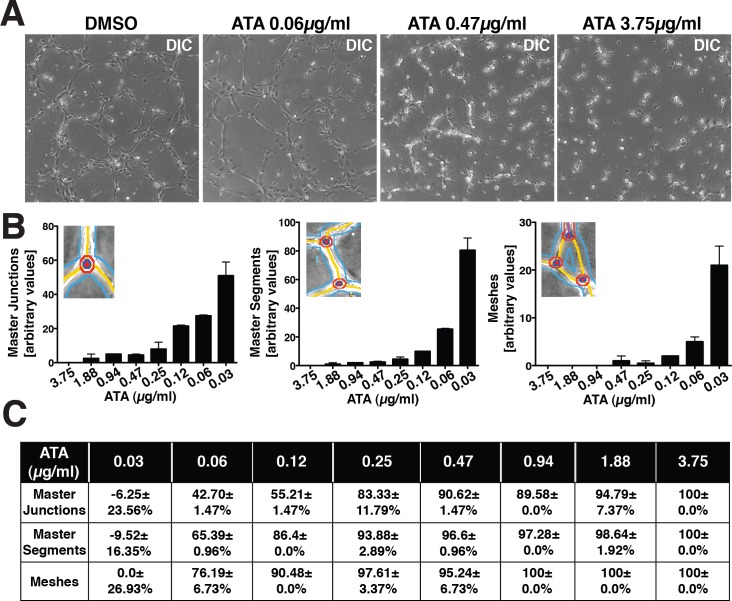
Whereas high levels of MAC lead to cell lysis, sublytic levels of MAC lead to mitogenesis and cytokine release.13–15 Approximately 10% of AMD patients progress to wet AMD, a condition associated with elevated levels of cytokines, such as VEGF. Wet AMD is a consequence in part to migration of choroidal endothelial cells invading the subretinal space in response to elevated VEGF. These endothelial cells eventually form immature blood vessels that leak fluids, a characteristic of macular edema. To examine whether ATA may inhibit endothelial tube formation, we performed standard HUVEC tube formation assays in the presence of different concentrations of ATA. At 0.06, 0.47, and 3.75μg/mL ATA, we observed an inhibition of tube formation by HUVECs (Fig. 3A). We quantified the ability of HUVECs to form master junctions, master segments, or meshes in the presence and absence of varying concentrations (0.03–3.75 μg/mL) of ATA. We found that ATA inhibits formation of master junctions, master segments, and meshes in a dose-dependent manner (Fig. 3B). Complete inhibition of all three aspects of tube formation was observed at 3.75 μg/mL ATA, with significant attenuation being observed at concentrations as low as 0.06 μg/mL (Fig. 3C). We conclude that ATA inhibits tube formation by HUVECs in vitro.
Figure 3.
ATA inhibits tube formation by endothelial cells in vitro. (A) Representative DIC images of tube formation by HUVECs in the presence of DMSO or ATA at three different concentrations (0.06 μg/mL, 0.47 μg/mL, and 3.75 μg/mL). (B) Quantification of the total number of master junctions, master segments, or meshes observed in the network of tubes formed by HUVECs in the presence of varying concentrations of ATA (0.03 μg/mL–3.75 μg/mL). An illustration of a master junction, master segment, and a mesh is indicated in the inserts. (C) Table summarizing the reduction in number of master segments, master junctions, and meshes in tubes formed in the presence of varying concentrations of ATA relative to DMSO. Quantification reveals a dose-dependent inhibition of master junctions, master segments, and meshes. This study was performed twice in duplicate for each concentration of ATA and DMSO.
ATA Inhibits Choroidal Neovascularization in a Model of AMD
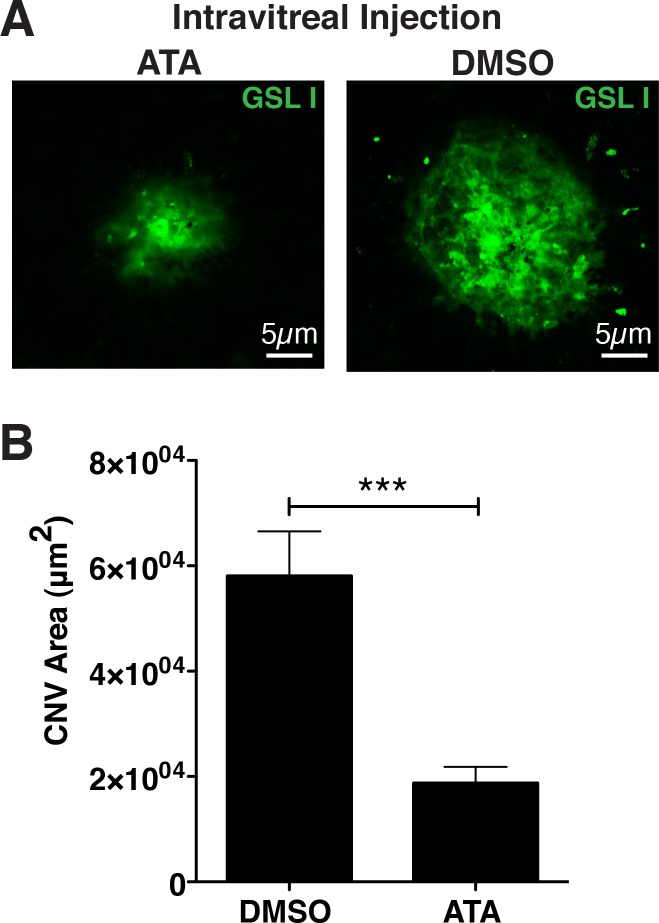
The most widely used model of CNV or wet AMD involves the use of laser-induced damage to the Bruch's membrane and RPE, resulting in elevated levels of cytokines and consequent endothelial cell migration into the subretinal space.27 This model is highly quantifiable and useful for the testing of inhibitors of neovascularization. For example, antibodies targeting VEGF inhibit laser-induced CNV.28 To determine whether ATA may influence the size of CNV spots following a laser burn, 1.5 μg of ATA in DMSO or DMSO alone was injected into the vitreous of C57Bl6/J mice immediately following laser-induced injury. Seven days postinjection, mice were killed and the RPE/choroid excised and endothelial cells stained with FITC-conjugated GSL I (Fig. 4A). We found that the average size of CNV observed in mice injected with DMSO was 5.81 ± 0.84 × 104 μm2, whereas the average size of CNV observed in mice injected with ATA was 1.88 ± 0.31 × 104 μm2, resulting in approximately a 67.7% ± 14.2% (P < 0.0001) reduction in CNV attributable to ATA (Fig. 4B). We hence conclude that ATA inhibits laser-induced CNV in a model of wet AMD.
Figure 4.
ATA inhibits CNV in a model of AMD. (A) Fluorescence micrographs of GSL I staining of representative laser-induced CNV spots in murine eyecups, following intravitreal injection of either 1.5 μg ATA in DMSO or DMSO alone. (B) Quantification of the area of CNV shows a significant reduction (67.7% ± 14.2%, P < 0.0001) in size of CNV in those eyes injected with ATA relative to those injected with DMSO. n = 38 spots/14 eyes for ATA and n = 29 spots/14 eyes for DMSO.
ATA Inhibits the Deposition of Murine Membrane Attack Complex In Vivo
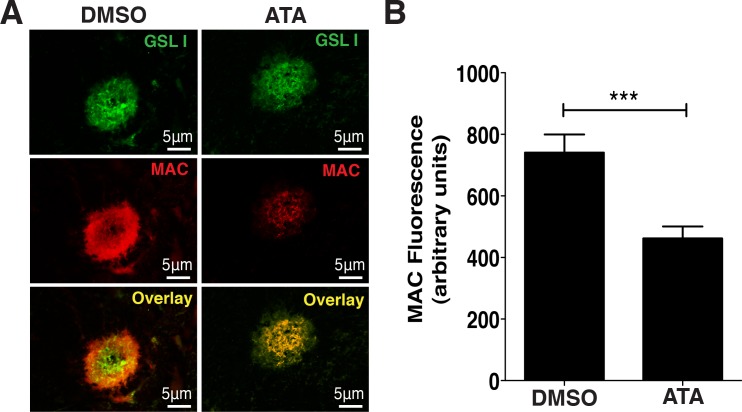
In the studies described above, we found that ATA inhibits the deposition of human MAC in vitro. To determine whether ATA could also inhibit murine MAC in vivo, we stained CNV complexes generated from laser burns in the above studies with an antibody against murine MAC (Fig. 5A). We found that ATA-injected mice had approximately 37.7% ± 9.2% (P < 0.0001) less MAC deposition in the area of the CNV relative to DMSO-injected eyes (Fig. 5B). Deposition of MAC was not directly correlated to the size of the CNV, as CNV spots of roughly similar size had significantly different levels of MAC. We conclude that ATA inhibits deposition of murine MAC in the laser-induced model of wet AMD.
Figure 5.
ATA inhibits the deposition of murine MAC in vivo. (A) Fluorescence micrographs of representative laser-induced CNV spots in murine eyecups costained with GSL I and an antibody against MAC reveals reduced MAC staining following intravitreal injection of ATA relative to injection with DMSO. (B) Quantification of MAC staining indicates a significant reduction (37.7% ± 9.2%, P < 0.0001) in staining intensity in eyes injected with ATA relative to those injected with DMSO. n = 38 spots/14 eyes for ATA, and n = 29 spots/14 eyes DMSO.
ATA Inhibits Macrophage Infiltration in Laser-Induced CNV
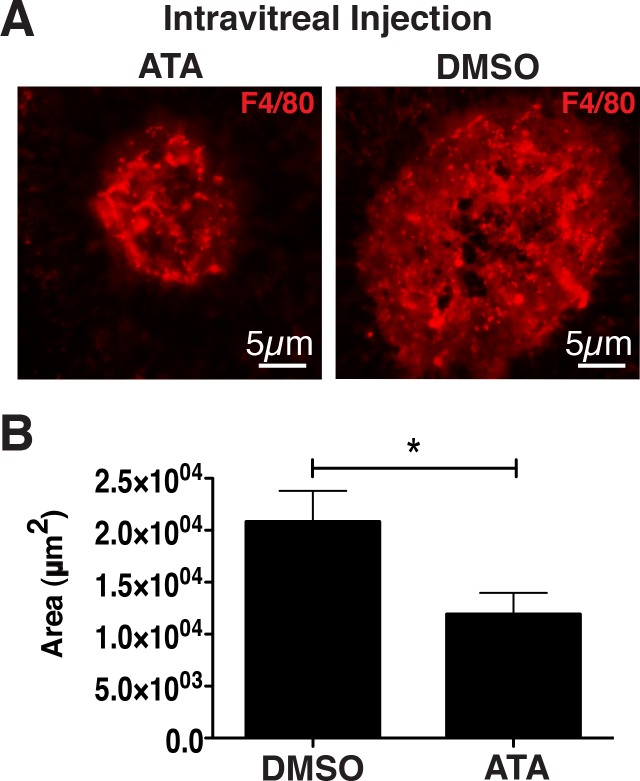
Activation of complement results in the production of C3a and C5a anaphylatoxins that bind to their respective receptors C3aR and C5aR on macrophages.20 Infiltration of macrophages has been documented in the eyes of AMD patients as well as in laser-induced CNV.21,22 Blocking of C3aR or C5aR by genetic or chemical approaches has been previously shown to reduce the size of laser-induced CNV29 and the generation of C5a may lead to the activation of endothelial cells in AMD.30 To determine whether ATA may attenuate infiltration of macrophages in laser-induced CNV, we stained laser-induced CNV spots with antibody against murine F4/80 (Fig. 6A). We determined that the average area of F4/80 staining observed in the CNV of mice injected with DMSO was 2.10 ± 0.29 × 104 μm2, whereas the average area of F4/80 staining observed in the CNV of mice injected with ATA was 1.2 ± 0.2 × 104 μm2, resulting in approximately a 42.68% ± 18.60% (P < 0.03) reduction in F4/80 staining attributable to ATA (Fig. 6B). We hence conclude that ATA inhibits macrophage infiltration in the laser-induced model of wet AMD.
Figure 6.
ATA inhibits macrophage infiltration in laser-induced CNV. (A) Fluorescence micrographs of representative laser-induced CNV spots in murine eyecups stained with an antibody against the macrophage marker, F4/80, indicating a reduction in macrophage staining in eyes injected with ATA relative to eyes injected with DMSO. (B) Quantification of the area of F4/80 staining indicated a significant reduction (42.68% ± 18.60%, P < 0.03) in the area of macrophage-staining in laser-induced CNV spots of eyes injected with ATA relative to those of eyes injected with DMSO. n = 18 spots/8 eyes for ATA, and n = 25 spots/10 eyes DMSO. F4/80, macrophage marker.
Discussion
In this study, we report for the first time that ATA, a small molecule inhibitor of MAC, attenuates complement activation and CNV in a model of exudative AMD. We also report for the first time that ATA inhibits MAC deposition and macrophage infiltration in laser-induced CNV. There are currently no therapies available for the dry or nonexudative form of AMD and each of the FDA-approved therapies for exudative AMD target VEGF. The cause of elevated levels of VEGF in the eyes of AMD patients is largely unknown. One hypothesis is that VEGF upregulation is a consequence of complement activation. Such hypothesis is consistent with the observations that individuals who have an inability to adequately regulate complement are predisposed to dry and/or wet AMD,8–11,16 and such individuals have elevated levels of MAC in their choroid and RPE,16 suggesting that complement activation has reached completion. Low levels of MAC are known to signal cells to secrete cytokines, such as VEGF.13–15 Individuals who are unable to complete the final step of complement activation (i.e., form the MAC) are partially protected from the development of exudative AMD.17 Finally, because the ultimate goal of MAC is to lyse cells, elevated levels of MAC are consistent with the observation that advanced AMD patients have areas of geographic atrophy (i.e., loss of RPE cells). Hence, the results of the studies described herein may have relevance toward the future development of therapies for nonexudative as well as exudative AMD.
Although the role of complement in AMD is not yet well understood, there is significant consensus that attenuation of complement activation in AMD may be of therapeutic value. Hence, multiple clinical trials targeting various parts of the complement pathway are currently under way. Although still at early stages, the goals of these trials are to examine the efficacy of targeting complement C3, C5, or Factor D by using a peptide, aptamer, or antibody, respectively.24
There are several reasons why targeting of MAC may be preferred relative to targeting of other parts of the complement pathway. Chief among these is that complement plays key roles in tissue homeostasis.31 For example, in addition to its key role in the host immune response, complement is also required for synaptogenesis, lipid metabolism, glucose uptake, triglyceride synthesis, and so forth. Altering cellular homeostasis through the use of drugs that target the complement pathway may lead to alterations in tissue homeostasis. The inability to produce MAC seems to have no significant side effects. For example, individuals in the Japanese population who fail to form MAC due to a polymorphism in C9 have a mortality rate no higher than the general population.32 Hence, targeting of MAC may be a preferred approach for the development of therapies for AMD.
Previously, we found that glycophosphatidylinositol-anchored CD59 protein, a naturally occurring inhibitor of MAC, expressed on RPE cells by an adenovirus vector can protect against the formation of human MAC on murine RPE ex vivo.23 Subsequently, we found that a soluble nonmembrane-associated CD59 delivered by an adeno-associated virus vector attenuates CNV in a model of exudative AMD following intravitreal injection. ATA has recently been shown to inhibit complement specifically at the stage of MAC formation.26 Relative to the use of proteins, such as CD59, there are some advantages to the use of small molecules as drugs. In general, production of small molecules is more cost-effective relative to protein therapeutics or gene therapy approaches. Also, although gene therapies generally persist for substantially longer than the half-lives of proteins, such as avastin or lucentis, small molecules are generally easier to produce in slow-release devices that may be placed in the vitreous compartment.
As discussed above, there is significant evidence suggesting that inhibition of MAC formation may be potentially therapeutic for exudative as well as nonexudative forms of AMD.19,23,33,34 The hypothesis discussed above suggests that MAC formation occurs before the release of cytokines, such as VEGF. Hence, it is reasonable to suggest that inhibition of MAC may prevent the progression of nonexudative to exudative AMD. Although many molecules are known to inhibit CNV in the laser model of exudative AMD, only CD59 has been previously shown to inhibit CNV through the inhibition of MAC.19 In those studies, CD59 reduced MAC and CNV by 53% and 62%, respectively, by using the same laser protocol as that used in the current study. In the current study, ATA inhibited MAC and CNV by 38% and 64%, respectively, suggesting that ATA or its analogs are yet another potent group of molecules worthy of further development as a therapy for AMD. ATA may have certain advantages and disadvantages relative to CD59 as a drug and these properties remain to be studied. Future studies will also examine the toxicity of ATA following intravitreal delivery. Prior studies indicate that ATA is well tolerated when fed to animals.26 The mode of delivery, intravitreal injection, is a current standard of care. Patients suffering from exudative AMD receive intravitreal injections every 4 to 12 weeks. Such injections can lead to complications such as increased intraocular pressure, retinal detachment, and endophthalmitis, but in the vast majority of cases they are well tolerated.
Although it has previously been shown that ATA can inhibit endothelial cell activation,35 formation of the C3 convertase of the alternative pathway,36 and MAC deposition,26 ATA also inhibits apoptosis37 and protein-nucleic acid interactions.38 ATA also has been shown to stimulate tyrosine phosphorylation, including the Jak2/STAT5 pathway39 and to inhibit calpain.40 Although it has been previously shown by us and others that the use of an inhibitor of MAC, such as CD59, can attenuate laser-induced CNV, it is likely that the additional properties of ATA may also be contributing toward the effects described in this study. For example, the antiangiogenic activity of ATA has been shown to be reduced in the presence of heparin and basic fibroblast growth factor (FGF)35; ATA also has been shown to bind acidic FGF, causing destabilization and reduction of its mitogenic activity.41 In addition, it has recently been reported that ATA also binds complement Factor D, preventing cleavage of the tissue-bound properdin-C3b-Factor B complex into the active C3 convertase enzyme properdin-C3b-Factor Bb.36 ATA may thus be inhibiting the alternative pathway of complement at two different stages. Inhibition of either the anaphylatoxins29 or the MAC independently34 results in a reduction of CNV. Future studies will dissect the relative contribution of the anaphylatoxins and the MAC in laser-induced CNV.
In summary, in this study we have found that ATA can protect cells from complement-mediated cell lysis, attenuate human MAC deposition in vitro, inhibit tube formation by endothelial cells, inhibit laser-induced CNV in mice, inhibit murine MAC deposition in vivo, and inhibit macrophage infiltration in laser-induced CNV. Our results suggest that ATA or its analogs warrant further investigation as a molecule for inhibiting complement-mediated pathology of AMD.
Acknowledgments
We thank Kerstin Birke, PhD, for helpful discussions during the course of this study.
Supported by grants from The Ellison Foundation, The Virginia B. Smith Trust, The National Institutes of Health/NEI (EY021805 and EY013837), The Department of Defense/TATRC, The Fireman Foundation (all to RK-S), and grants to the Department of Ophthalmology at Tufts University School of Medicine from the Lions Eye Foundation and Research to Prevent Blindness.
Disclosure: E. Lipo, None; S.M. Cashman, None; R. Kumar-Singh, None
References
- 1. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572 [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114: 253–262 [DOI] [PubMed] [Google Scholar]
- 3. Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012; 96: 752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Jong PT. Age-related macular degeneration. N Engl J Med. 2006; 355: 1474–1485 [DOI] [PubMed] [Google Scholar]
- 5. Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab. Curr Med Res Opin. 2011; 27: 1465–1475 [DOI] [PubMed] [Google Scholar]
- 6. van Wijngaarden P, Qureshi SH. Inhibitors of vascular endothelial growth factor (VEGF) in the management of neovascular age-related macular degeneration: a review of current practice. Clin Exp Optom. 2008; 91: 427–437 [DOI] [PubMed] [Google Scholar]
- 7. Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012; 33: 295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421 [DOI] [PubMed] [Google Scholar]
- 10. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424 [DOI] [PubMed] [Google Scholar]
- 12. Davies A, Lachmann PJ. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol Res. 1993; 12: 258–275 [DOI] [PubMed] [Google Scholar]
- 13. Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994; 179: 985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halperin JA, Taratuska A, Nicholson-Weller A. Terminal complement complex C5b-9 stimulates mitogenesis in 3T3 cells. J Clin Invest. 1993; 91: 1974–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunchithapautham K, Rohrer B. Sublytic membrane-attack-complex (MAC) activation alters regulated rather than constitutive vascular endothelial growth factor (VEGF) secretion in retinal pigment epithelium monolayers. J Biol Chem. 2011; 286: 23717–23724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011; 93: 565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishiguchi KM, Yasuma TR, Tomida D, et al. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 508–512 [DOI] [PubMed] [Google Scholar]
- 18. Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013; 229: 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cashman SM, Ramo K, Kumar-Singh R. A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration. PLoS One. 2011; 6: e19078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009; 46: 2753–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001; 20: 385–414 [DOI] [PubMed] [Google Scholar]
- 22. Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 3578–3585 [DOI] [PubMed] [Google Scholar]
- 23. Ramo K, Cashman SM, Kumar-Singh R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest Ophthalmol Vis Sci. 2008; 49: 4126–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013; 13: 438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HK, Kim JE, Wi HC, et al. Aurintricarboxylic acid inhibits endothelial activation, complement activation, and von Willebrand factor secretion in vitro and attenuates hyperacute rejection in an ex vivo model of pig-to-human pulmonary xenotransplantation. Xenotransplantation. 2008; 15: 246–256 [DOI] [PubMed] [Google Scholar]
- 26. Lee M, Guo JP, Schwab C, McGeer EG, McGeer PL. Selective inhibition of the membrane attack complex of complement by low molecular weight components of the aurin tricarboxylic acid synthetic complex. Neurobiol Aging. 2012; 33: 2237–2246 [DOI] [PubMed] [Google Scholar]
- 27. Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010; 29: 500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campa C, Kasman I, Ye W, Lee WP, Fuh G, Ferrara N. Effects of an anti-VEGF-A monoclonal antibody on laser-induced choroidal neovascularization in mice: optimizing methods to quantify vascular changes. Invest Ophthalmol Vis Sci. 2008; 49: 1178–1183 [DOI] [PubMed] [Google Scholar]
- 29. Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006; 103: 2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 5336–5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013; 25: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagata M, Hara T, Aoki T, et al. Inherited deficiency of ninth component of complement: an increased risk of meningococcal meningitis. J Pediatr. 1989; 114: 260–264 [DOI] [PubMed] [Google Scholar]
- 33. Birke K, Lipo E, Birke MT, Kumar-Singh R. Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration. PLoS One. 2013; 8: e76766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bora NS, Kaliappan S, Jha P, et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007; 178: 1783–1790 [DOI] [PubMed] [Google Scholar]
- 35. Gagliardi AR, Collins DC. Inhibition of angiogenesis by aurintricarboxylic acid. Anticancer Res. 1994; 14: 475–479 [PubMed] [Google Scholar]
- 36. Lee M, Guo JP, McGeer EG, McGeer PL. Aurin tricarboxylic acid self-protects by inhibiting aberrant complement activation at the C3 convertase and C9 binding stages. Neurobiol Aging. 2013; 34: 1451–1461 [DOI] [PubMed] [Google Scholar]
- 37. Beery R, Haimsohn M, Wertheim N, et al. Activation of the insulin-like growth factor 1 signaling pathway by the antiapoptotic agents aurintricarboxylic acid and Evans blue. Endocrinology. 2001; 142: 3098–3107 [DOI] [PubMed] [Google Scholar]
- 38. Hallick RB, Chelm BK, Gray PW, Orozco EM Jr. Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977; 4: 3055–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rui H, Xu J, Mehta S, et al. Activation of the Jak2-Stat5 signaling pathway in Nb2 lymphoma cells by an anti-apoptotic agent, aurintricarboxylic acid. J Biol Chem. 1998; 273: 28–32 [DOI] [PubMed] [Google Scholar]
- 40. Posner A, Raser KJ, Hajimohammadreza I, Yuen PW, Wang KK. Aurintricarboxylic acid is an inhibitor of mu- and m-calpain. Biochem Mol Biol Int. 1995; 36: 291–299 [PubMed] [Google Scholar]
- 41. Lozano RM, Rivas G, Gimenez-Gallego G. Destabilization, oligomerization and inhibition of the mitogenic activity of acidic fibroblast-growth factor by aurintricarboxylic acid. Eur J Biochem. 1997; 248: 30–36 [DOI] [PubMed] [Google Scholar]