Abstract
Exposure to stressors at formative stages in the development of wildlife and humans can have enduring effects on health. Understanding which, when and how stressors cause enduring health effects is crucial because these stressors might then be avoided or mitigated during formative stages to prevent lasting increases in disease susceptibility. Nevertheless, the impact of early-life exposure to stressors on the ability of hosts to resist and tolerate infections has yet to be thoroughly investigated. Here, we show that early-life, 6-day exposure to the herbicide atrazine (mean ± s.e.: 65.9±3.48 µg l−1) increased frog mortality 46 days after atrazine exposure (post-metamorphosis), but only when frogs were challenged with a chytrid fungus implicated in global amphibian declines. Previous atrazine exposure did not affect resistance of infection (fungal load). Rather, early-life exposure to atrazine altered growth and development, which resulted in exposure to chytrid at more susceptible developmental stages and sizes, and reduced tolerance of infection, elevating mortality risk at an equivalent fungal burden to frogs unexposed to atrazine. Moreover, there was no evidence of recovery from atrazine exposure. Hence, reducing early-life exposure of amphibians to atrazine could reduce lasting increases in the risk of mortality from a disease associated with worldwide amphibian declines. More generally, these findings highlight that a better understanding of how stressors cause enduring effects on disease susceptibility could facilitate disease prevention in wildlife and humans, an approach that is often more cost-effective and efficient than reactive medicine.
Keywords: Batrachochytrium dendrobatidis, developmental window, glucocorticoids, herbicide, resistance, tolerance
1. Introduction
Stressors can have profound and enduring effects on health when experienced during formative stages of life [1–4]. Understanding which, when and how stressors cause these enduring health effects is crucial because these stressors might then be avoided or mitigated during formative stages to prevent lasting increases in disease. Surprisingly, we know little about how early-life exposure to stressors affects the risk of infectious diseases later in life. Indeed, a review on later-life outcomes of early-life exposures [4] provides several examples of stress early in life having enduring effects on non-infectious disease outcomes, but offers no examples of early-life exposures affecting infectious diseases later in life (but see [5]).
There are several mechanisms by which early-life exposure to stressors might affect disease later in life. First, vertebrate exposure to stress hormones (e.g. glucocorticoids) during key developmental windows can cause lasting alterations in the functioning of their hypothalamic–pituitary–adrenal (HPA) axis, affecting their immune system into adulthood [1–3]. These changes to the endocrine or immune system of hosts might affect infection resistance (ability to prevent or clear infections), tolerance (ability to minimize the fitness consequences of infections [6–9]) or both. The distinction between resistance and tolerance is important, because tolerant individuals can be superspreaders [3], disproportionately facilitating pathogen transmission, whereas resistant hosts can impose strong selection pressures on parasites for increased virulence [6]. Second, the susceptibility of many species to pathogens can depend on their size and developmental stage. Thus, early-life exposure to stressors that alter growth or development might also alter the risk of infectious disease later in life [10]. Distinguishing these mechanisms is important. On the one hand, if stressors affect host susceptibility as a function of individual size or development (i.e. quality/condition), then any stressor that affects these traits could affect disease risk. On the other hand, if the mechanism is mediated by specific effects of a stressor (e.g. a toxin) on the immune system, any enduring effect of stressor exposure could be limited to parasites controlled by affected immune parameters.
Regardless of the mechanism, it is critical that we understand the effects of early-life exposure to stressors on infectious disease. Such data are rare but could explain recent emergence of some zoonoses, as well as greater prevalence of some infections in anthropogenically modified areas [3]. Effects of stressors on disease are particularly important for amphibians because they are the most threatened of all vertebrate taxa, and many of their declines have been linked to disease [11,12], most prominently Batrachochytrium dendrobatidis (Bd). Bd is a chytrid fungus implicated in hundreds of amphibian declines in the past four decades, and whose distribution and consequences for hosts are influenced by abiotic stressors [13–17].
We set out to test whether early-life exposure to the herbicide atrazine—the second most commonly used pesticide in the USA, and possibly the world [18]—has immediate and/or enduring effects on Bd-induced mortality of amphibians, and, if so, to identify the mechanisms driving these effects. We exposed Cuban tree frog tadpoles (Osteopilus septentrionalis), at two different windows in their development, to an ecologically relevant exposure (6-day exposure to 65.9 ± 3.48 µg l−1, mean ± s.e.; see the electronic supplementary material, Results) of atrazine (see the electronic supplementary material, figure S1). We then challenged the frogs with Bd, either immediately after atrazine exposure (as tadpoles) or a mean of 46 days later (post-metamorphosis), to evaluate the short- and long-term effects of atrazine on amphibian resistance and tolerance to Bd (see the electronic supplementary material, figure S1).
We formulated several specific hypotheses based on the biology of amphibian–Bd interactions, and documented effects of atrazine and early-life stressors on amphibian hosts. Frogs tend to be more sensitive to chytridiomycosis at smaller sizes and as they approach metamorphosis [19,20], and atrazine is known to reduce amphibian size and have variable effects on development [21]. Moreover, stressors experienced early in tadpole development tend to slow metamorphosis, whereas stressors experienced later in tadpole development can expedite metamorphosis [21]. Hence, we postulated that early-life exposure to atrazine would affect Bd-induced mortality by altering the size and developmental stage at which the frogs were exposed to Bd. In addition, in a variety of vertebrates, atrazine exposure early in life disrupts reproductive hormone regulation and morphology [22–24], alters the HPA axis and stress hormone levels, and has enduring negative effects on performance and immunity [21,25–32]. Thus, we also hypothesized that, even when controlling for size and developmental stage, atrazine exposure would have immediate and persistent effects on Bd-induced mortality mediated by changes to host physiology. Finally, atrazine has been shown to affect amphibian resistance and tolerance to macroparasites [21,25,26,33], and thus we hypothesized that it would affect both resistance and tolerance to Bd.
2. Material and methods
(a). Experimental design and animal care
Five weeks before the start of the experiment, we established 60 outdoor mesocosms (1.8 m diameter, 60 cm deep, approx. 1100 l) at a facility in southeastern Hillsborough County, FL, USA, each containing 800 l of water and inoculations of local zooplankton, phytoplankton and periphyton, and covered with 60% shade cloth. Forty tanks received 50 O. septentrionalis tadpoles (all at Gosner stages [25–28,34]) from five clutches collected from pools at the University of South Florida (USF) campus (the clutches were mixed to homogenize genetic variation before being distributed among the tanks). These pools were filled with municipal water and could not receive any run-off that might contain contaminants. This experiment was conducted in outdoor tanks, so that the food resources of the tadpoles might be more natural than in a laboratory.
We implemented a completely randomized 2×2×2×2 factorial design with five replicates of each treatment (see the electronic supplementary material, figure S1). Tanks received either 25 ml of ethanol solvent or atrazine (Chemservice, West Chester, PA; technical grade, purity more than 98%; see the electronic supplementary material, Results) dissolved in 25 ml of ethanol. Rohr and co-workers have conducted 10 previous studies examining the effects of atrazine on amphibians and parasites (most reviewed in [21,35–40]). These studies quantified various traits of the amphibians and parasites [27,28,35–41], but never detected any immediate or later-life effects of the solvent on any measured trait. Thus, a water control was not included in our design, and we assume the effects of atrazine did not depend on the presence of the solvent (i.e. no atrazine-by-solvent interactions). To quantify actual atrazine concentrations, water samples were taken from every tank 1 h after dosing and atrazine was quantified using the Abraxis (Abraxis LLC, Warminster, PA) ELISA microtiter plate kit, with each sample processed in duplicate (measured concentrations were 65.9 ± 3.48 mg l−1, mean ± s.e.). Atrazine has a long half-life [21] and was shown to have negligible breakdown over a 7-day period [41].
To evaluate whether there were developmental windows of susceptibility to atrazine, tadpoles were exposed to the solvent control or atrazine for 6 days at one of two periods during their development, either in the first week (Gosner stage 26–28) or second week (Gosner stage 35–37) of the experiment (see electronic supplementary material, figure S1). These two time points were chosen because they roughly corresponded to the pre- and pro-metamorphic developmental windows in which the stress hormone corticosterone can affect the tadpole development of some amphibian species [42]. After exposure to atrazine or solvent, we characterized Gosner stage [34] and snout–vent lengths (SVLs) of all tadpoles, and exposed them to Bd or not (see below). To test for short- and long-term effects of atrazine exposure on disease risk, the pathogen treatments occurred less than 18 h or a mean of 46 days after the end of the chemical exposure period. The latter Bd exposure time was post-metamorphosis. If a tank of tadpoles was designated to be exposed to the Bd treatment post-metamorphosis, then all the tadpoles from the tank were counted and transferred to an adjacent tank after the 6-day atrazine- or solvent-exposure period and then reared to metamorphosis. These adjacent tanks were established the same way as the ‘exposure’ tanks but were free of atrazine or solvent. Metamorphs were pulled from the tanks every other day and maintained in the laboratory (12 h light cycle, 23°C) on vitamin- and mineral-dusted crickets (fed ad libitum) until their use in the Bd-exposure portion of the experiment. In summary, there were 60 mesocosms total; 40 served as the primary tanks for the 2×2×2 portion of the experiment (atrazine or solvent × early or late in development×immediate or delayed Bd exposure), and 20 mesocosms had freshwater to house the delayed Bd exposure animals in the absence of atrazine or solvent.
(b). Bd exposure and quantification
Tadpoles were exposed to 2 ml of the Bd+ or Bd− (prepared identically to Bd+ but lacked Bd zoospores) inocula in 80 ml of artificial spring water (ASW) [43] for 2 days (see the electronic supplementary material, Methods for more details). They were then transferred along with the inoculum water to 1 l cups filled with fresh ASW and fed organic spinach ad libitum. Metamorphic frogs were exposed to the Bd by pipetting 2 ml of 3 × 104 zoospores ml−1 onto the frog's ventral side. Excess inoculum was collected in each frog's vented plastic container (350 ml), which contained 125 ml of dampened autoclaved soil collected from the USF campus. In general, five tadpoles or metamorphs were exposed to Bd from each tank, but these numbers varied depending on survival. Post-metamorphic frogs were fed five vitamin- and mineral-dusted crickets (5–15 mm) twice per week, and tadpoles were fed organic spinach ad libitum. During the Bd+ or Bd− exposure periods, tadpoles and metamorphs were maintained at 23°C on a 12 h light cycle and their survival was monitored daily for 14 days. At the end of the Bd+ or Bd− exposure periods, all surviving animals were euthanized with an overdose of MS-222 and individually preserved in 70% ethanol for later quantification of Bd. We used the quantitative PCR procedure described by Kriger et al. [44] to quantify Bd from tadpole mouthparts (dissected) and hindlimbs (one hindlimb was removed from tadpoles that had developed hindlimbs; see the electronic supplementary material, Methods).
(c). Statistical analyses
Statistical analyses were conducted using R statistical software [45], and all probability values were calculated using type II sums of squares. Measured atrazine concentration was analysed using a full-factorial model (‘lm’ function, base package) with developmental window and timing of Bd exposure as the crossed factors. Survival to metamorphosis (binomial error) and time to metamorphosis (normal error) were analysed using generalized linear mixed-effects models (‘lmer’ function, ‘nlme’ package) with individual frogs nested within each tank (a random factor), and testing for the main and interactive effects of atrazine treatment and developmental window. Survival of O. septentrionalis during the 6-day atrazine- or solvent-exposure period was analysed identically as survival to metamorphosis except that the analysis also included the factor timing of Bd treatment and associated interaction terms. We also used generalized linear mixed-effects models to analyse survival during the Bd-exposure period (a binomial response; ‘lmer’ function, ‘nlme’ package). Given that a portion of the amphibians from each mesocosm were or were not exposed to Bd, individual frogs were again nested within each tank (a random factor) to ensure proper replication within the analysis. In this model, we tested for all main effects (atrazine treatment, developmental window, timing of Bd treatment, Bd treatment) as well as two-, three- and four-way interactions.
We also evaluated whether any differential mortality associated with the treatments could be attributed to changes in resistance or tolerance to Bd. To evaluate the effects of treatments on resistance to Bd, we used generalized mixed-effects models with Bd load as the response variable (‘glmmADMB’ function, ‘glmmADMB’ package), individuals nested within tanks (a random effect), and a negative binomial error distribution. For tadpoles, Gosner stage, SVL, time since Bd exposure, atrazine, window and an atrazine-by-window interaction were the tested factors. For metamorphs, we tested all the same factors except that Gosner stage was excluded. Significance of factors in all generalized linear models was determined using log-likelihood ratio tests.
The fitness effects of parasitism can be broken down into two components: (i) the cost of parasite exposure, which is the difference in fitness between organisms that were not exposed to parasites and those that were exposed but not infected, and (ii) tolerance, the change in host fitness as a function of parasite burden [8]. Tolerance analyses seek to test whether any significant effect of treatments on a fitness proxy can be accounted for or nullified by pathogen abundance. Hence, after controlling for the vigour (survival in the absence of parasite exposure), tolerance analyses seek to evaluate whether two groups of hosts with identical parasite abundances differ in fitness [6–8]. Assuming no change in parasite virulence over the course of infection, the host with the greater fitness is more tolerant of the infection. In each of the two tolerance analyses described in this paragraph, we conducted analyses including and excluding animals that were exposed to Bd and died despite not getting infected, but results were similar regardless of alternative approaches (see the electronic supplementary material, Results). Excluding uninfected but exposed animals isolates the effects of treatments on tolerance, whereas including them considers the effect of treatments on the combination of tolerance and the cost of parasite exposure [8].
One criticism of traditional tolerance analyses is that researchers often cannot distinguish between host or parasite traits driving differences in fitness among hosts with similar parasite burdens [46]. This complication was avoided in our experiment, because Bd was never exposed to atrazine, only the host was exposed. Thus, any difference in Bd-induced mortality between atrazine- and solvent-exposed groups with similar Bd loads must be due to changes in hosts, not Bd. In other words, tolerance, a host trait, is the appropriate term here.
To test for the effects of treatments on tolerance, we repeated the binomial analyses described earlier for frog survival during the Bd-exposure period but only conducted the analysis on animals exposed to Bd and included log Bd load as a covariate. Additionally, we used the same predictors and dataset as above but conducted a censored Cox proportional hazards model to evaluate whether treatments affected how long frogs survived (see electronic supplementary material, Results). Finally, to evaluate the effects of treatments on cost of exposure, we compared the survival (binomial response) of frogs that were challenged with Bd but did not get infected versus frogs that were not challenged with Bd. If atrazine-exposed frogs that were challenged with Bd but did not get infected were still more likely to die than atrazine-exposed frogs that were not challenged with Bd (i.e. an atrazine-by-Bd interaction), then this would indicate that atrazine increased the costs of exposure to Bd.
3. Results and discussion
None of the factors we manipulated had significant effects on tadpole survival during the 6-day atrazine or solvent (i.e. control) exposure period (d.f. = 1; atrazine: χ2 = 0.139, p = 0.709; window: χ2 = 0.654, p = 0.419; timing of Bd exposure: χ2 = 2.018, p = 0.156; interactions: χ2 < 0.788, p > 0.374; electronic supplementary material, figure S2). Atrazine also had no significant effects on survival to metamorphosis (d.f. = 1; atrazine: χ2 = 0.171, p = 0.680; atrazine×window: χ2 = 0.003, p = 0.958; electronic supplementary material, figure S2). Additionally, in the absence of Bd exposure, neither atrazine (χ2 = 0.024, d.f. = 1, p = 0.876) nor any interactions of other factors with atrazine were significant predictors of mortality (χ2 < 0.460, d.f. = 1, p > 0.497; figure 1; electronic supplementary material, figures S2 and S3).
Figure 1.
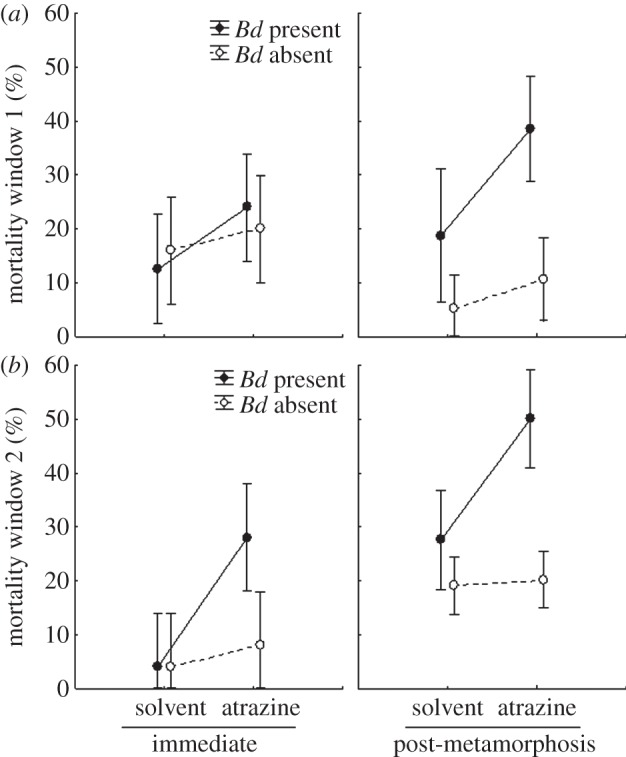
Effects of atrazine exposure on the survival of Cuban tree frogs (Osteopilus septentrionalis). Atrazine exposure occurred (a) early or (b) later in tadpole development (windows 1 and 2, respectively). To test for short-term and long-term effects of atrazine on disease resistance, exposure to inocula with or without the pathogen Batrachochytrium dendrobatidis (Bd) occurred either immediately after the atrazine exposure (immediate) or after frog metamorphosis (post-metamorphosis), which was a mean of 46-days after the last day of atrazine exposure. Shown are means ± 95% CIs (n = 5).
Although atrazine exposure early in life had no significant effects on survival in the absence of Bd, it significantly increased mortality in the presence of Bd (figure 1), resulting in a significant Bd-by-atrazine interaction (table 1). Indeed, risk of death was two times more likely for individuals exposed to atrazine and Bd (35.1% mortality) than those exposed to solvent and Bd (15.7% mortality; figure 1). Mortality in the solvent-treated animals during this two-week exposure period was only 11% (figure 1). There was no significant interaction term that included both atrazine and timing of Bd exposure (table 1), which indicates that the Bd-induced mortality was of a similar magnitude when frogs were exposed to Bd immediately after the 6-day exposure to atrazine or later in life (mean = 46 days; figure 1). Thus, there was no evidence of recovery from early-life exposure to atrazine. Similarly, in a previous study, there was no evidence of amphibian recovery to behavioural alterations induced by early-life exposure to atrazine [28]. Given the lack of recovery from atrazine, the long-term increase in amphibian susceptibility to Bd associated with early-life exposure to atrazine should outweigh any adverse direct effect of atrazine on the short-lived (approx. 24 h) Bd zoospores [40].
Table 1.
Statistical results from a generalized linear mixed-effects model examining the effects of atrazine exposure (presence or absence) at two times in Cuban tree frog tadpole development (early or late) on their survival (binomial response) in the presence or absence of the pathogen Batrachochytrium dendrobatidis (Bd). To test for short-term and long-term effects of atrazine on disease resistance, Bd exposure occurred immediately after the atrazine exposure or a mean of 46 days after the atrazine exposure, the latter of which was after metamorphosis. A subset of the tadpoles from a tank were exposed to Bd+ or Bd− inocula, and thus these individuals were nested within tank and treated as a random factor to ensure proper error structure.
| effect | d.f. | χ2 | p* |
|---|---|---|---|
| Bd (present or absent) | 1 | 12.50 | <0.001 |
| timing of Bd exposure (immediate or post-metamorphosis) | 1 | 5.91 | 0.015 |
| developmental window (early or late) | 1 | 0.10 | 0.758 |
| atrazine (present or absent) | 1 | 2.10 | 0.147 |
| Bd×timing of Bd | 1 | 1.39 | 0.238 |
| Bd×developmental window | 1 | 0 | 0.985 |
| Bd×atrazine | 1 | 4.90 | 0.027 |
| timing of Bd×developmental window | 1 | 3.38 | 0.066 |
| timing of Bd×atrazine | 1 | 1.05 | 0.305 |
| developmental window×atrazine | 1 | 0.75 | 0.386 |
| Bd×timing of Bd×developmental window | 1 | 1.49 | 0.222 |
| Bd×developmental window×atrazine | 1 | 0.22 | 0.637 |
| Bd×timing of Bd×atrazine | 1 | 0 | 0.983 |
| timing of Bd×developmental window×atrazine | 1 | 0.24 | 0.625 |
| four-way interaction | 1 | 0.08 | 0.772 |
*Log-likelihood ratio test based on type II sums of squares.
There was also no significant interaction term that included atrazine and developmental window (table 1). In other words, the increase in Bd-induced death associated with early-life exposure to atrazine was independent of when atrazine exposure occurred during development (figure 1); both exposure windows had similar negative outcomes with respect to death. However, we caution against concluding that amphibians lack developmental windows of sensitivity to atrazine, because an actual window of sensitivity could have spanned the end of the first window and the beginning of the second window, precluding detection, and because the entire larval stage could be a developmental window.
We explored several potential mechanisms for how atrazine increased Bd-induced death. For instance, frogs are known to be more sensitive to Bd both at smaller sizes and as they approach metamorphosis [19,20], and thus it is possible that the effects of atrazine on Bd-induced mortality were simply a function of atrazine affecting frog size and/or development. Indeed, death was significantly more likely when frogs were exposed to Bd after metamorphosis than as tadpoles (table 1 and figure 1) and atrazine exposure accelerated tadpole development when it occurred in window two but not window one (atrazine × window: Wilk's F2,95 = 3.318, p = 0.040; figure 2a). Consequently, many tadpoles exposed to atrazine in window two (but not window one) began to metamorphose during the Bd-exposure period (figure 1). Further, although atrazine- and solvent-exposed frogs were of similar size when exposed to Bd as tadpoles, when challenged with Bd as metamorphs, atrazine-exposed frogs were generally smaller than solvent-exposed frogs (atrazine×timing of Bd: χ2 = 4.521, d.f. = 1, p = 0.034; figure 2b; MANOVA on mass and SVL: χ2 = 3.427, d.f. = 1, p = 0.064). As predicted, tadpole developmental stage (coefficient ± s.e.: 0.193 ± 0.076; χ2 = 7.468, d.f. = 1, p = 0.006) and SVL (coefficient ± s.e.: −0.609 ± 0.225; χ2 = 8.077, d.f. = 1, p = 0.004) were indeed significant positive and negative predictors of death from Bd, respectively. These findings indicate that early-life exposure to atrazine reduced amphibian size and accelerated their development (window 2 only), which, in turn, increased their likelihood of dying from Bd exposure.
Figure 2.
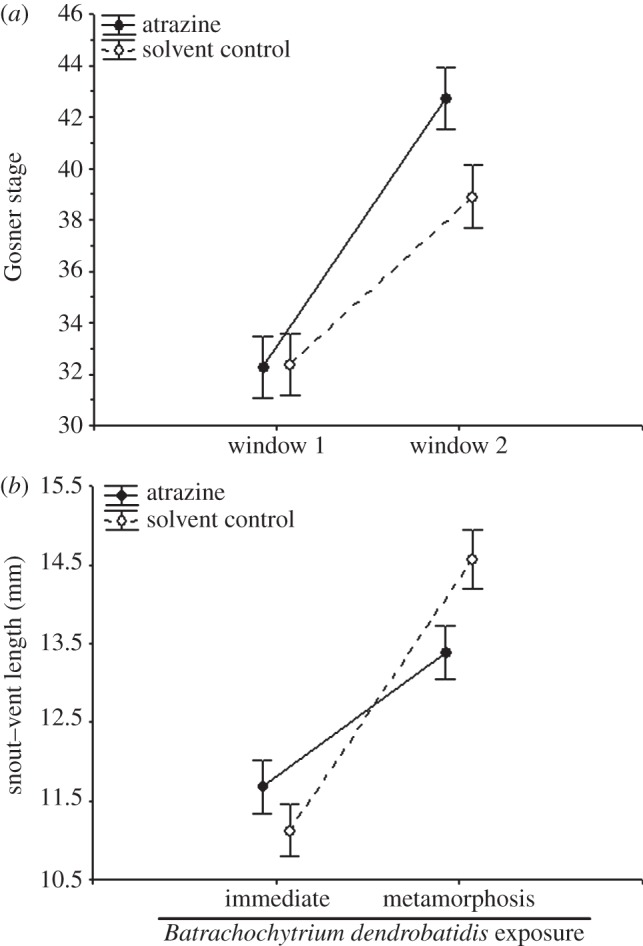
Effect of atrazine exposure on (a) the Gosner developmental stage (tadpoles only) and (b) snout–vent length of Osteopilus septentrionalis at the time of exposure to Batrachochytrium dendrobatidis (Bd). Both the atrazine-by-developmental window (a) and the atrazine-by-timing of Bd exposure (b) (immediately after the chemical treatment or 46 days later after metamorphosis) interactions are significant (p < 0.05). See Results for statistics. Shown are means ± 1 s.e. In (b), the means were calculated averaging across developmental windows.
Although atrazine effects on size and development explain some of the susceptibility to Bd, there might also be significant effects of atrazine on Bd-induced mortality independent of effects on size and development, perhaps through atrazine-mediated dysregulation of the endocrine and immune systems. To test this hypothesis, we evaluated whether atrazine still had significant effects on Bd-induced mortality after controlling for developmental stage and SVL (a measure of size). Atrazine remained a significant positive predictor of mortality risk when these covariates were added to the model (tadpoles: coefficient ± s.e.: 0.756 ± 0.814; χ2 = 4.15, d.f. = 1, p = 0.042; overall mortality: coefficient ± s.e.: 0.686 ± 0.935; χ2 = 7.23, d.f. = 1, p = 0.007). Thus, despite atrazine accelerating development that resulted in exposure to Bd at more susceptible developmental stages (i.e. near metamorphosis for tadpoles) and sizes [19,20], these effects alone were not adequate to explain the increase in Bd-induced mortality associated with atrazine, suggesting that atrazine also had persistent effects on components of host physiology that contributed to the observed Bd-induced mortality.
Changes to host resistance or tolerance are two physiological mechanisms by which atrazine could increase Bd-induced death [6–8]. Resistance, or the inverse of Bd load on the frogs, depended on whether Bd exposure occurred as tadpoles or after metamorphosis (χ2 = 72.68, d.f. = 1, p < 0.001), but it did not depend significantly on SVL (coefficient ± s.e.: 0.121 ± 0.094; χ2 = 1.64, d.f. = 1, p = 0.200), developmental window (mean log10 genome equivalents ± s.e.: window 1: 1.615 ± 0.246; window 2: 1.459±0.287; χ2 = 3.10, d.f. = 1, p = 0.078), time since Bd exposure (coefficient ± s.e.: 0.026 ± 0.056; χ2 = 0.20, d.f. = 1, p = 0.655), atrazine (control: 1.642 ± 0.271; atrazine: 1.432 ± 0.262; χ2 = 0.02, d.f. = 1, p = 0.888; figure 3a) or any interactions with atrazine (χ2 < 0.80, d.f. = 1, p > 0.370). Hence, despite atrazine-exposed frogs having significantly greater Bd-induced mortality than solvent-exposed frogs, there was no evidence that the atrazine-exposed frogs were less resistant to Bd. By contrast, tolerance differed significantly among the atrazine- and solvent-exposed frogs (figure 3b,c); at the same Bd load, atrazine-exposed frogs were more likely to die than solvent-exposed frogs (binomial model: χ2 = 6.95, d.f. = 1, p = 0.008; figure 3b,c). The effect of atrazine on tolerance of infections was independent of developmental window and exposure period (χ2 < 0.951, d.f. = 1, p > 0.329) and was not caused by frog size (SVL: χ2 = 0.116, d.f. = 1, p = 0.733). Much of the effect of atrazine on tolerance was driven by atrazine elevating the cost of exposure to Bd [8], because atrazine-exposed frogs that were challenged with Bd but did not get infected were still more likely to die than atrazine-exposed frogs that were not challenged with Bd (see the electronic supplementary material, Results and figure S4). This effect on cost of exposure could be caused by a recently discovered chemical released by Bd that can cause pathology in the absence of infection [47] or collateral damage caused by inflammation [6,7], as early-life exposure to atrazine was recently shown to cause hyperinflammation in trout [48].
Figure 3.
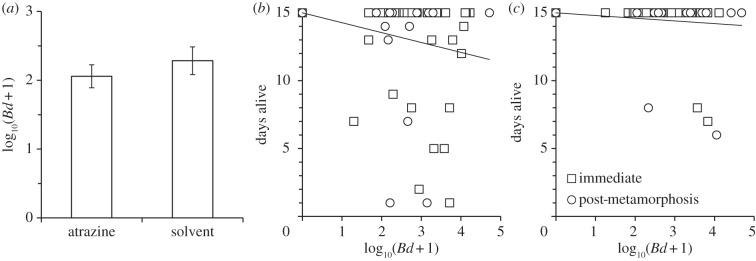
Effects of atrazine on the resistance and tolerance to Batrachochytrium dendrobatidis (Bd). Resistance (a) and tolerance (atrazine-exposed (b), solvent-exposed (c)) of Osteopilus septentrionalis frogs to Bd when challenged with Bd immediately after the atrazine exposure or a mean of 46 days later (post-metamorphosis). Animals that died before the end of the experiment, but had zero Bd load, were excluded to isolate the effects of tolerance from cost of exposure [8], though results were similar when these individuals were included in the analysis (electronic supplementary material, Results). Shown are means (±s.e.) in (a). Given that there was no atrazine-by-exposure period interaction for tolerance (p = 0.681), the load for the post-metamorphic frogs in (b,c) was scaled (multiplied by 1.7) to match that of the pre-metamorphic frogs to facilitate visualizing the overall effect of atrazine, and a single best-fit line is provided for the two exposure periods.
In summary, we showed that 6-day exposure to an environmentally realistic concentration of atrazine early in the life of an amphibian was associated with long-term increases in mortality from a chytrid fungus implicated in worldwide amphibian declines [11,12]. We found no evidence of recovery from this exposure, as the magnitude of the atrazine effect on Bd-induced mortality was similar whether frogs were challenged with Bd immediately after atrazine exposure or 46 days later. Hence, amphibians might need to be exposed to atrazine only briefly as larvae for atrazine to cause persistent increases in their risk of Bd-induced mortality, suggesting that the role of atrazine in amphibian declines warrants further, careful investigation. In addition, the 6-day exposure to atrazine was associated with reduced amphibian size that was only apparent later in life (see [30] for similar effect), an effect that can reduce fitness by diminishing reproductive output [49]. These findings are consistent with several studies demonstrating that atrazine can affect physiology, behaviour, growth, immunity and survival of mammals, fish and amphibians [21–30], and with a recent study demonstrating that atrazine exposure causes negative effects on genes involved in early immunity [48]. However, this study is unique because it demonstrates that early-life exposure to atrazine can have enduring negative effects on tolerance to infections.
Similar to our findings, short-term atrazine exposure reduced tadpole tolerance to trematode infections [26], but in other studies, atrazine altered resistance to infections [21,25]. Effects of early-life exposure to stressors are complex [50], and we suspect that the effects of atrazine on these two defence strategies are context-dependent. In conclusion, the findings presented here suggest that understanding and preventing exposure of susceptible populations to early-life conditions that chronically compromise the ability of individuals to cope with infections represents an important and underused approach to disease management that should greatly enhance both wildlife and human health.
Acknowledgements
We thank the extensive group of undergraduate researchers who helped with this work and the Rohr laboratory and two anonymous reviewers for comments. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Data used in this study are embargoed for 1 year for additional analyses and associated manuscripts, after which they will be made available on the website of the corresponding author. Data enquiries can also be made through an email request to the corresponding author.
Funding statement
This work was supported by grants from the US Department of Agriculture (NRI 2008-00622 20, 2008-01785, 2013-04712), US Environmental Protection Agency (STAR R83-3835, CAREER 83518801) and National Science Foundation (EF-1241889) to J.R.R.
References
- 1.Glaser R, Kiecolt-Glaser JK. 2005. Science and society: stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (doi:10.1038/nri1571) [DOI] [PubMed] [Google Scholar]
- 2.Matthews SG. 2002. Early programming of the hypothalamo-pituitary–adrenal axis. Trends Endocrinol. Metab. 13, 373–380 (doi:10.1016/s1043-2760(02)00690-2) [DOI] [PubMed] [Google Scholar]
- 3.Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. 2010. The effects of anthropogenic global changes on immune functions and disease resistance. Ann. N. Y. Acad. Sci. 1195, 129–148 [DOI] [PubMed] [Google Scholar]
- 4.Boekelheide K, et al. 2012. Predicting later-life outcomes of early-life exposures. Environ. Health Perspect. 120, 1353–1361 (doi:10.1289/ehp.1204934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budischak SA, Belden LK, Hopkins WA. 2008. Effects of malathion on embryonic development and latent susceptibility to trematode parasites in ranid tadpoles. Environ. Toxicol. Chem. 27, 2496–2500 (doi:10.1897/08-018.1) [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941 (doi:10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears BF, Rohr JR, Allen JE, Martin LB. 2011. The economy of inflammation: when is less more? Trends Parasitol. 27, 382–387 (doi:10.1016/j.pt.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 8.Rohr JR, Raffel TR, Hall CA. 2010. Developmental variation in resistance and tolerance in a multi-host-parasite system. Funct. Ecol. 24, 1110–1121 (doi:10.1111/j.1365-2435.2010.01709.x) [Google Scholar]
- 9.Raberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raffel TR, Hoverman JT, Halstead NT, Michel P, Rohr JR. 2010. Parasitism in a community context: trait-mediated interactions with competition and predation. Ecology 91, 1900–1907 (doi:10.1890/09-1697.1) [DOI] [PubMed] [Google Scholar]
- 11.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 (doi:10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 12.Wake DB, Vredenburg VT. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11 466–11 473 (doi:10.1073/pnas.0801921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. USA 107, 8269–8274 (doi:10.1073/pnas.0912883107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson C, Benard MF, Shaffer HB, Parker JM, O'Leary C, Conlon JM, Rollins-Smith LA. 2007. Effects of chytrid and carbaryl exposure on survival, growth, and skin peptide defenses in foothill yellow-legged frogs. Environ. Sci. Technol. 41, 1771–1776 (doi:10.1021/es0611947) [DOI] [PubMed] [Google Scholar]
- 15.Parris MJ, Baud DR. 2004. Interactive effects of a heavy metal and chytridiomycosis on Gray Treefrog larvae (Hyla chrysoscelis). Copeia 344–350 [Google Scholar]
- 16.Raffel TR, Halstead NT, McMahon T, Romansic JM, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151 (doi:10.1038/nclimate1659) [Google Scholar]
- 17.Gahl MK, Pauli BD, Houlahan JE. 2011. Effects of chytrid fungus and a glyphosate-based herbicide on survival and growth of wood frogs (Lithobates sylvaticus). Ecol. Appl. 21, 2521–2529 (doi:10.1890/10-2319.1) [DOI] [PubMed] [Google Scholar]
- 18.Kiely T, Donaldson D, Grube A. 2004. Pesticide industry sales and usage: 2000 and 2001 market estimates. Washington, DC: US Environmental Protection Agency [Google Scholar]
- 19.Carey C, Bruzgul JE, Livo LJ, Walling ML, Kuehl KA, Dixon BF, Pessier AP, Alford RA, Rogers KB. 2006. Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3, 5–21 (doi:10.1007/s10393-005-0006-4) [Google Scholar]
- 20.Garner TWJ, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA, Fisher MC. 2009. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118, 783–791 (doi:10.1111/j.1600-0706.2008.17202.x) [Google Scholar]
- 21.Rohr JR, McCoy KA. 2010. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Perspect. 18, 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes TB, et al. 2010. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl Acad. Sci. USA 107, 4612–4617 (doi:10.1073/pnas.0909519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes TB, et al. 2011. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 127, 64–73 (doi:10.1016/j.jsbmb.2011.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crain DA, Guillette LJ, Rooney AA, Pickford DB. 1997. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect. 105, 528–533 (doi:10.1289/ehp.97105528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohr JR, et al. 2008. Agrochemicals increase trematode infections in a declining amphibian species. Nature 455, 1235–1239 (doi:10.1038/nature07281) [DOI] [PubMed] [Google Scholar]
- 26.Koprivnikar J. 2010. Interactions of environmental stressors impact survival and development of parasitized larval amphibians. Ecol. Appl. 20, 2263–2272 (doi:10.1890/09-1558.1) [DOI] [PubMed] [Google Scholar]
- 27.Rohr JR, Sager T, Sesterhenn TM, Palmer BD. 2006. Exposure, postexposure, and density-mediated effects of atrazine on amphibians: breaking down net effects into their parts. Environ. Health Perspect. 114, 46–50 (doi:10.1289/ehp.8405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohr JR, Palmer BD. 2005. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ. Toxicol. Chem. 24, 1253–1258 (doi:10.1897/04-448R.1) [DOI] [PubMed] [Google Scholar]
- 29.Rowe AM, Brundage KM, Schafer R, Barnett JB. 2006. Age-dependent decrease in BALB/c mouse immune function following early life exposure to the herbicide atrazine. J. Immunol. 176, S165–S165 [Google Scholar]
- 30.Paetow LJ, McLaughlin JD, Cue RI, Pauli BD, Marcogliese DJ. 2012. Effects of herbicides and the chytrid fungus Batrachochytrium dendrobatidis on the health of post-metamorphic northern leopard frogs (Lithobates pipiens). Ecotoxicol. Environ. Saf. 80, 372–380 (doi:10.1016/j.ecoenv.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 31.Laws SC, et al. 2009. Chlorotriazine herbicides and aetabolites activate an ACTH-dependent release of corticosterone in male wistar rats. Toxicol. Sci. 112, 78–87 (doi:10.1093/toxsci/kfp190) [DOI] [PubMed] [Google Scholar]
- 32.Rayner JL, Enoch RR, Wolf DC, Fenton SE. 2007. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long–Evans rats. Toxicol. Appl. Pharmacol. 218, 238–248 (doi:10.1016/j.taap.2006.11.020) [DOI] [PubMed] [Google Scholar]
- 33.Rohr JR, Raffel TR, Sessions SK, Hudson PJ. 2008. Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 18, 1743–1753 (doi:10.1890/07-1429.1) [DOI] [PubMed] [Google Scholar]
- 34.Gosner N. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 35.Rohr JR, Palmer BD. 2013. Climate change, multiple stressors, and the decline of ectotherms. Conserv. Biol. 27, 741–751 (doi:10.1111/cobi.12086) [DOI] [PubMed] [Google Scholar]
- 36.Rohr JR, Sesterhenn TM, Stieha C. 2011. Will climate change reduce the effects of a pesticide on amphibians?: partitioning effects on exposure and susceptibility to pollution. Glob. Change Biol. 17, 657–666 (doi:10.1111/j.1365-2486.2010.02301.x) [Google Scholar]
- 37.Staley ZR, Rohr JR, Harwood VJ. 2010. The effect of agrochemicals on indicator bacteria densities in outdoor mesocosms. Environ. Microbiol. 12, 3150–3158 (doi:10.1111/j.1462-2920.2010.02287.x) [DOI] [PubMed] [Google Scholar]
- 38.Staley ZR, Rohr JR, Harwood VJ. 2011. Test of direct and indirect effects of agrochemicals on the survival of fecal indicator bacteria. Appl. Environ. Microbiol. 77, 8765–8774 (doi:10.1128/Aem.06044-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staley ZR, Senkbeil JK, Rohr JR, Harwood VJ. 2012. Lack of direct effects of agrochemicals on zoonotic pathogens and fecal indicator bacteria. Appl. Environ. Microbiol. 78, 8146–8150 (doi:10.1128/aem.01815-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon TA, Romansic JM, Rohr JR. 2013. Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environ. Sci. Technol. 47, 7958–7964 (doi:10.1021/es401725s) [DOI] [PubMed] [Google Scholar]
- 41.Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, Sager T, Sih A, Palmer BD. 2004. Multiple stressors and salamanders: effects of an herbicide, food limitation, and hydroperiod. Ecol. Appl. 14, 1028–1040 (doi:10.1890/03-5087) [Google Scholar]
- 42.Glennemeier KA, Denver RJ. 2002. Developmental changes in interrenal responsiveness in anuran amphibians. Integr. Comp. Biol. 42, 565–573 (doi:10.1093/icb/42.3.565) [DOI] [PubMed] [Google Scholar]
- 43.Cohen LM, Neimark H, Eveland LK. 1980. Schistomsoma mansoni: response of cercariae to a thermal gradient. J. Parasitol. 66, 362–364 (doi:10.2307/3280843) [PubMed] [Google Scholar]
- 44.Kriger KM, Hines HB, Hyatt AD, Boyle DG, Hero JM. 2006. Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Dis. Aquat. Org. 71, 141–148 (doi:10.3354/dao071141) [DOI] [PubMed] [Google Scholar]
- 45.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
- 46.Little TJ, Shuker DM, Colegrave N, Day T, Graham AL. 2010. The coevolution of virulence: tolerance in perspective. PLoS Pathog. 6 e1001006 (doi:10.1371/journal.ppat.1001006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon TA, Brannelly LA, Chatfield MWH, Johnson PTJ, Maxwell JB, McKenzie VJ, Richards-Zawacki CL, Venesky MD, Rohr JR. 2013. Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc. Natl Acad. Sci. USA 110, 210–215 (doi:10.1073/pnas.1200592110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shelley LK, Ross PS, Miller KM, Kaukinen KH, Kennedy CJ. 2012. Toxicity of atrazine and nonylphenol in juvenile rainbow trout (Oncorhynchus mykiss): effects on general health, disease susceptibility and gene expression. Aquat. Toxicol. 124, 217–226 (doi:10.1016/j.aquatox.2012.08.007) [DOI] [PubMed] [Google Scholar]
- 49.Reading CJ. 2007. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 151, 125–131 (doi:10.1007/s00442-006-0558-1) [DOI] [PubMed] [Google Scholar]
- 50.Guillette LJ, Iguchi T. 2012. Life in a contaminate world. Science 337, 1614–1615 (doi:10.1126/science.1226985) [DOI] [PubMed] [Google Scholar]


