Abstract
Concepts act as a cornerstone of human cognition. Humans and non-human primates learn conceptual relationships such as ‘same’, ‘different’, ‘larger than’, ‘better than’, among others. In all cases, the relationships have to be encoded by the brain independently of the physical nature of objects linked by the relation. Consequently, concepts are associated with high levels of cognitive sophistication and are not expected in an insect brain. Yet, various works have shown that the miniature brain of honeybees rapidly learns conceptual relationships involving visual stimuli. Concepts such as ‘same’, ‘different’, ‘above/below of’ or ‘left/right are well mastered by bees. We review here evidence about concept learning in honeybees and discuss both its potential adaptive advantage and its possible neural substrates. The results reviewed here challenge the traditional view attributing supremacy to larger brains when it comes to the elaboration of concepts and have wide implications for understanding how brains can form conceptual relations.
Keywords: concept learning, visual cognition, insect cognition, honeybee, Apis mellifera
1. Introduction
Concepts are considered as ‘the glue that holds our mental life together … in that they tie our past experiences together to our present interactions with the world’ [1, p. 1]. They act as a cornerstone of human cognition and underlie analogy, language or mathematical abilities, among others [2–6]. For instance, humans and non-human primates learn relational concepts such as ‘same’, ‘different’, ‘larger than’, ‘better than’, among others. In all cases, the relationships have to be encoded by the brain independently of the physical nature of the objects linked by the relation [2–6].
Relational concepts differ from categories as the latter rely on the presence of distinctive physical features of objects to be categorized, whereas the former rely on relationships between stimuli rather than on physical features of the stimuli [5]. Consequently, relational concepts are associated with high levels of cognitive sophistication and are not therefore, expected in an insect brain. Yet, works on honeybees have shown that their miniature brains can rapidly learn conceptual relationships between visual stimuli [7–10].
Here, we review evidence supporting the existence of relational-concept learning in bees and ask the following questions: (i) which are the neurobiological mechanisms underpinning this capacity? (ii) what do bees gain from this concept learning? Is it just an epiphenomenon inculcated by experimental procedures, or is it an adaptive trait, relevant in a visual and ecological context? (iii) what do we mean when we speak about ‘concept learning’ in bees? Is this linguistic label appropriate?
2. The honeybee Apis mellifera: a model for the study of higher-order forms of associative learning
Insects have historically fascinated biologists because they offer the possibility of studying sophisticated behaviours [11–14] and simultaneously accessing the neural bases of such behaviours [15–18]. Among insects, the honeybee has emerged as a powerful model for the study of associative learning [12,14,15,19–21]. In a natural context, bees learn and memorize the local cues characterizing the places of interest, which are essentially the hive and the food sources [22–25].
Several protocols have been developed to access honeybee learning and memory in controlled conditions. Here, we will focus on visual learning protocols in which free-flying, individually marked honeybees are trained to choose visual targets paired with sucrose solution as the equivalent of nectar reward [26–30] (figure 1). In these experiments, bees have to be trained and tested individually to achieve a precise control of their experience.
Figure 1.

An individually marked free-flying bee collecting sucrose solution on a concentric-disc pattern.
Using this protocol, it has been shown that bees are capable of higher-order forms of visual learning [12,14,21]: they categorize both artificial patterns [31–35] and pictures of natural scenes [36] (see reference [37] for a review), they navigate complex mazes [38,39], exhibit top-down modulation of their visual perception [40], or use spatial configuration to recognize complex pictures [31,41–43].
3. Conceptual learning in honeybees
Various reports have indicated that honeybees learn relational concepts such as ‘sameness/difference’ [9,10], ‘above/below’ [8] and the mastering of two rules simultaneously, ‘above/below’ (or left/right) and ‘different of’ [7].
(a). Sameness/difference concepts
The learning of the concepts of sameness and difference was demonstrated through the protocols of delayed matching to sample (DMTS) and delayed non-matching to sample (DNMTS), respectively [9]. In these protocols, an animal is presented a non-reinforced sample and has afterwards to choose among two or more stimuli, one of which corresponds to the sample previously shown. If trained in a DMTS, then the animal has to choose the stimulus matching the sample to obtain a reward, irrespective of the physical features of the stimulus shown; if trained in a DNMTS, then it has to choose the opposite to the sample to obtain the reward.
Honeybees were trained to enter a Y-maze to collect sucrose solution on one of the arms of the maze (figure 2a); the position of the reward changed randomly between the arms of the maze from visit to visit. In a first experiment, individually marked bees were trained following a DMTS protocol to determine whether they were capable of learning a concept of sameness. Bees were presented with a changing non-rewarded sample (i.e. one of two different colour discs—‘colour group’—or one of two different black-and-white gratings, vertical or horizontal—‘pattern group’) at the entrance of a maze (figure 2b). The bee was rewarded only if it chose the stimulus identical to the sample once within the maze. Bees trained with colours and presented in transfer tests with black-and-white gratings that they had not experienced before chose the grating identical to the sample at the entrance of the maze. Similarly, bees trained with the gratings and tested with colours in transfer tests chose the novel colour corresponding to that of the sample (figure 2c). Transfer was not limited to different types of visual stimuli (pattern versus colour), but occurred also between odours and colours [9]. Bees also mastered a DNMTS task, thus showing that they learn a rule of difference between stimuli as well [9].
Figure 2.
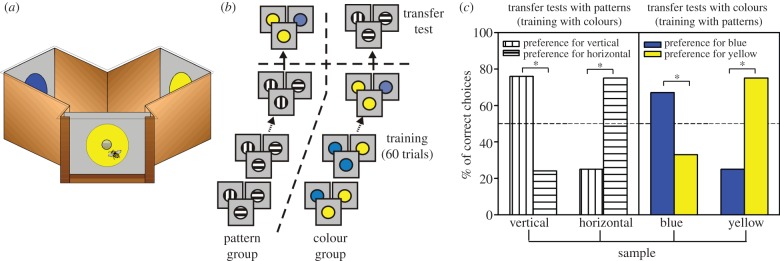
Sameness learning in honeybees [9]. (a) Y-maze used to train bees in a delayed matching-to-sample task. Bees entered into the maze to collect sugar solution on one of the back walls of the maze. A sample was shown at the entrance before bees accessed the arms of the maze. (b) Training protocol. A group of bees were trained during 60 trials with black-and-white, vertical and horizontal gratings (pattern group); another group was trained with colours, blue and yellow (colour group). After training, both groups were subjected to a transfer test with novel stimuli (patterns for bees trained with colours, colours for bees trained with patterns). (c) Performance of the pattern and the colour group in the transfer tests. Both groups chose the novel stimulus corresponding to the sample although they had no experience with such test stimuli.
These results were the first to document that bees learn rules relating stimuli in their environment. They were later verified in a study showing that the working memory underlying the solving of the DMTS task lasts for approximately 5 s [10], a period that coincides with the duration of other visual and olfactory short-term memories characterized in simpler forms of associative learning in bees [18]. DMTS was also used in categorization experiments aimed at studying the capacity of bees to group images according to broad classes such as ‘radial flower’, ‘plant stems’ or ‘landscapes’ [36] and in experiments on numerosity (reviewed in reference [44]).
(b). Above/below concepts
For many animals that operate in complex natural environments, spatial concepts such as right, left, above or below of are of crucial importance to generate appropriate relational displacements and orientation. The capacity to learn an above/below relationship between visual stimuli and to transfer it to novel stimuli that are perceptually different from those used during the training was studied in free-flying bees [8]. Bees were trained to fly into a Y-maze and choose visual stimuli presented above or below a horizontal bar. Training followed a differential conditioning procedure in which one spatial relation (e.g. ‘target above bar’) was associated with sucrose solution, whereas the other relation (e.g. ‘target below bar’) was associated with quinine solution. One group of bees was rewarded on the ‘target above bar’ relation, whereas another group was rewarded on the ‘target below bar’ relation. After completing the training, bees were subjected to a non-rewarded transfer test in which a novel target stimulus (not used during the training) was presented above or below the bar. Despite the novelty of the test situation, bees responded appropriately: if trained for the above relationship, then they chose the novel stimulus above the bar, and if trained for the below relationship, then they chose the novel stimulus below the bar [8].
Yet, because during the training all stimuli used appeared always in the same region of the visual field (e.g. upper field for bees trained to ‘above’ and lower field for bees trained to ‘below’), instead of learning a conceptual relationship, bees could have simply relied on the statistic distributions of images, which are different for the two problems. A series of snapshots acquired during training would determine an average stimulus which would be spatially distinct for the above and the below training. Furthermore, if bees relied on a simple cue like the centre of gravity of the patterns [45], which would be associated with reward, then the problem becomes elemental.
Both interpretations are ruled out by a second experiment in which instead of using a salient horizontal bar as referent, two discriminable stimuli positioned one above the other were used, so that one acted as the target and the other as the referent (figure 3a). The target varied from trial to trial in order to promote the extraction of an abstract above/below relationship [8]. As before, one group of bees was trained to select the ‘target above referent’ relationship (‘above group’) and another group the ‘target below referent’ relationship (‘below group’). Bees rapidly learned their respective relationship and when subjected to a first transfer test in which a novel stimulus was introduced as target, they preferred the trained spatial relationship (figure 3b). In a second transfer test, the referent was located in the middle of the background both for the rewarded and the non-rewarded stimulus, so that it could not help the bees choosing between them. In this case, bees still chose appropriately the stimulus pair presenting the spatial relationship for which they were trained (figure 3b), thus showing that the relative position of both target and referent was learned.
Figure 3.
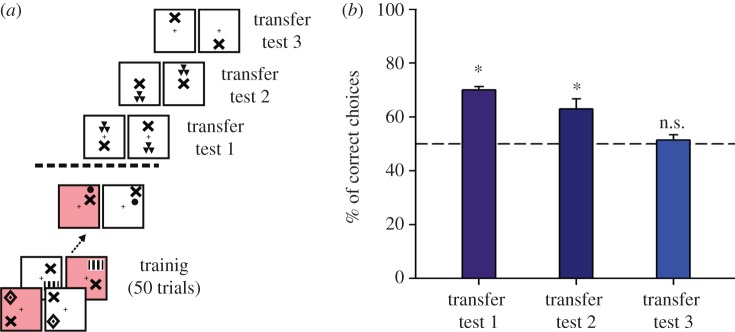
Above/below learning in honeybees [8]. (a) Training protocol. A group of bees were trained during 50 trials to fly into a Y-maze to choose black patterns on a white background. Patterns were a variable target disposed above/below a constant referent. Half of the bees were rewarded on the ‘target above referent’ relation whereas the other half was rewarded on the ‘target below referent’ relation. The referent was either the disc or the cross, depending on the group of bees trained. In the example shown, the referent is the cross and the relationship rewarded during training, indicated in pink, is ‘above’ (‘above the cross’). After training, three types of transfer tests were performed. (b) Performance in the transfer tests. ‘Correct choices’ indicate here choice of the previously rewarded relationship (‘above the cross’). Bees learned the concept of ‘above/below’ and transferred it to novel stimuli fulfilling the learned relationship (transfer test 1). Transfer tests 2 and 3 showed that neither the spatial location of the referent on the background nor the centre of gravity of stimuli was used as a discrimination cue to resolve the task.
Even more important was a third transfer test in which only the referent was presented in the upper or the lower part of the background to determine whether its absolute position was an orientation cue used by the bees, instead of its position relative to the target, which was now absent. Had the bees relied on the centre of gravity of stimuli or the statistic distribution of images, they should choose again correctly despite the absence of the target; if, however, the relationship between target and referent mediated the bees' choice, then performance should collapse. Choice in this test was random (figure 3b), thus showing that bees did indeed learn a spatial relationship between target and referent [8].
(c). Mastering two concepts simultaneously
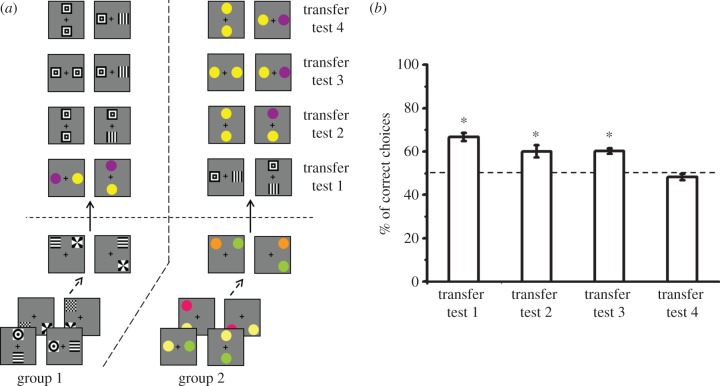
Processing of various different concepts was recently studied in experiments [7] in which bees were shown to rapidly master two concepts simultaneously, one based on spatial relationships (above/below and right/left), and another based on difference (figure 4).
Figure 4.
Simultaneous mastering of two concepts in honeybees [7]. Bees learned to use two concepts simultaneously: ‘above/below’ (or right/left) and ‘difference’. (a) Bees were trained with sucrose reward in a Y-maze to choose the stimulus presenting two different patterns (group 1) or two different coloured discs (group 2) in an above/below (or right/left) relationship depending on the group of bees. Appearances and relative position of the patterns varied from trial to trial. (b) After 30 training trials, four transfer tests were performed. In transfer test 1, ‘correct choices’ indicate choice of the previously rewarded spatial relationship; in transfer tests 2 and 3 and 4, the term indicates choice of the stimulus with 2 different images. In transfer test 1, bees transferred their choice to unknown stimuli presenting the appropriate spatial relationship despite belonging to a different modality. Transfer tests 2–4 demonstrated that bees also learned that the stimuli had to present two different images. Bees used both rules simultaneously.
Bees were trained to discriminate visual stimuli composed of two images linked by a specific spatial relationship (either above/below or right/left depending on bees). To obtain the sucrose reward, the bees had to choose the appropriate spatial relationship, irrespective of the images defining the relationship. Importantly, the two images composing a stimulus were always different (figure 4a). After training, subsequent tests showed that bees learned the rule based on the spatial relation, so that they transferred their choice to novel images never seen before if these subtended the appropriate relationship (figure 4). Moreover, they also extracted from the training the fact that the two images were different, so that they preferred the appropriate relationship defined by two different images to the same relationship defined by two identical images (figure 4). Notably, if the inappropriate relationship was presented, in one case defined by two different images and in the other by two identical images, bees preferred the stimulus with the two different images where, at least, the rule of difference was preserved. Finally, in a conflictive situation in which the bees had to choose between the appropriate spatial relationship defined by identical images and the inappropriate relationship defined by different images, the bees demonstrated no preference (figure 4). These three tests showed that bees were able to master simultaneously two different concepts: the spatial concept and the difference concept. They assigned them the same weight in their decision-making process so that their choice was guided by the presence of both or at least one of the concepts. As a consequence, performance collapsed in the conflictive situation.
As in the previous study [8], a series of internal within-subject controls and simulation algorithms allowed researchers to exclude confounding low-level cues such as the global centre of gravity, the global orientation of the stimuli, or the retinotopic similarity between the rewarded stimuli.
These results thus demonstrate that the miniature brains of bees can extract and use at least two different concepts simultaneously [7]. Interestingly, training was conceived to inculcate explicitly the spatial concept (e.g. above/below) but not the concept of difference, which was nevertheless perceived and extracted by bees. Monkeys have been trained to switch between two concepts, sameness and difference, presented in a random succession [46]. Concept alternation was possible, because, in each trial, a contextual cue indicated which concept had to be applied. Although this experiment has not yet been performed with bees, their capacity to use contextual information [48,49] and more than one concept at a time [7] suggests that concept alternation could be at the bees' reach.
4. Neurobiological insights into conceptual learning in bees
The experiments on primates reported above combined behavioural measurements in DMTS and DNMTS trials with electrophysiological recordings in the prefrontal cortex (PFC) [46,49]. PFC neurons were found which are concept-selective, i.e. which exhibit greater activity during sameness trials or during difference trials, regardless of which visual sample was used [46]. In the case of insects, with no PFC, search for neural structures supporting concept learning could focus on neural architectures sharing some parallels with the PFC, despite obvious differences in brain size and neuron number.
The PFC integrates distinct anatomical areas that are interconnected with a broad variety of cortical and subcortical brain areas involved in sensory, motor, emotional and reward processing [49]. PFC neurons are thus activated by diverse stimuli from virtually all sensory modalities, prior to and during the execution of motor responses, during memory for previously encountered stimuli, and in anticipation of expected events [50]. In addition, PFC neurons can convey information about internal factors such as motivation and attention [51].
In the honeybee, mushroom bodies (MBs) are higher-order associative brain structures allowing the combination of information pertaining to different sensory modalities (figure 5). Each bee MB consists of approximately 170 000 tightly packed, parallel neurons, the Kenyon cells. Multi-sensory input (olfactory, visual, mechanosensory, gustatory) is compartmentalized [52–54] (figure 5b,c), and many MB extrinsic neurons are multi-modal [55–57]. The multi-modal convergence existing at the level of the bee MBs supports integration of sensory information across various modalities and MB subcompartments, and suits the MBs for relational associations.
Figure 5.
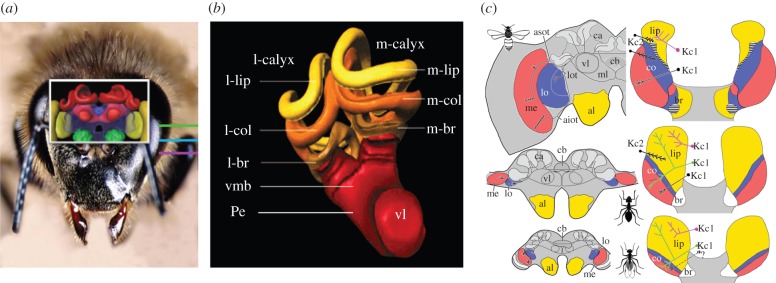
(a) Three-dimensional reconstruction of a honeybee brain. The mushroom bodies (MBs) are shown in red. (b) Three-dimensional reconstruction of a honeybee MB in frontal view (vmb) (kindly provided by R. Menzel, Free University of Berlin). Two calyces a lateral (l) and a medial one (m), fuse in a single peduncle (Pe). The somata of the Kenyon cells (KCs), which integrate the MB are located in the calyx bowl. The dendrites of the KC form the calyx which is subdivided in three main regions; the lip, the collar (col) and the basal ring (br). The axons of the KC subdivide and form the α or vertical lobe (vl), the β or medial lobe, and the γ layer (not shown). (c) Multi-modal representation in the MB calyces (kindly provided by Wulfila Gronenberg, University of Arizona). Top: the brain of a honeybee worker Apis mellifera; middle: the brain of a carpenter ant worker Camponotus floridanus; bottom: the brain of a male reproductive C. floridanus. Male ants do not forage and have MBs that are approximately half the size of those of a worker ant. The visual regions medulla (me) and lobula (lo) are shown in red and blue, respectively. The primary olfactory neuropiles, the antennal lobes (al), are shown in yellow; cb, central body; asot, anterior–superior optic tract; aiot, anterior–inferior optic tract; ca, calyces; ml, medial lobe. For each brain, a detail of the sensory afferences at the level of the calyces is shown using the yellow (antennal lobe), red (medulla) and blue (lobula) colour code. Kc1 are the ‘normal’ (‘spiny’) Kenyon cells that give rise to the vertical and medial lobes; Kc2 are the ‘clawed’ Kenyon cells that give rise to a γ layer in the MB lobes.
Like the primate PFC [58], MBs are also associated with memory, attention and reinforcement systems. MBs have been historically characterized as a substrate for associative memories (LTM) [59–61]. Yet, the inhibitory feedback loops on the MBs may also serve to inhibit responses to irrelevant information [62], thereby enhancing attention to relevant cues or relations [63,64]. Moreover, reinforcement signalling via aminergic neurons is also associated with MBs, not only in the bee [65,66] but also in other insects [67–72].
Does this mean that all insects endowed with MBs should be capable of conceptual learning? The case of the fruit fly suggests that the answer to this question may be negative. Fruit flies present unimodal (olfactory) MBs. They lack therefore MBs capable of integrating information across sensory modalities and allowing the extraction of sensory-independent regularities as those underlying concepts. Despite their tight association with elemental forms of memory [16,17,73–75], reinforcement systems [69,71,76] and attention-like processes [63,64], the fly MBs lack the multi-sensoriality that would be required to encode conceptual rules that are valid across distinct sensory modalities. Admittedly, concepts could be established within a single modality. Yet, demonstrating the existence of a given concept based on unimodal stimuli raises the problem of how to characterize the animal's performance in a transfer test with novel stimuli. Positive transfer could be explained in terms of pure stimulus generalization (based on physical features) rather than on relational learning, thus questioning the conceptual interpretation.
Multi-modal MBs can be found not only in honeybees but also in other Hymenoptera [77,78] such as wasps, ants and solitary bees (see figure 5c for bees and ants). In these insects, MBs receive multiple sensory inputs via parallel, layered channels. A large number of combinations of sensory submodalities can thus be processed and compared at the output level, depending on which combinations of ‘channels’ the dendrites of particular output neurons probe [78].
Are ants and wasps capable of conceptual problem-solving? The answer to this question is that we do not know, because experiments on concept learning are not known for other species of Hymenoptera. Yet, here we would like to argue that MB multi-modality is necessary but not enough to ensure conceptual problem-solving. Ecological and evolutionary factors may constitute important constraints for the development of this capacity.
5. An evolutionary and ecological scenario for conceptual learning in bees
First, multi-modal representation in the MBs may vary significantly among social insects depending on lifestyle so that in some cases a dominant sensory input may overshadow other sensory cues [78] (figure 5c). Second, further aspects could be required to extract conceptual relationships. In particular, the combination of navigation abilities [79] and the presence of a flexible visual pattern recognition system [14] may be important for conceptual learning. This suggestion does not imply that concept learning should be restricted to animals that ally both capacities, but rather that these abilities are cornerstones onto which concept learning might develop.
Both conditions are met by honeybees, which are central-place foragers (i.e. their foraging trips start and end at the same fixed point in space: the hive) and are endowed with flexible strategies allowing them to recognize and generalize visual patterns both at flowers and at the nest. Visual pattern recognition in bees goes beyond rigid retinotopic matching that precludes recognition when slight changes in orientation or angle of view are introduced. Bees and wasps extract relevant features of images and combine them in specific configural representations either for flower-like stimulus recognition, in the case of bees, or for interindividual recognition based on visual masks, in the case of some wasp species [31,33,80,81]. Bees can flexibly generalize their choice to visual images sharing such configurations despite drastic variations in other spatial details and positioning in the visual field [36]. These capacities may intervene in the extraction and recognition of relational concepts, where the focus of attention is the relationship binding visual features.
In their foraging bouts, bees use sky-based information as a navigation compass. Prominent landmarks and landscape information also define routes and support navigation [24,25,82,83]. In this context, mastering spatial relationships to build generic representations around the hive or the food source may be particularly useful. Extracting relationships such as ‘same’, ‘different’, ‘to the right (or the left) of or ‘above (below) of’ may help maintaining routes in a changing environment where variations in the aspect of landmarks may be otherwise disturbing.
Additionally, the condition of moving in a complex and structured environment, with numerous and variable landmarks, may be important. If this is the case, then concept learning would be limited or absent in central-place foragers that perform their foraging trips in unstructured environments devoid of landmarks (such as the Sahara desert in the case of Cataglyphis fortis ants; see reference [84] for review), where visual pattern recognition may not be intensely solicited and the possibility of relating landmarks through conceptual relationships would be rather reduced. For these insects, sky-compass-based information can provide the essential toolkit to navigate efficiently, even if landmarks may be used for navigation. Similarly, central-place foragers with reduced daily or seasonal foraging activities may not have evolved conceptualization capacities as their opportunities to extract spatial relationships between objects in their environment, even if structured, would be limited.
We posit then that both extrinsic factors, such as the ecological characteristics of the environment and intrinsic factors, such as the presence of flexible visual pattern recognition strategies, developed navigation abilities and intense foraging activities, may be determinant for the development of conceptual learning in insects.
6. What do we mean when we speak about concept learning in bees
We have seen that honeybees may behave as if they were guided by different kinds of concepts inculcated by specific training procedures, that such concepts admit a plausible ecological scenario and that neural structures exist in the bee brain that could support concept learning.
Yet, a fundamental question is whether the nature of concepts elaborated by bees is comparable to that of humans? This question is difficult to answer because of the problem of accessing the contents of an animal's mind. Although we may not be able to ascribe in a straightforward way a conceptual content to a bee's mind, we can nevertheless assume that there exists a content that can be correctly ascribed to describe efficiently the multiple discriminations reviewed in this chapter, in particular if, as discussed here, alternative, low-level explanations can be ruled out for these discriminations. Given that bees choose on the basis of relationships between stimuli, that such relations may bind variable objects whose physical nature becomes irrelevant during the problems to be solved, and that bees transfer their choice to novel situations never seen before if the learned relationships can be detected, a content revolving around simple discrimination learning has to be ruled out. Such discrimination learning would be stimulus specific and would preclude the kind of transfer observed in the experiments reviewed here, particularly in the very first experience with novel stimuli [85]. As this was not the case, it thus seems appropriate to assume that the proposition ‘bees may use concepts to solve discrimination problems’ is safe and tracks what bees do, irrespective of the nature of their conceptual knowledge.
This conclusion may create the idea that concepts may not represent a higher-order form of mental representation. This argument has some caveats: do we claim that concepts are lower-form representations just because bees possess them? In other words, if bees solve conceptual discriminations, does it have necessarily to be on the basis of ‘simple’ representations? It is sometimes assumed that ‘simple’ and ‘miniature’ nervous systems such as those of insects implement cognitive faculties by radically different mechanisms compared with vertebrates, rather relying on innate routines and elemental forms of associative learning. However, constructing a great division between simple and advanced nervous systems will lead us astray, because the basic logical structure of the processes underlying spontaneity, decision-making, planning and communication is similar in many respects in big and small brains [11,86].
7. Conclusion
Concept learning, described as a higher-order form of learning and considered as a cornerstone of human cognition [1,4,87], is a capacity that is now well documented in honeybees. Several forms of conceptual learning have been demonstrated so far in these insects and further studies could add new forms to the list of concepts that bees can master. The fact that only these insects have been shown to solve learning sets leading to concept formation does not make of honeybees a cognitive exception among insects. Other insect species sharing essential traits such as multi-modal MBs, flexible visual pattern recognition strategies and intense and frequent central-place navigation activities in structured environments, may perhaps be capable of comparable performances. This suggestion should be, therefore, viewed as a call for comparative research, especially around the useful dimensions of mushroom body organization and foraging ecology that may underlie concept learning. Negative results should be made available even if their dissemination is difficult as it is critical to know what animals cannot do as well as what they can.
The case of honeybees reveals that minimal neural architectures are capable of extracting the regularities underlying concept formation. In the absence of a PFC, structures that are more simple in terms of their number of neurons, but not necessarily in terms of their functional principles, can support conceptual knowledge in the bee brain.
The essential task is therefore to identify and characterize the circuits that mediate concept learning in the bee brain. The fact that the behaviour of tethered bees flying stationary can be now studied [88] opens new possibilities to reproduce conceptual learning under these conditions and to use MB blockade [89] to study the potential relation between these structures and this form of problem-solving. If the honeybee has reaffirmed its model status for behavioural studies on concept learning, then it can also play a significant role in unravelling the neural bases of this capacity.
Acknowledgements
We thank four anonymous reviewers for comments and M. Rowlands and J.M. Roy for inspiring discussions. Thanks are also due to R. Menzel and W. Gronenberg for kindly providing figure 5b and c, respectively.
Funding statement
This work was supported by the French National Research Agency (ANR), the Institut Universitaire de France, the French Research Council (CNRS), and the University Paul Sabatier.
References
- 1.Murphy GL. 2002. The big book of concepts. Cambridge, MA: MIT Press [Google Scholar]
- 2.Halford GS, Wilson WH, Phillips S. 2010. Relational knowledge: the foundation of higher cognition. Trends Cogn. Sci. 14, 497–505 (doi:10.1016/j.tics.2010.08.005) [DOI] [PubMed] [Google Scholar]
- 3.Doumas LAA, Hummel JE, Sandhofer CM. 2008. A theory of the discovery and predication of relational concepts. Psychol. Rev. 115, 1–43 (doi:10.1037/0033-295X.115.1.1) [DOI] [PubMed] [Google Scholar]
- 4.Lamberts K, Shanks D. 1997. Knowledge, concepts, and categories. Cambridge, MA: Psychology Press [Google Scholar]
- 5.Zentall TR, Galizio M, Critchfied TS. 2002. Categorization, concept learning, and behavior analysis: an introduction. J. Exp. Anal. Behav. 78, 237–248 (doi:10.1901/jeab.2002.78-237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zentall TR, Wasserman EA, Lazareva OF, Thompson RKR, Rattermann MJ. 2008. Concept learning in animals. Comp. Cogn. Behav. Rev. 3, 13–45 [Google Scholar]
- 7.Avarguès-Weber A, Dyer AG, Combe M, Giurfa M. 2012. Simultaneous mastering of two abstract concepts by the miniature brain of bees. Proc. Natl Acad. Sci. USA 109, 7481–7486 (doi:10.1073/pnas.1202576109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avarguès-Weber A, Dyer AG, Giurfa M. 2011. Conceptualization of above and below relationships by an insect. Proc. R. Soc. B 278, 898–905 (doi:10.1098/rspb.2010.1891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. 2001. The concepts of 'sameness’ and ‘difference’ in an insect. Nature 410, 930–933 (doi:10.1038/35073582) [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Bock F, Si A, Tautz J, Srinivasan MV. 2005. Visual working memory in decision making by honey bees. Proc. Natl Acad. Sci. USA 102, 5250–5255 (doi:10.1073/pnas.0501440102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008 (doi:10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan MV. 2010. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 55, 267–284 (doi:10.1146/annurev.ento.010908.164537) [DOI] [PubMed] [Google Scholar]
- 13.Giurfa M. 2013. Cognition with few neurons: higher-order learning in insects. Trends Neurosci. 36, 285–294 (doi:10.1016/j.tins.2012.12.011) [DOI] [PubMed] [Google Scholar]
- 14.Avarguès-Weber A, Deisig N, Giurfa M. 2011. Visual cognition in social insects. Annu. Rev. Entomol. 56, 423–443 (doi:10.1146/annurev-ento-120709-144855) [DOI] [PubMed] [Google Scholar]
- 15.Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193, 801–824 (doi:10.1007/s00359-007-0235-9) [DOI] [PubMed] [Google Scholar]
- 16.Busto GU, Cervantes-Sandoval I, Davis RL. 2010. Olfactory learning in Drosophila. Physiology 25, 338–346 (doi:10.1152/physiol.00026.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RL. 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28, 275–302 (doi:10.1146/annurev.neuro.28.061604.135651) [DOI] [PubMed] [Google Scholar]
- 18.Menzel R. 1999. Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323–340 (doi:10.1007/s003590050392) [Google Scholar]
- 19.Avarguès-Weber A, Mota T, Giurfa M. 2012. New vistas on honey bee vision. Apidologie 43, 244–268 (doi:10.1007/s13592-012-0124-2) [Google Scholar]
- 20.Menzel R, Giurfa M. 1999. Cognition by a mini brain. Nature 400, 718–719 (doi:10.1038/23371) [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Si A, Pahl M. 2012. Visually guided decision making in foraging honeybees. Front. Neurosci. 6, 88 (doi:10.3389/fnins.2012.00088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzel R. 1985. Learning in honey bees in an ecological and behavioral context. In Experimental behavioral ecology and sociobiology (eds Hölldobler B, Lindauer M.), pp. 55–74 Stuttgart, Germany: Gustav Fischer [Google Scholar]
- 23.Menzel R, Greggers U, Hammer M. 1993. Functional organization of appetitive learning and memory in a generalist pollinator, the honey bee. In Insect learning ecology and evolutionary perspectives (eds Papaj DR, Lewis AC.), pp. 79–125 New York, NY: Chapman & Hall [Google Scholar]
- 24.Collett TS, Zeil J. 1998. Places and landmarks: an arthropod perspective. In Spatial representation in animals (ed. Healy S.), pp. 18–53 Oxford, UK: Oxford University Press [Google Scholar]
- 25.Collett TS. 1996. Insect navigation en route to the goal: multiple strategies for the use of landmarks. J. Exp. Biol. 199, 227–235 (doi:10.1016/0022-0981(95)00202-2) [DOI] [PubMed] [Google Scholar]
- 26.Giurfa M, Menzel R. 1997. Insect visual perception: complex abilities of simple nervous systems. Curr. Opin. Neurobiol. 7, 505–513 (doi:10.1016/S0959-4388(97)80030-X) [DOI] [PubMed] [Google Scholar]
- 27.von Frisch K. 1914. Der Farbensinn und Formensinn der Biene. Zool. Jahrb Abt Allg Zool. Physiol. Tiere 37, 1–238 [Google Scholar]
- 28.Wehner R. 1981. Spatial vision in arthropods. In Handbook of sensory physiology VIc (ed. Autrum HJ.), pp. 287–616 Berlin, Germany: Springer [Google Scholar]
- 29.Lehrer M. 1997. Honeybees’ visual spatial orientation at the feeding site. In Detection and communication in arthropods (ed. Lehrer M.), pp. 115–144 Basel, Switzerland: Birkhäuser [Google Scholar]
- 30.Srinivasan MV. 1994. Pattern recognition in the honeybee: recent progress. J. Insect Physiol. 40, 183–194 (doi:10.1016/0022-1910(94)90041-8) [Google Scholar]
- 31.Avarguès-Weber A, Portelli G, Benard J, Dyer A, Giurfa M. 2010. Configural processing enables discrimination and categorization of face-like stimuli in honeybees. J. Exp. Biol. 213, 593–601 (doi:10.1242/jeb.039263) [DOI] [PubMed] [Google Scholar]
- 32.Giurfa M, Eichmann B, Menzel R. 1996. Symmetry perception in an insect. Nature 382, 458–461 (doi:10.1038/382458a0) [DOI] [PubMed] [Google Scholar]
- 33.Stach S, Benard J, Giurfa M. 2004. Local-feature assembling in visual pattern recognition and generalization in honeybees. Nature 429, 758–761 (doi:10.1038/nature02594) [DOI] [PubMed] [Google Scholar]
- 34.van Hateren JH, Srinivasan MV, Wait PB. 1990. Pattern recognition in bees: orientation discrimination. J. Comp. Physiol. A 167, 649–654 [Google Scholar]
- 35.Horridge GA, Zhang SW. 1995. Pattern vision in honeybees (Apis mellifera): flower-like patterns with no predominant orientation. J. Insect Physiol. 41, 681–688 (doi:10.1016/0022-1910(95)00021-L) [Google Scholar]
- 36.Zhang SW, Srinivasan MV, Zhu H, Wong J. 2004. Grouping of visual objects by honeybees. J. Exp. Biol. 207, 3289–3298 (doi:10.1242/jeb.01155) [DOI] [PubMed] [Google Scholar]
- 37.Benard J, Stach S, Giurfa M. 2006. Categorization of visual stimuli in the honeybee Apis mellifera. Anim. Cogn. 9, 257–270 (doi:10.1007/s10071-006-0032-9) [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Mizutani A, Srinivasan MV. 2000. Maze navigation by honeybees: learning path regularity. Learn. Mem. 7, 363–374 (doi:10.1101/lm.32900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang SW, Bartsch K, Srinivasan MV. 1996. Maze Learning by honeybees. Neurobiol. Learn. Mem. 66, 267–282 (doi:10.1006/nlme.1996.0069) [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Srinivasan M. 1994. Prior experience enhances pattern discrimination in insect vision. Nature 368, 330–332 (doi:10.1038/368330a0) [Google Scholar]
- 41.Dyer AG, Neumeyer C, Chittka L. 2005. Honeybee (Apis mellifera) vision can discriminate between and recognise images of human faces. J. Exp. Biol. 208, 4709–4714 (doi:10.1242/jeb.01929) [DOI] [PubMed] [Google Scholar]
- 42.Dyer AG, Rosa MGP, Reser DH. 2008. Honeybees can recognise images of complex natural scenes for use as potential landmarks. J. Exp. Biol. 211, 1180–1186 (doi:10.1242/jeb.016683) [DOI] [PubMed] [Google Scholar]
- 43.Dyer AG, Vuong QC. 2008. Insect brains use image interpolation mechanisms to recognise rotated objects. PLoS ONE 3, e4086 (doi:10.1371/journal.pone.0004086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahl M, Si A, Zhang S. 2013. Numerical cognition in bees and other insects. Front. Psychol. 4, 162 (doi:10.3389/fpsyg.2013.00162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernst R, Heisenberg M. 1999. The memory template in Drosophila pattern vision at the flight simulator. Vision Res. 39, 3920–3933 (doi:10.1016/S0042-6989(99)00114-5) [DOI] [PubMed] [Google Scholar]
- 46.Wallis JD, Anderson KC, Miller EK. 2001. Single neurons in prefrontal cortex encode abstract rules. Nature 411, 953–956 (doi:10.1038/35082081) [DOI] [PubMed] [Google Scholar]
- 47.Mota T, Giurfa M, Sandoz JC. 2011. Color modulates olfactory learning in honeybees by an occasion-setting mechanism. Learn. Mem. 18, 144–155 (doi:10.1101/lm.2073511) [DOI] [PubMed] [Google Scholar]
- 48.Collett TS, Fauria K, Dale K, Baron J. 1997. Places and patterns: a study of context learning in honeybees. J. Comp. Physiol. A 181, 343–353 (doi:10.1007/s003590050120) [Google Scholar]
- 49.Miller EK, Nieder A, Freedman DJ, Wallis JD. 2003. Neural correlates of categories and concepts. Curr. Opin. Neurobiol. 13, 198–203 (doi:10.1016/S0959-4388(03)00037-0) [DOI] [PubMed] [Google Scholar]
- 50.Freedman DJ, Miller EK. 2008. Neural mechanisms of visual categorization: insights from neurophysiology. Neurosci. Biobehav. Rev. 32, 311–329 (doi:10.1016/j.neubiorev.2007.07.011) [DOI] [PubMed] [Google Scholar]
- 51.Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (doi:10.1146/annurev.neuro.24.1.167) [DOI] [PubMed] [Google Scholar]
- 52.Mobbs PG. 1982. The brain of the honeybee Apis mellifera I. The connections and spatial organization of the mushroom bodies. Phil. Trans. R. Soc. Lond. B 298, 309–354 (doi:10.1098/rstb.1982.0086) [Google Scholar]
- 53.Ehmer B, Gronenberg W. 2002. Segregation of visual input to the mushroom bodies in the honeybee (Apis mellifera). J. Comp. Neurol. 451, 362–373 (doi:10.1002/cne.10355) [DOI] [PubMed] [Google Scholar]
- 54.Strausfeld NJ. 2002. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J. Comp. Neurol. 450, 4–33 (doi:10.1002/cne.10285) [DOI] [PubMed] [Google Scholar]
- 55.Rybak J, Menzel R. 1998. Integrative properties of the Pe1-neuron, a unique mushroom body output neuron. Learn. Mem. 5, 133–145 [PMC free article] [PubMed] [Google Scholar]
- 56.Grünewald B. 1999. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee Apis mellifera. J. Comp. Physiol. A 185, 565–576 (doi:10.1007/s003590050417) [Google Scholar]
- 57.Homberg U, Erber J. 1979. Response characteristics and identification of extrinsic mushroom body neurons of the bee. Z. Naturforsch 34, 612–615 [Google Scholar]
- 58.Barbey AK, Patterson R. 2011. Architecture of explanatory inference in the human prefrontal cortex. Front. Psychol. 2, 162 (doi:10.3389/fpsyg.2011.00162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menzel R, Erber J, Masuhr T. 1974. Learning and memory in the honeybee. In Experimental analysis of insect behaviour (ed. Barton-Browne L.), pp. 195–217 Berlin, Germany: Springer [Google Scholar]
- 60.Erber J, Masuhr T, Menzel R. 1980. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiol. Entomol. 5, 343–358 (doi:10.1111/j.1365-3032.1980.tb00244.x) [Google Scholar]
- 61.Menzel R, Müller U. 1996. Learning and memory in honeybees: from behavior to neural substrates. Annu. Rev. Neurosci. 19, 379–404 (doi:10.1146/annurev.ne.19.030196.002115) [DOI] [PubMed] [Google Scholar]
- 62.Farris SM. 2011. Are mushroom bodies cerebellum-like structures? Arth. Struct. Dev. 40, 368–379 (doi:10.1016/j.asd.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 63.Miller SM, Ngo TT, van Swinderen B. 2011. Attentional switching in humans and flies: rivalry in large and miniature brains. Front. Hum. Neurosci. 5, 188 (doi:10.3389/fnhum.2011.00188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Swinderen B, Andretic R. 2011. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc. R. Soc. B 278, 906–913 (doi:10.1098/rspb.2010.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammer M, Menzel R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156 [PMC free article] [PubMed] [Google Scholar]
- 66.Vergoz V, Roussel E, Sandoz JC, Giurfa M. 2007. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE 2, e288 (doi:10.1371/journal.pone.0000288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizunami M, Unoki S, Mori Y, Hirashima D, Hatano A, Matsumoto Y. 2009. Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol. 7, 46 (doi:10.1186/1741-7007-7-46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10 495–10 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, et al. 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512–516 (doi:10.1038/nature11304) [DOI] [PubMed] [Google Scholar]
- 70.Aso Y, et al. 2012. Three dopamine pathways induce aversive odor memories with different stability. PLOS Genet. 8, e1002768 (doi:10.1371/journal.pgen.1002768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke CJ, et al. 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492, 433–437 (doi:10.1038/nature11614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Unoki S, Matsumoto Y, Mizunami M. 2005. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 22, 1409–1416 (doi:10.1111/j.1460-9568.2005.04318.x) [DOI] [PubMed] [Google Scholar]
- 73.Waddell S, Quinn WG. 2001. What can we teach Drosophila? What can they teach us? Trends Genet. 17, 719–726 (doi:10.1016/S0168-9525(01)02526-4) [DOI] [PubMed] [Google Scholar]
- 74.Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275 (doi:10.1038/nrn1074) [DOI] [PubMed] [Google Scholar]
- 75.Keene AC, Waddell S. 2007. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8, 341–354 (doi:10.1038/nrn2098) [DOI] [PubMed] [Google Scholar]
- 76.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. 2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 (doi:10.1016/j.cub.2010.06.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strausfeld NJ. 2012. Arthropod brains: evolution, functional elegance, and historical significance, p. 848 Cambridge, MA: Belknap Press [Google Scholar]
- 78.Gronenberg W. 2001. Subdivisions of hymenopteran mushroom body calyces by their afferent supply. J. Comp. Neurol. 436, 474–489 (doi:10.1002/cne.1045) [DOI] [PubMed] [Google Scholar]
- 79.Chittka L, Jensen K. 2011. Animal cognition: concepts from apes to bees. Curr. Biol. 21, R116–R119 (doi:10.1016/j.cub.2010.12.045) [DOI] [PubMed] [Google Scholar]
- 80.Tibbetts EA. 2002. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B 269, 1423–1428 (doi:10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheehan MJ, Tibbetts EA. 2011. Specialized face learning is associated with individual recognition in paper wasps. Science 334, 1272–1275 (doi:10.1126/science.1211334) [DOI] [PubMed] [Google Scholar]
- 82.Chittka L, Geiger K. 1995. Can honeybees count landmarks? Anim. Behav. 49, 159–164 (doi:10.1016/0003-3472(95)80163-4) [Google Scholar]
- 83.Chittka L, Geiger K, Kunze J. 1995. The influences of landmarks on distance estimation of honey bees. Anim. Behav. 50, 23–31 (doi:10.1006/anbe.1995.0217) [Google Scholar]
- 84.Wehner R. 2003. Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588 (doi:10.1007/s00359-003-0431-1) [DOI] [PubMed] [Google Scholar]
- 85.Thomas RK, Noble LM. 1988. Visual and olfactory learning in rats: what evidence is necessary to show conceptual behavior? Anim. Learn. Behav. 16, 157–163 (doi:10.3758/BF03209059) [Google Scholar]
- 86.Menzel R, Giurfa M. 2001. Cognitive architecture of a mini-brain: the honeybee. Trends Cognit. Sci. 5, 62–71 (doi:10.1016/S1364-6613(00)01601-6) [DOI] [PubMed] [Google Scholar]
- 87.Murphy GL. 2010. What are categories and concepts? In The making of human concepts (eds Mareschal D, Quinn PC, Lea SEG.), pp. 11–28 New York, NY: Oxford University Press [Google Scholar]
- 88.Luu T, Cheung A, Ball D, Srinivasan MV. 2011. Honeybee flight: a novel ‘streamlining’ response. J. Exp. Biol. 214, 2215–2225 (doi:10.1242/jeb.050310) [DOI] [PubMed] [Google Scholar]
- 89.Devaud JM, Blunk A, Podufall J, Giurfa M, Grünewald B. 2007. Using local anaesthetics to block neuronal activity and map specific learning tasks to the mushroom bodies of an insect brain. Eur. J. Neurosci. 26, 3193–3206 (doi:10.1111/j.1460-9568.2007.05904.x) [DOI] [PubMed] [Google Scholar]