Abstract
Species with broader geographical ranges are expected to be ecological generalists, while species with higher heat tolerances may be relatively competitive at more extreme and increasing temperatures. Thus, both traits are expected to relate to increased survival during transport to new regions of the globe, and once there, establishment and spread. Here, we explore these expectations using datasets of latitudinal range breadth and heat tolerance in freshwater and marine invertebrates and fishes. After accounting for the latitude and hemisphere of each species’ native range, we find that species introduced to freshwater systems have broader geographical ranges in comparison to native species. Moreover, introduced species are more heat tolerant than related native species collected from the same habitats. We further test for differences in range breadth and heat tolerance in relation to invasion success by comparing species that have established geographically restricted versus extensive introduced distributions. We find that geographical range size is positively related to invasion success in freshwater species only. However, heat tolerance is implicated as a trait correlated to widespread occurrence of introduced populations in both freshwater and marine systems. Our results emphasize the importance of formal risk assessments before moving heat tolerant species to novel locations.
Keywords: macroecology, invasion risk assessment, biogeography, species traits, equatorward range boundary, thermal physiology
1. Introduction
The introduction of species by humans to geographical regions outside their native ranges is influencing ecosystems from the deep sea to the poles [1]. While some introduced species have had minimal or even positive impacts beyond their system of origin [2], others spread rapidly and have wide-ranging direct and indirect negative impacts [3]. Introduced species have been implicated in causing biodiversity loss [4], regime shifts [5] and extinctions [6], all of which can impact human resources and economic activity [7]. Furthermore, there is evidence that non-native species may fare better than native species in a warming climate [8]. This observation begs the question of whether aspects of heat tolerance, in particular, are related to invasion success.
Species with greater ecological generality and the capacity to tolerate more extreme abiotic conditions may be more likely to be transported, by virtue of their inhabiting broad, native geographical ranges. Moreover, these species may also have a greater probability of matching between their environmental tolerances and conditions in novel habitats, effectively increasing their capacity to survive transport, colonize and establish in new locations, and spread to inhabit broad, introduced distributions [9]. Indeed, recent experimental and meta-analytical studies of both marine and freshwater species indicate that introduced species are distinguished by broader native latitudinal ranges, as well as tolerance of environmental variability and extreme heat at both the whole organism and cellular levels [10–14]. At warmer environmental temperatures, the growth rate, recruitment success and survivorship of introduced species can also be higher, leading to a competitive advantage over native species [15–19]. Therefore, traits conferring successful navigation of the various stages of the invasion pathway may additionally allow introduced species to fare better in a warmer climate.
Here, we investigate whether native and introduced aquatic invertebrates and fishes can be distinguished by geographical range attributes and physiological tolerances [20]. We first test the expectation that for any given location, introduced species will have broader ranges and greater heat tolerances than co-occurring related (i.e. from the same taxonomic order) native species. If introduced species tend to originate nearer the equator than native species from the same locations, we would expect the heat tolerances of introduced species to be relatively higher than co-occurring natives [19]. Hence to advance our understanding of the mechanism behind any differences in heat tolerances between native and introduced species we account for the latitudinal position of each species’ geographical range (quantified as the source geographical range for introduced species) in our analyses.
Next, to better understand if geographical range breadth and heat tolerance play a role in successful invasion, we synthesize additional data on geographical range extent and heat tolerance of three groups: introduced species with either limited or extensive establishment and spread, and species not known to occur outside their native range. We test two sets of predictions with a global dataset. First, if broad native latitudinal ranges and high thermal tolerances are pre-requisites for successful transport, colonization and establishment in novel locations, then all introduced species able to colonize outside their native range, regardless of spreading success following initial establishment, will have broader latitudinal ranges and greater heat tolerances in comparison to native species. Second, if the establishment and spread of introduced species is facilitated by these traits, then those species achieving widespread occurrence following establishment will have broader source ranges and higher thermal tolerances than both native species and introduced species with limited non-native distributions. We provide support for a positive relationship between invasion success and wider source geographical range sizes in freshwater species. However, ability to establish and spread extensively is related to higher heat tolerances in both marine and freshwater species.
2. Material and methods
(a). Data collection and inclusion criteria
We gathered data from published reports (1927–2011) of thermal tolerance in ectothermic animals from aquatic environments. Data were obtained by literature searches (ISI Web of Knowledge and Google Scholar) with a combination of search terms ‘marine’ OR ‘estuarine’ OR ‘freshwater’ OR ‘aquatic’ AND ‘CTmax’ OR ‘upper temperature limit’ OR ‘heat tolerance’ OR ‘thermal tolerance’ OR ‘thermal limit’. We compiled taxonomic details and latitudinal range limits using additional online searches (citations are reported in datasets S1 and S2 and the majority of contributions are from FishBase [21] and the Global Invasive Species Database (http://www.issg.org/database)). The equatorward range limits of species whose ranges extend both north and south of the equator were set to zero, and their geographical range breadths were calculated for the hemisphere in which they occurred at the highest absolute latitude.
Those studies quantifying thermal tolerances of native and introduced species from the same habitats and with the same methods were included in the first analysis, distinguished as the ‘co-occurring’ dataset (see electronic supplementary material, dataset S1). This analysis allowed us to address the prediction that if ectothermic animals from any given shallow aquatic habitat are sampled, introduced species will have wider ranges and higher heat tolerances. This dataset comprises 15 published studies plus the experiments described herein (n = 16 studies).
In the second (‘global’) dataset, thermal tolerance data for 215 species of freshwater and marine fishes and invertebrates were compiled (see electronic supplementary material, dataset S2). The native status of species from the co-occurring dataset reflected if the species was native to the particular study location, and was changed for the global analysis if this species was introduced in another region of the globe. We first ensured that the reported occurrences of species in novel locations were before 2003. We then classified introduced species as having extensive or limited distributions based on the geographical extent of their occurrence in novel locations.
Species with extensive introduced ranges (n = 69) displayed high establishment and spreading potential: these species are distinguished by having established populations in five or more novel regions, typically on multiple continents. Asterias amurensis (northern Pacific sea star) and Charybdis japonica (paddle crab) have spread rapidly to span more than one degree of latitude in a new locality and are therefore included in the ‘extensive’ category. Species classified as having limited non-native ranges (n = 38) were restricted in geographical extent, such as to an island or bay, with limited potential for establishment and spread (such as has occurred for several bait fishes, e.g. Agosia chrysogaster). A cut-off of four or less established populations was selected to distinguish ‘limited’ non-native distributions.
(b). Thermal tolerance experiments
Heat tolerance data for aquatic species are more extensive in Northern Hemisphere species. To increase the representation of species from austral habitats, we assessed the thermal tolerances (critical or lethal limit) of six invasive species (Chiton glaucs, Physa actua, Sabella spallanzanii, Mytilus galloprovincialis, Crassostrea gigas and Asterias amurensis) and four native Australian marine invertebrates (Ischnochiton australis, Gyraulus cf. gilberti, Sabellastarte australiensis and Patiriella brevispina). Animals were hand-collected between April and July 2011 from three locations. Following collection, specimens were transported to the laboratory in a temperature-controlled container in water from the collection site (13–14°C). On arrival, each species was held (unfed) in a flow-through aquarium system maintained at 16°C for 12–48 h prior to experimentation. During experiments, 10–24 individuals of each species (as reported in electronic supplementary material, table S1) were placed in separate containers with fresh or salt water (as appropriate) and immersed in a temperature-controlled water bath (1°C ± 0.5 accuracy). Temperature was raised at a rate of 1°C per hour from 20°C up to the temperature at which all animals in the experimental trial had reached the behavioural endpoint identified in pilot experiments. At every temperature increment, responsiveness was assessed. Finally, after bringing the temperature down to 16°C, animals were then re-assessed for recovery. The mean temperature at which animals became unresponsive during rapid heating is reported in electronic supplementary material, dataset S1. Introduced species were manipulated under a permit issued by the Victorian State Government Department of Primary Industries (NP207).
(c). Statistical analyses
To quantify the relationships of geographical range extent and heat tolerance with invasion success, we conducted analyses separately for the co-occurring and global datasets (see electronic supplementary material, datasets S1 and S2). To do so, we fit explanatory models using linear modelling and maximum-likelihood techniques in R [22]. Prior to analyses we conducted collinearity diagnostics by calculating generalized variance inflation factors (GVIF) for fixed effects (described below) considered for inclusion in each global model. Fixed effects were excluded when GVIF values exceeded a value of two. The constancy of variance and normality of both the random and fixed effects was confirmed using visual inspection.
We ran six separate analyses where range attributes and heat tolerances were first compared between native and introduced species that co-occurred and, second, for a larger dataset that divided introduced species by the extent of their global introduced distributions (see electronic supplementary material, tables S2 and S3). On the basis of known factors likely to influence our response variables, we included habitat (freshwater, marine) and taxon (fish, invertebrate) as fixed effects. Additional covariates for analyses of range attributes included the latitude at which animals were collected (study latitude) and the mid-latitude of the native or, for introduced species, source geographical ranges (to account for possibility that introduced species may live closer to the equator and therefore be relatively heat tolerant). We also considered the interactions between origin and habitat, and between origin and study latitude.
When heat tolerance was the response variable, experiment-related factors that influence thermal tolerance estimates were included as fixed factors [19]: metric category (lethal: temperature at which mortality occurs; critical: temperature at which motor function is lost or ‘critical thermal maximum’), heating protocol (rapid: more than 1°C change per day; slow: less than 1°C change per day), life stage (juvenile, adult), pre-experimental acclimation temperature, absolute latitude of specimen collection and the interaction between thermal tolerance endpoint and protocol. Finally, Hemisphere (Northern, Southern) was included as an additional fixed factor to ensure that inclusion of our experimental data did not bias our findings.
Model selection consisted of assessing whether the inclusion of random effects (nested taxonomy: class within family within genus) and study (co-occurring analysis only) was justified by examining the contributed variance components for each. We excluded random effects that explained less than 1% of the overall variance. Including taxonomy controlled for variation in the response variable due to any similarities in geographical range size or heat tolerance that might be present owing to shared phylogenetic history, approximated by taxonomic grouping (see electronic supplementary material, table S2). A study identifier controlled for variation in heat tolerance owing to experimental protocols (see electronic supplementary material, table S2b).
Multimodel inference produced model-averaged parameter estimates and unconditional standard errors using AICc for all factors included in the full model (see electronic supplementary material, table S2). The 80% confidence model set (see electronic supplementary material, table S3) was calculated with the package ‘MuMIn’ [23] and the function model.avg.
3. Results
Latitudinal range breadth distinguishes introduced and native species in freshwater systems only. When sampled from the same locations, introduced freshwater species tend to have wider source latitudinal ranges in one hemisphere (by 13.5° of latitude) than co-occurring native species (figure 1a and electronic supplementary material, table S1a). In comparison, the range breadths of marine native and introduced species are similar (figure 1a). In contrast to range breadth, heat tolerance is related to the geographical extent of introduction for both freshwater and marine species. Introduced aquatic species are more heat tolerant (e.g. by 1.7°C for equatorial species assessed with a rapid heating protocol and critical thermal limits, electronic supplementary material, table S1b) than co-occurring related natives (figure 1b).
Figure 1.
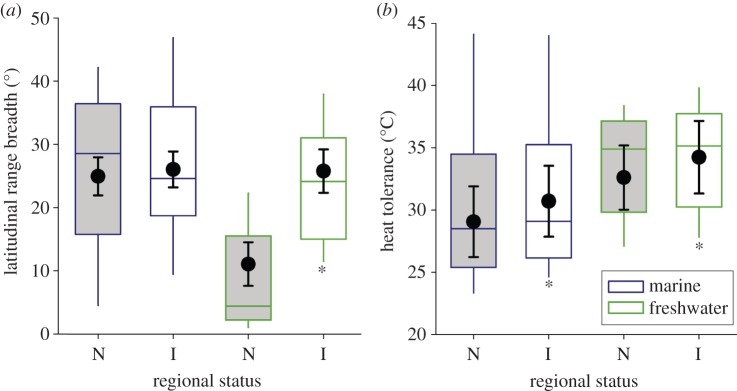
(a) Range breadth and (b) heat tolerance of native (N is the reference treatment, shaded grey) and introduced (I) invertebrates (n = 34 species in each habitat) and fish (n = 34 species) collected from the same locations in marine and freshwater habitats. Box plots display data from 16 studies in both hemispheres. Mixed model coefficients (black circles) and unconditional standard errors are averaged from the set of best models (see methods) where taxonomy (for range breadth) and study (for heat tolerance) were included as random effects. Asterisks indicate where the 95% CI in the difference between introduced versus the reference excluded zero after accounting for other fixed factors and covariates (model results are reported in electronic supplementary material, table S2a and b).
Redefining introduced status on the basis of global versus regional occurrence patterns for a larger dataset resulted in a similar latitudinal distribution of data for native and introduced species, although with greater representation at mid-latitudes (figure 2a). Both groups of freshwater introduced species have broader latitudinal ranges in comparison to native species, on average by, respectively, 5.6° latitude for those with limited distributions and 12.4° latitude for introduced species with more widespread non-native distributions (figure 2b), a difference that is statistically supported (see electronic supplementary material, table S2c). By contrast, the range breadths of marine native and introduced species overlap (figure 2b). While more widely distributed introduced species tend to occur 3.5° latitude closer to the equator than native species, the confidence interval for this difference crosses zero (figure 3a and electronic supplementary material, table S2e). In fact, the broader latitudinal ranges of introduced freshwater species relate primarily to the poleward location of the geographical range; freshwater species with widespread distributions occur an average of 11.7° latitude closer to a pole than native species (figure 3b and electronic supplementary material, table S2f). While the latitudinal position of equatorward range limits were similar in the Northern and Southern Hemispheres, we found that, on average, the poleward range limit of species from the Southern Hemisphere was 5.2° latitude closer to the equator than species from the Northern Hemisphere. This is presumably because land is limited at higher latitudes in the Southern Hemisphere, but suggests similar patterns in the two hemispheres.
Figure 2.
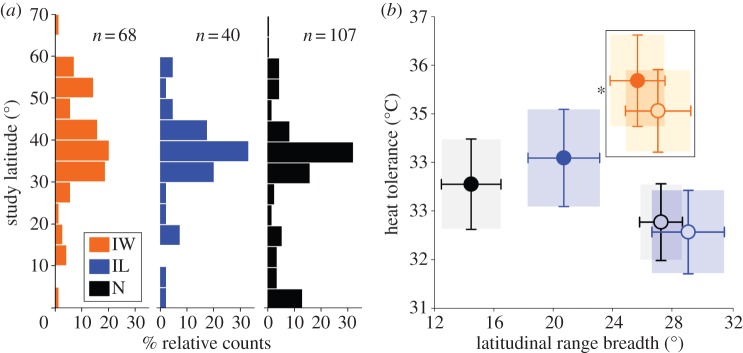
(a) Distribution of study latitude for species categorized as having widespread (IW) and limited (IL) introduced distributions, and native species (N). (b) Averaged mixed model predictions for heat tolerance versus range breadth in invertebrates and fish from marine (open circles) and freshwater habitats (filled circles) where the bars are the unconditional standard error, highlighted by shaded boxes (model summaries are in electronic supplementary material, table S2c and d). Range breadths of introduced freshwater species with widespread distributions are broader than natives (asterisks indicate a 95% CI in the difference between introduced and native species that exclude zero), while range breadths do not differ between native and introduced species in the ocean. The heat tolerance of widely occurring introduced species is higher than native species in both freshwater and marine habitats (black box, as supported by the 95% CI), while introduced species with limited distributions have similar heat tolerance to natives in both habitats. Predictions represent the majority of the data: 35°N latitude, and in the case of heat tolerance, an experimental protocol estimating critical limits using a rapid heating protocol at an acclimation temperature of 20°C.
Figure 3.
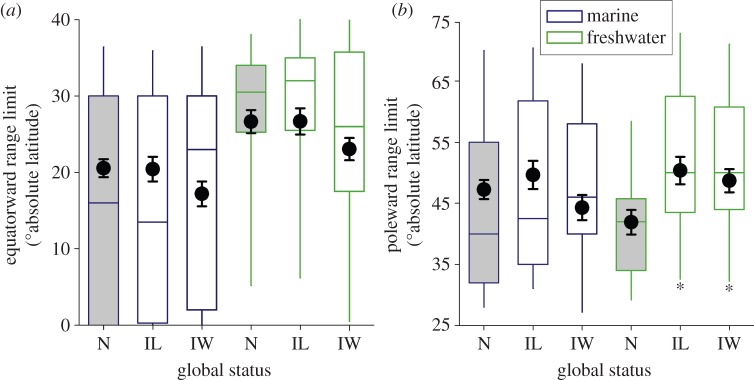
Absolute latitude of the (a) equatorward and (b) poleward range limits in native (N is the reference treatment, shaded grey) and species with limited (IL) and widespread (IW) introduced distributions from marine and freshwater habitats. Box plots display the distribution of data for 215 species (figure 2a). Mixed model coefficients (black circles) and unconditional standard errors are averaged from the set of best models where class, family and genus were included as nested random effects. Asterisks indicate where the 95% CI in the difference between introduced and native species groups excluded zero after accounting for other fixed factors and covariates (model results are reported in electronic supplementary material, tables S2e and f).
Introduced species with widespread introductions are significantly more heat tolerant than those with limited distributions, as well as native species, in both the Northern and Southern Hemispheres (e.g. by 2.2°C for equatorial species assessed with a rapid heating protocol and critical thermal limits, electronic supplementary material, table S2d). Thus, those introduced species achieving widespread distributions are generally distinguished by their heat tolerance, whereas introduced freshwater species are further differentiated by the latitudinal extent of their range, owing to greater poleward proximity (figure 3). The higher heat tolerances of widespread introduced species cannot therefore be fully explained by introduced species having geographical ranges which are located closer to the equator.
4. Discussion
Here, we find that geographical range attributes and heat tolerance in aquatic ectotherms differ between native and introduced species. While freshwater species with widespread occurrence are distinguished by their broad latitudinal source ranges, the capacity to tolerate heat is common to both freshwater and marine species that have extensive introduced distributions. Moreover, elevated heat tolerance in introduced species is not simply because these species originate from source geographical ranges that fall closer to the equator where the climate is warmer in comparison to native species. Thus, although we have not measured unsuccessful introductions, our findings are consistent with the hypothesis that physiology may underpin successful transport of species to new locations, and once there, their survival, establishment and spread. Our analysis therefore extends previously observed patterns to the global scale and illustrates important differences between marine and freshwater species in the traits correlated with successful introductions.
In freshwater systems, the latitudinal range breadths of introduced species are broader than native species. While species that with more extensive distributions may be more likely to be transported elsewhere [9], species with broader source geographical ranges are also expected to achieve this breadth owing to greater ecological generality. Biological traits such as wider diet breadth, habitat generality and greater dispersal potential [24] may confer a competitive advantage for those species introduced to a new range [25,26]. This may be particularly true for freshwater species, as native freshwater fishes and invertebrates are distinguished by having restricted latitudinal ranges in comparison to their introduced counterparts. However, native and introduced marine species tend to have similar geographical range breadths and latitudinal position. This finding suggests that geographical range attributes may be less important as a predictor for invasion success in the ocean, possibly because dispersal and habitat connectivity are greater in marine versus terrestrial and freshwater systems [27,28]. Habitat-related differences in the mechanisms driving widespread geographical introduction will be important to test in future studies.
In contrast to geographical range attributes—which differ between marine and freshwater species—heat tolerance is generally elevated in introduced aquatic species that have achieved widespread non-native distributions when compared with those with limited distributions. Our findings therefore implicate heat tolerance as a mechanism that could underlie successful introductions in aquatic systems. Importantly, we show that introduced species displaying extensive establishment and spread are relatively heat tolerant, whereas species that have colonized but failed to establish, have been eradicated, or those that display limited spreading following establishment have comparable heat tolerances to native species. This may be in part because widely introduced species also tend to occur 3.5° in latitude closer to the equator in comparison with other species, however, the confidence windows among native and introduced species overlap. Therefore, given that our analyses account for study latitude, there appears to be a strong role for species-specific heat tolerance as a mechanism for the success of introduced species that is not simply a by-product of occurring slightly closer to the equator in their source geographical range. While heat tolerance may confer a benefit during transport or colonization to a subset of introduced species, higher thermal limits appear to generally differentiate those species that become the most widespread, many of which have been introduced to multiple continents. Thus, species that have been widely introduced may provide the opportunity to consider how regional climate differences and factors such as climate change velocity [28] relate to spreading rates, and thus to identify possible mechanisms underpinning successful introduction.
The mechanisms conferring heat tolerance range from cellular adaptations to organismal behaviours [29] and may differ among introduced species. An open question is whether species with broad introduced ranges tend to be those with a particular set of heat tolerance mechanisms. Regardless of mechanism, higher heat tolerance may enable the occupation of fringe habitats [11], resulting in reduced competition. For instance, introduced infaunal invertebrates in riverine ecosystems occur in relatively warm microhabitats [30]. Performance-related processes such as growth and reproduction may also enhance the performance of introduced species in a warmer climate [10,16,17], suggesting that the competitive advantages for heat tolerance species may be multi-faceted under climate change.
In searching for thermal tolerance information from a wide range of species, we found that experimental data are relatively more common from temperate latitudes (figure 3a). A recent meta-analysis of climate-related performance in native and introduced species also found a majority of studies were conducted in temperate mid-latitude locations [14], where mean environmental temperatures may be less extreme than in tropical regions [19]. Inclusion of heat tolerance data from equatorial latitudes, presently lacking possibly owing to spatial bias in research effort and publication, may reveal that the difference between native and introduced species declines in tropical systems where species live, on average, closer to their upper thermal limit [19,31].
Information on juvenile life stages comprises another gap in thermal tolerance data; although we included life stage as a fixed effect in our model, the majority of data concerned adult stages. Testing for invasion-related heat tolerance in juveniles is a further direction that might indicate how relative heat tolerance at different life-history stages influences how species success various at different stages of the invasion pathway. Moreover, cold tolerance data are less available than data on heat tolerance. Yet because the poleward spread of species will presumably be limited by winter extremes, it is important to examine whether cold tolerance confers advantages to introduced species, as well as overall thermal niche breadth [32]. For instance, some tropical marine invertebrates possess low acute cold tolerance and may therefore be capable of spreading to higher latitudes [33,34]. Moreover, because introduced freshwater species with widespread non-native occurrence tended to have source distributions that were 11.7° of latitude closer to a pole in comparison to native species, these species are also likely to possess greater cold tolerance and capacity to cope with seasonality [19]. We therefore suggest that the study of invasion dynamics at species’ equatorward and poleward latitudes, at different life stages and at lower temperatures, should be prioritized.
Our results indicate that heat tolerance is an important physiological trait which managers can use to predict the potential of arriving species or new colonists to establish viable populations and spread, such as by following standardized experimental protocols to directly compare the physiological tolerances of introduced and native species. Risk assessments that include metrics of relative heat tolerance may consequently offer an important indicator of invasion risk, including under climate warming. Additionally, because range breadth tends to be greater in species with greater dispersal capacity [26], limiting dispersal pathways in introduced freshwater species is a key management strategy [35].
Here, we provide strong global support that heat tolerance is directly related to the geographical extent of introduction in aquatic ectothermic animals. Our findings corroborate previous studies investigating the potential for introduced species to spread in a warmer climate [14,17,18]. We further provide the novel understanding that heat tolerance could be a primary mechanism facilitating successful introductions, rather than being indirectly related to geographical range characteristics. Heat tolerance may be especially important in determining the impacts of extreme high temperature events predicted to increase in frequency and severity over the next decade, which can significantly impact community structure [36]. Further research in the field of conservation physiology to link experimental heat tolerance metrics with real-world animal responses to environmental variability are also important [37]. Moreover, the physiological and demographic responses of species to environmental variability depend upon the velocity and variability of temperature change [28] in concert with changes in abiotic and biotic factors, such as resource availability [12]. As the distributional and performance responses of species are idiosyncratic among ecosystems [14], approaches to identify traits that promote colonization, establishment and spread may need to be habitat-specific to provide general predictive capacity of invasion extent and success.
Acknowledgements
L. McGrath and R. Watson from the Victorian Marine Science Consortium, and P. Elliott from the Woodridge Marine Discovery Centre, assisted with animal collection and hosted the experiments. We thank A. Bellgrove, S. Guggenheimer, L. Laurenson, C. Magilton, T. Mathews, S. McKelvie, J. McKelvie, J. McIntire, S. Mill, D. Mills, S. Rowe and J. Wills for support and assistance to C.M.M. during completion of the initial literature review and experiments.
Data accessibility
The supporting data for this article are included in the electronic supplementary material.
References
- 1.Steneck RS, Carlton JT. 2001. Human alterations of marine communities: students beware. In Marine community ecology (eds Bertness MD, Gaines SD, Hay ME.), pp. 445–468 Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Rodriguez L. 2006. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol. Invas. 8, 927–939 (doi:10.1007/s10530-005-5103-3) [Google Scholar]
- 3.Parker IM, et al. 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biol. Invas. 1, 3–19 (doi:10.1023/A:1010034312781) [Google Scholar]
- 4.Molnar JL, Gamboa RL, Revenga C, Spalding MD. 2008. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 6, 485–492 (doi:10.1890/070064) [Google Scholar]
- 5.Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. 2004. Regime shifts, resilience, and biodiversity in ecosystems management. Annu. Rev. Ecol. Syst. 35, 557–581 (doi:10.1146/annurev.ecolsys.35.021103.105711) [Google Scholar]
- 6.Clavero M, Garcia-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends. Ecol. Evol. 20, 110 (doi:10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 7.Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G. 2002. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive. Proc. R. Soc. Lond. B 269, 2407–2413 (doi:10.1098/rspb.2002.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends. Ecol. Evol. 14, 135–139 (doi:10.1016/S0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- 9.Theoharides KA, Dukes JS. 2007. Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol. 176, 256–273 (doi:10.1111/j.1469-8137.2007.02207.x) [DOI] [PubMed] [Google Scholar]
- 10.Zerebecki RA, Sorte CJB. 2011. Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS ONE 6, e14806 (doi:10.1371/journal.pone.0014806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenz M, et al. 2011. Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: results from a globally replicated study. Environ. Res. 111, 943–952 (doi:10.1016/j.envres.2011.05.001) [DOI] [PubMed] [Google Scholar]
- 12.Knapp S, Kühn I. 2012. Origin matters: widely distributed native and non-native species benefit from different functional traits. Ecol. Lett. 15, 696–703 (doi:10.1111/j.1461-0248.2012.01787.x) [DOI] [PubMed] [Google Scholar]
- 13.Goodwin BJ, McAllister AJ, Fahrig L. 1999. Predicting invasiveness of plant species based on biological information. Conserv. Biol. 13, 422–426 (doi:10.1046/j.1523-1739.1999.013002422.x) [Google Scholar]
- 14.Sorte CJB, et al. 2012. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 16, 261–270 (doi:10.1111/ele.12017) [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Haack RA, Zhang R. 2011. Does global warming increase establishment rates of invasive alien species? A centurial time series analysis. PLoS ONE 6, e24733 (doi:10.1371/journal.pone.0024733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. 2002. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl. Acad. Sci. USA 99, 15 497–15 500 (doi:10.1073/pnas.242437499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorte CJB, Williams SL, Zerebecki RA. 2010. Ocean warming increases threat of invasive species in a marine fouling community. Ecology 91, 2198–2204 (doi:10.1890/10-0238.1) [DOI] [PubMed] [Google Scholar]
- 18.Walther G-R, et al. 2009. Alien species in a warmer world: risks and opportunities. Trends. Ecol. Evol. 24, 686–693 (doi:10.1016/j.tree.2009.06.008) [DOI] [PubMed] [Google Scholar]
- 19.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 (doi:10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolar CS, Lodge DM. 2001. Progress in invasion biology: predicting invaders. Trends. Ecol. Evol. 16, 199–204 (doi:10.1016/S0169-5347(01)02101-2) [DOI] [PubMed] [Google Scholar]
- 21.Froese R, Pauly D. (ed.) 2000. FishBase 2000: concepts, design and data sources. Los Baños: ICLARM Contribution 1594, ICLARM [Google Scholar]
- 22.R Development CT 2013. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 23.Bartoń K. 2009. MuMIn: Multi-model inference. R package version 0122. See http://CRAN.R-project.org/package=MuMIn. Vienna, Austria: R Foundation for Statistical Computing
- 24.Kinlan BP, Gaines SD. 2003. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020 (doi:10.1890/01-0622) [Google Scholar]
- 25.Feeley KJ, Silman MR. 2010. Land-use and climate change effects on population size and extinction risk of Andean plants. Global Change Biol. 16, 3215–3222 (doi:10.1111/j.1365-2486.2010.02197.x) [Google Scholar]
- 26.Lester SE, Ruttenberg BI, Gaines SD, Kinlan BP. 2007. The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758 (doi:10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]
- 27.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology, global consequences and control. Ecol. Appl. 10, 689–710 (doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [Google Scholar]
- 28.Burrows MT, et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (doi:10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]
- 29.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 30.Verbrugge L, Schipper A, Huijbregts M, Van der Velde G, Leuven R. 2011. Sensitivity of native and non-native mollusc species to changing river water temperature and salinity. Biol. Invasions 14, 1187–1199 (doi:10.1007/s10530-011-0148-y) [Google Scholar]
- 31.Nguyen KDT, Morley SA, Lai C-H, Clark MS, Tan KS, Bates AE, Peck LS. 2011. Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS ONE 6, e29340 (doi:10.1371/journal.pone.0029340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bykova O, Sage RF. 2012. Winter cold tolerance and the geographic range separation of Bromus tectorum and Bromus rubens, two severe invasive species in North America. Global Change Biol. 18, 3654–3663 (doi:10.1111/gcb.12003) [Google Scholar]
- 33.Davenport J, Wong TM. 1992. Effects of temperature and aerial exposure on three tropical oyster species, Crassostrea belcheri, Crassostrea iradelei and Saccostrea cucullata. J. Therm. Biol. 17, 135–139 (doi:10.1016/0306-4565(92)90023-9) [Google Scholar]
- 34.Lai CH, Morley SA, Tan KS, Peck LS. 2011. Thermal niche separation in two sympatric tropical intertidal Laternula (Bivalvia: Anomalodesmata). J. Exp. Mar. Biol. Ecol. 405, 68–72 (doi:10.1016/j.jembe.2011.05.014) [Google Scholar]
- 35.Johnson LE, Ricciardi A, Carlton JT. 2001. Overland dispersal of aquatic invasive species: a risk assessment of transient recreational boating. Ecol. Appl. 11, 1789–1799 (doi:10.1890/1051-0761(2001)011[1789:ODOAIS]2.0.CO;2) [Google Scholar]
- 36.Smale D, Wernberg T. 2013. Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B 280, 20122829 (doi:10.1098/rspb.2012.2829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Fernández D, Aragón P, Bilton DT, Lobo JM. 2012. Assessing the congruence of thermal niche estimations derived from distribution and physiological data. A test using diving beetles. PLoS ONE 7, e48163 (doi:10.1371/journal.pone.0048163) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supporting data for this article are included in the electronic supplementary material.


