Abstract
Disentangling the relative roles of males, females and their interactive effects on competitive fertilization success remains a challenge in sperm competition. In this study, we apply a novel experimental framework to an ideally suited externally fertilizing model system in order to delineate these roles. We focus on the chinook salmon, Oncorhynchus tshawytscha, a species in which ovarian fluid (OF) has been implicated as a potential arbiter of cryptic female choice for genetically compatible mates. We evaluated this predicted sexually selected function of OF using a series of factorial competitive fertilization trials. Our design involved a series of 10 factorial crosses, each involving two ‘focal’ rival males whose sperm competed against those from a single ‘standardized’ (non-focal) rival for a genetically uniform set of eggs in the presence of OF from two focal females. This design enabled us to attribute variation in competitive fertilization success among focal males, females (OF) and their interacting effects, while controlling for variation attributable to differences in the sperm competitive ability of rival males, and male-by-female genotypic interactions. Using this experimental framework, we found that variation in sperm competitiveness could be attributed exclusively to differences in the sperm competitive ability of focal males, a conclusion supported by subsequent analyses revealing that variation in sperm swimming velocity predicts paternity success. Together, these findings provide evidence that variation in paternity success can be attributed to intrinsic differences in the sperm competitive ability of rival males, and reveal that sperm swimming velocity is a key target of sexual selection.
Keywords: genetic compatibility, interacting phenotypes, polyandry, multiple mating, cryptic female choice
1. Introduction
Sperm often have to compete for fertilization with ejaculates from rival males [1]. Consequently, sperm competition has been credited with the rapid evolution and spectacular diversity of ejaculate traits observed across numerous taxa [2,3], thus dispelling the notion that sperm cells are shaped solely by natural selection to function as ‘DNA-delivery machines’ [4]. Accompanying the evidence for sexual selection on ejaculates is corresponding evidence that selection can favour female reproductive traits that serve to bias fertilizations towards either ‘preferred’ [5] or genetically compatible mates [6–8]. Sperm competition can therefore be underpinned by effects attributable to both sexes, including relative differences in the sperm competitive ability of rival males [9–11], consistent biases in fertilization rates that are mediated by female effects [12], and complex interactions involving the genotypes of competing males and females [13–15]. Consequently, disentangling these processes, and in particular the relative importance of males, females and their interacting effects in sperm competition, poses a significant empirical challenge [16,17].
Externally fertilizing taxa offer highly versatile and experimentally powerful models for partitioning sources of additive and non-additive variation in fitness traits. In these systems, the use of factorial sperm competitive assays makes it possible to attribute variation in competitive fertilization success among males, females and their interacting effects, while controlling for stochasticity in sperm competitiveness owing to the random sampling of (non-focal) rival competitors [17,18]. In this paper, we apply these methods for the first time in the context of sperm competition using the chinook salmon, Oncorhynchus tshawytscha, a fish exhibiting external fertilization in which males and females mate multiply [19]. Previous work on O. tshawytscha and other salmonid fishes has revealed that ovarian fluid (OF), which forms an extracellular matrix surrounding externally spawned eggs, upregulates sperm motility [20] and differentially mediates the swimming velocities of sperm from conspecific males. Specifically, sperm tested in the presence of OF from certain females perform better (swim faster) than when tested with others, and such patterns can be non-transitive across different male–female pairings [21–24]. As relative differences in sperm swimming velocity have been shown to be associated with sperm competitiveness in salmonid fishes [25], OF has been implicated as a potential arbiter of cryptic female choice in these taxa [23]. Thus, any attempt to attribute variation in sperm competitiveness to either intrinsic male effects and/or male-by-female (and male-by-male) genotypic interactions must also consider the potential role that OF plays in mediating sperm competition [8].
Our study exploits and modifies the experimental framework proposed for cross-classified designs by Garcia-Gonzalez & Evans [17]. This framework is especially amenable to externally fertilizing taxa where it is possible to partition sources of variation in sperm competition among male and female effects, while accounting for stochasticity attributable to variation in the sperm competitive ability of non-focal rivals [18]. In applying this design, we incorporate the important modification of manipulating the identity of OF donors while maintaining a standard (genetic) maternal background (eggs) against which rival sperm compete for fertilizations (figure 1). In this way, the success of individual focal sperm competitors can be attributed to male effects (e.g. attributable to intrinsic differences in the sperm competitive ability of focal males), female effects (attributable to differences in OF) and male-by-female interaction (compatibility mediated exclusively by the differential effects of OF on sperm competitive ability).
Figure 1.
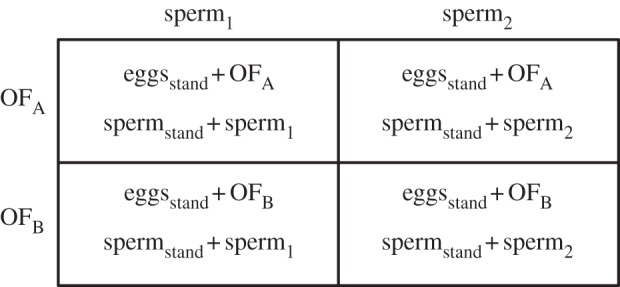
A single block of the cross-classified design to test effects of ovarian fluid (OF) on competitive fertilization success. The genetic identity of females within each block is standardized by using eggs from a single female (eggsstand) across all four crosses (note that eggsstand are bathed in OF from two different females). A different standard female was used in each block of the design. The competitive fertilization ability of sperm from two focal males (sperm1 and sperm2) was tested against sperm from a single standardized rival male (spermstand), so that sperm competitiveness across different female OF backgrounds was not influenced by stochastic effects attributable to differences among non-focal rival males. Again, different focal and standard males were used in each block. Each competitive fertilization trial was performed in replicate, thus generating eight in vitro fertilization trials for each block. Paternity success from each cross was estimated using microsatellite DNA markers (see main text).
2. Material and methods
(a). Study species and maintenance
Chinook salmon were caught in a trap located on the Kaiapoi River, which forms part of the Waimakariri River system, Canterbury, New Zealand. The Waimakariri River is one of the main rivers inhabited by chinook salmon in New Zealand [26]. We used sexually mature (2- to 3-year-old) salmon captured during their natural spawning season (April–May). Once caught, all individually marked fish were measured for body size (fork length = length from the tip of snout to the end of the middle caudal fin rays) and maintained in a hatchery raceway using standard husbandry procedures at Salmon Smolt NZ, Canterbury, New Zealand. Briefly, fish were maintained in the hatchery raceway's natural river (12.5–13°C) water, which was circulated throughout the hatchery. Fish were caught over a two-week period prior to the commencement of the study, which then took 20 days to complete. During the artificial spawning trials (see below), a small fin clip was taken from each fish and stored in 95% ethanol for DNA extraction. All animals were collected and maintained according to the standards of the Animal Ethics Committee for the University of Otago, New Zealand (permit no. AEC/13/10).
(b). Experimental overview
We used a factorial design to test whether OF mediates sexual selection for compatible mates, and to separate these effects from variation in the intrinsic fertilizing capabilities of focal males (figure 1). Beforehand, however, we confirmed that male-by-female OF–sperm velocity interactions, reported previously in O. tshawytscha [23], were detectable in the experimental stock used for this study, and under the fertilization conditions imposed in our subsequent competitive fertilization assays. For these preliminary trials, we tested sperm velocity from six males crossed with OF from six females in all (n = 36) combinations.
In our subsequent experiment, we conducted a series (n = 10) of 2×2 factorial crosses, each comprising an ejaculate from one of two focal males (sperm1 or sperm2), an ejaculate from a single and unique standard rival male against which sperm from each of the focal males competed (spermstand), OF from one of two focal females (OFA or OFB) and eggs from a single standard female (eggsstand). Thus, in each of these experimental ‘blocks’, ejaculates from each of two focal males competed against the sperm from a single standard male for fertilization of a standard egg batch in the presence of OF from each of two distinct focal females (figure 1). We performed two replicate trials within each block (i.e. each in vitro fertilization trial was performed twice), and used unique combinations of males and females among the 10 blocks. This design enabled us to attribute variance in paternity success to intrinsic differences between focal males, variation in OF and their potentially interacting effects (see §2g). We also conducted in vitro computer-assisted sperm analyses (CASA) for all males used in these trials, and estimated pairwise genetic relatedness for focal sperm competitors and standard females, in order to generate potential predictors of male reproductive success.
(c). Test for ovarian fluid–sperm interactions
We initially conducted sperm velocity assays to test for the interacting effects of OF and male identity in generating variation in sperm velocity. This was necessary because in this study, OF from each focal female had to be partitioned across four fertilization trials within each block of the design (see below), requiring us to use OF at a lower concentration (OF : river H2O = 1 : 10 = 10% OF) in the competitive fertilization assays (see below) to that used previously (50% OF) to demonstrate OF–sperm interaction in O. tshawytscha [23]. In other respects, our methods for this preliminary experiment closely follow those described previously for O. tshawytscha [23]. Briefly, we conducted a factorial experiment involving sperm from six males and OF solutions from six females (OF diluted to 10% with raceway water). Sperm velocity estimates for all n = 36 unique combinations of sperm and OF were performed in replicate. The sperm velocity assays followed the methods described below for the competitive fertilization trials (§2f). As these trials revealed significant OF–sperm interactions (see Results and discussion), we proceeded to test for interacting and main effects of males and OF using a 10% OF solution in our subsequent competitive fertilization trials.
(d). Competitive fertilization trials
In each block, ejaculates from the two focal males and standard rival male were stripped manually, diluted in phosphate-buffered saline and counted to determine sperm density using an improved Neubauer haemocytometer. Sperm samples from the three males could then be standardized, so that we could extract equal numbers of sperm from each male prior to fertilizations (each sample contained 1×108 sperm). Although we ensured that sperm numbers were equalized between rival males, we acknowledge that sperm volumes would have systematically varied between individuals, thus potentially influencing competitive fertilization success (e.g. through the effects of seminal fluid). We were careful to ensure that milt samples used in subsequent trials were not contaminated by urine or water (which can activate sperm) during stripping; sperm samples were therefore stored ‘dry’ (i.e. non-activated) until required for fertilizations (samples stored at 4°C and used within 5 h of collection) and sperm velocity assays (see §2f).
To obtain OF from the two focal females in each block, females were killed, so that eggs and OF could be expelled from the body cavity and sieved to separate OF from the eggs. The same process was used to extract eggs from the standard female in each block, but, in this case, eggs were retained and OF was eventually discarded (see below). Extreme care was taken to ensure that no eggs were damaged during this process. Within each block, the eggs from the standard female (suspended in their natural OF) and the OF samples from the two focal females were stored at 4°C until required (within 5 h of collection). Just prior to the in vitro fertilization experiments, the standard eggs were drained of their natural OF and subsequently washed through a sieve with artificial OF solution (NaCl2 155 mM, KCl 3.1 mM, MgSO4 · 7H2O 1.3 mM, CaCl2 · 2H2O 3.4 mM, Tris base 20 mM, Tris–HCl 20 mM, pH 8.5) to remove traces of the original OF. Eggs from the standard female were then drained of artificial OF and split into four equal portions (approx. 100 eggs each), each placed into plastic beakers with 25 ml of OF (100% concentration) from one of the two focal females (i.e. either OFA or OFB; figure 1). Meanwhile, sperm from each focal male was mixed with the sperm from the standard male (in a plastic tube using a micropipette to gently mix the samples) to generate eight mixed sperm pools for the subsequent in vitro fertilization assays (i.e. 4 × [sperm1 + spermstand]; 4 × [sperm2 + spermstand]). Following a 5 min egg–OF incubation stage, the mixed sperm solutions (along with 225 ml natural freshwater obtained from the salmons’ raceway) were added to each egg pool according to the design depicted in figure 1. Thus, at fertilization OF concentrations were maintained at 10%. All fertilizations were performed twice, generating eight competitive IVF trials in each block. This procedure was repeated across all n = 10 blocks. Fertilized eggs were then placed on trays in heath racks and left undisturbed for 28 days in circulating hatchery water.
In separate in vitro fertilization trials, we conducted non-competitive fertilization assays using the two focal males and standard male in each block, and the eggs from the standard female. For each trial, we counted the number of developing embryos and eggs that were not fertilized. These trials confirmed that all individuals were reproductively fertile under non-competitive conditions (mean percentage fertilized±s.e. = 55±9%; range 10–98%, n = 18 replicated fertilization trials, on average 78–128 eggs counted per fertilization trial). Therefore, any observed biases in fertilization success in the competitive in vitro fertilization trials could not be attributed to infertility.
(e). Molecular analysis
To determine offspring paternity, we undertook microsatellite genotyping of unhatched embryos (approx. 48 embryos per family, taken at the ‘eyed’ stage, 28 days post-fertilization; total genotyped n = 1937). DNA was extracted from preserved tissue samples using a standard Chelex procedure [27]. For each competitive fertilization trial, the two putative sires, standard sire, standard dam and a haphazard sample of 48 progeny were genetically typed using a multiplex of nine highly polymorphic microsatellite markers (Ocl-1 [28], Omy-325, Ots-101, Ots-104, Ots-107, Ots-2 [29], Ots-3 [30], Ssa-197, Ssa-85 [31]). Paternity assignment was made via a maximum-likelihood approach using Cervus (v. 3.0) [32]. To estimate genetic relatedness between both the focal and standard sperm competitors and the standard female used in each block, we calculated the level of pairwise relatedness (R) using the Queller & Goodnight [33] index of relatedness. We used the Coancestry program [34] to calculate this relatedness index based on the similarity of alleles at the above nine microsatellite loci.
(f). Sperm assays
We used the same focal and standard males included in the factorial design described above to assess sperm swimming velocity using CASA software (v. 12, CEROS, Hamilton–Thorne Biosciences, Beverly, MA). Sperm assays were performed in freshwater to provide baseline velocity estimates for each male (assays are therefore comparable with other studies relating variation in sperm velocity to paternity success in salmonids [25]). Sperm were activated on 20 μ Leja slides (Leja Products B.V., Nieuw-Vennep, the Netherlands) on a temperature-controlled stage cooler (TS-4 Thermal Microscope Stage, Physitemp, USA) set to 12.5°C to match the temperature of the holding raceway at the hatchery. Activated sperm were video-recorded using a video camera (XC-ST50, Sony, Tokyo, Japan) mounted on an external phase contrast microscope (CX41, Olympus, Melville, NY) with a 10× negative phase objective. Sperm were recorded at 10 s post-activation. Sperm velocity parameters were measured twice for each milt sample; these measures included: (i) average path velocity (VAP, µm s−1), which estimates the average velocity of sperm cells over a smoothed cell path; (ii) straight line velocity (VSL, µm s−1), the average velocity on a straight line between the start and the endpoint of the track; and (iii) curvilinear velocity (VCL, µm s−1), the actual velocity along the sperm's trajectory. The threshold values for defining static cells were predetermined at 15 µm s−1 for VAP and VSL. Sperm velocity measures were based on an average of 159.3 ± 11.0 s.e. sperm tracks per sample. Across all samples tested in this study, VAP was strongly positively correlated with other velocity estimates (e.g. VAP–VCL, Pearson correlation r = 0.98, n = 59, p < 0.0001). We therefore focused on just VAP as an estimate of sperm swimming velocity (results remain unchanged irrespective of the measure). Within-sample repeatability (using linear mixed-effects methods [35]) for each male's two successive VAP measures was high (R = 0.95 ± 0.015 s.e., 95% CIs = 0.92–0.97). We used the mean of the two VAP values for each sperm sample in the subsequent analysis.
(g). Statistical analyses
Statistical analyses were performed using R v. 2.15.3 [36] within the linear mixed-effects package lme4 [37]. We carried out three separate analyses, the first of which tested for the interactive effects of OF and the identity of sperm donors on sperm velocity when OF were diluted to 10% (see §2c). In this initial model, VAP was entered as a normally distributed response variable, whereas the explanatory variables of male ID, OF ID and their interaction were coded as random effects. Likelihood-ratio tests were used to assess the significance of explanatory variables [38], where models with and without each random effect were compared using the log likelihood-ratio statistic (LLR), which is −2× the difference in log-likelihoods between the full and reduced models and approximately distributed as chi-squared with 1 d.f.
Our second mixed-effects model incorporated a binomial error distribution to assess the importance of focal males, OF and their interacting effects in predicting paternity success. In this analysis, a two-vector response variable comprised the number of eggs fertilized by the focal male and the number of eggs fertilized by the standard male in each competitive fertilization trial. Random variables for each model included focal male identity, focal female identity (i.e. the ID of the OF donor) and the interaction between focal female and focal male. As above, the significance of predictor variables was assessed using likelihood-ratio tests.
Our third mixed-effects model also incorporated a binomial error distribution to examine the relationship between the same response variable (relative paternity) and differences in sperm swimming speed (VAP) and differences in body size between the focal and standard males (both fitted as predictor variables). The difference in male body size was included in this analysis to account for possible size-related variation in sperm quality among males [39]. We also included differences between the relatedness of the focal male with the standard female, and the standard male with the standard female in each trial (i.e. ΔR) as a potential predictor of paternity success (see Results and discussion). In this analysis, a high ΔR-value would indicate that the focal male was more closely related to the standard female than his rival (standard male), and vice versa. To account for moderate overdispersion in this third model (dispersion parameter from uncorrected model; Ø = 1.73), we included observation-level random effects [40], which reduced the dispersion parameter to Ø = 1.01. We included ‘block’ (10 levels) in this analysis, rather than male ID (and OF ID), as a random effect to account for non-independence of subjects within each block. We avoided using focal male ID as a random effect, because this factor simultaneously explains variance in the response variable (paternity) and the predictor (VAP); thus variance in paternity explained by VAP was eroded in models in which focal male ID was included as a random effect. VAP was always associated with relative paternity in any model variant that did not include focal male ID as a random factor. To confirm this association between VAP and paternity, we supplemented our mixed-effects model by using a resampling approach in which independent assortments of focal males and OF donors (note that these are the diagonals in figure 1) were taken at random from each of the 10 blocks (10 000 iterations in total), thus avoiding the need for random effects in our analysis (see also [41]). These analyses, conducted using the PopTools Excel add-on [42], generated a distribution of 10 000 correlation coefficients for the relationship between differences in VAP between focal and standard sperm competitors and the proportion of offspring sired by focal males, from which we calculated the mean and 95% confidence limits (CLs). We carried out similar randomization trials to estimate mean and 95% CLs for partial correlation coefficients (controlling for body size). In both analyses, each of the 10 000 randomly assorted datasets contained n = 20 independent assortments of focal males and OF donors.
3. Results and discussion
Our initial tests for the interactive and main effects of males and OF revealed no significant main effects (p > 0.33) but highly significant interacting effects of male and OF on sperm swimming velocity (LLR = 37.1, d.f. = 1, p < 0.0001; figure 2). This result therefore indicates that under the conditions imposed in our subsequent sperm competition experiment, OF from individual female donors had the potential to differentially mediate sperm swimming velocity, and therefore also fertilization success, when ejaculates from two males compete to fertilize eggs.
Figure 2.
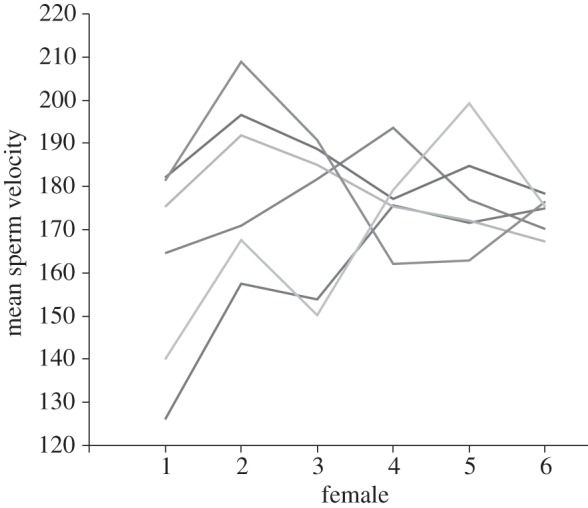
The interacting effects of ovarian fluids from six different females on sperm velocity (average path velocity; VAP) of six different male chinook salmon (O. tshawytscha). Lines represent the mean VAP values for each male.
Despite evidence for OF–sperm interactions, we detected no significant interactive effects of OF and males in the sperm competition experiment. Instead, we found a significant effect of focal male ID on relative paternity success, revealing that certain males produced ejaculates with higher average fertilization capacities under sperm competition than others (table 1). Although there is evidence that success in sperm competition can be repeatable across mating contexts [9–11], our study is novel in that it models variation in sperm competitiveness while experimentally accounting for potentially confounding sources of (stochastic) variation attributable to rival males [17,18]. This finding, in conjunction with the results from our subsequent mixed-effects model and resampling approaches (see below), leads us to conclude that under the experimental conditions imposed in our experiment, variation in relative paternity can be attributed exclusively to variation in the relative sperm competitive ability of focal males (see also [25]), independently of potentially confounding factors such as maternal effects, stochasticity attributable to rival male identity and genotypic male-by-male interactions.
Table 1.
Variation in paternity success in Oncorhynchus tshawytscha partitioned among the random effects of male, female (ovarian fluid, OF) and their interaction. The significance of each effect was tested using likelihood-ratio tests, where the log likelihood-ratio test statistic (LLR) is −2× the difference in log-likelihoods between hierarchically structured models (see main text). Significant p-values are indicated in italics.
| random effects | Var | LLR | p |
|---|---|---|---|
| Male | 1.13 | 47.52 | <0.0001 |
| OF | ∼0 | ∼0 | 0.99 |
| Male×OF | ∼0 | ∼0 | 0.99 |
In contrast to our findings for male effects, we detected no overall effect of OF-donor identity on relative paternity success, and no evidence for male-by-female interactions arising from the differential effects of OF on sperm competitiveness (table 1). The latter finding was unanticipated, given prior evidence from O. tshawytscha and other salmonid fishes that variation in sperm velocity is contingent on the interactive effects of sperm- and OF-donor identities [21–24], a finding that is also supported in this paper. As we note in our methods, we were constrained to use a concentration of 10% OF compared with higher concentrations used previously. It is possible, therefore, that OF–sperm interactions may become more apparent under higher OF concentrations. However, our findings in this study confirmed that sperm–OF interactions were highly significant at 10% OF, which approximates levels shown to upregulate sperm swimming velocity in salmonids (e.g. approx. 70% increase in motility from 0 to 10% OF in lake trout at 10 s post-activation [43]) and other externally fertilizing fishes (e.g. sticklebacks [44]). Moreover, recent work on salmonids has revealed that OF mediates the selection of gametes from conspecific males at just 1% OF—a 10-fold reduction in OF concentration compared with the present investigation [45]. Thus, in the present experiment, ‘intrinsic’ male effects appear to outweigh OF–sperm interactions, but it would nevertheless be interesting to explore the interacting effects of OF under a range of OF concentrations.
We envisage three scenarios that may account for the highly significant male effect for relative paternity success detected in our study. First, males may exhibit extrinsic differences in sperm competitive ability, such that differences in relative paternity success among focal males reflect intrinsic differences in their ability to fertilize eggs under sperm competition. According to this scenario, individual males will differ in the relative quality of their ejaculates, such that the outcome of sperm competition reflects a ‘loaded raffle’ favouring high-quality males [46,47]. Second, the male effects detected in our analysis could conceivably arise owing to differences in compatibility between the focal sperm competitors and the females used to generate standard egg batches for each block. According to this second scenario, in each block one of the focal sperm competitors may have been more compatible with the standard female than the other. Within the confines of the current experimental framework (which standardizes female genotype within each bock), such male-by-female genotypic interactions would have contributed towards the significant ‘male’ effect in the model. Third, patterns of variation in relative paternity may not reflect patterns of fertilization at conception, to the extent that post-zygotic (differential) embryo mortality may have driven the patterns we observed [48–51]. Our subsequent mixed-effects model, in which differences in male traits (sperm velocity and body size) and pairwise relatedness measures for focal males and the standard female in each block were regressed on relative paternity, enables us to address some of these possibilities. We found no evidence that patterns of pairwise relatedness, which are known to mediate sperm velocity in lake trout [21] and guppies [8], predicted relative paternity in O. tshawytscha (table 2). Thus, compatibility effects (scenario 2) involving the focal males and standard females appear unlikely, at least to the extent that these are attributable to relatedness. Instead, we found that focal males with relatively faster-swimming sperm than their standard rivals sired relatively higher numbers of offspring (table 2; figure 3). This finding was subsequently corroborated by our resampling analysis, in which the correlation between VAP and paternity success was tested across 10 000 reassembled datasets (each comprising n = 20 independent assortments of focal males and OF donors). This analysis revealed a positive and significant correlation between sperm swimming velocity and paternity success (mean r = 0.35; 95% CLs = 0.25–0.43), a relationship that was not attributable to variation in body size (mean partial r controlling for body size = 0.32; 95% CLs = 0.22–0.42). The observed positive association between sperm swimming velocity and relative paternity accords with evidence from the Atlantic salmon Salmo salar [25], thus supporting the first scenario outlined above. However, we cannot discount the third scenario, effectively captured by the ‘good sperm’ process [52], whereby offspring from genetically superior males (who produce relatively competitive ejaculates) exhibit relatively higher post-zygotic survival, thus generating the ‘paternity’ biases detected in this study (theory and evidence reviewed in [50,53]).
Table 2.
Results from generalized linear mixed-effects model testing the relationship between relative paternity success and differences between male traits (focal minus standard rival males; fork length = body size; VAP = sperm velocity) and the difference in the Queller & Goodnight [33] estimate of relatedness between the two rival sperm competitors and the standard female (ΔR). Significant p-values are indicated in italics.
| fixed effects | estimate | s.e. | z-value | p-value |
|---|---|---|---|---|
| intercept | 0.24 | 0.21 | 1.12 | 0.262 |
| fork length | −0.03 | 0.02 | −1.20 | 0.231 |
| VAP | 0.007 | 0.003 | 2.12 | 0.028 |
| ΔR (relatedness) | 0.14 | 0.67 | 0.21 | 0.837 |
Figure 3.
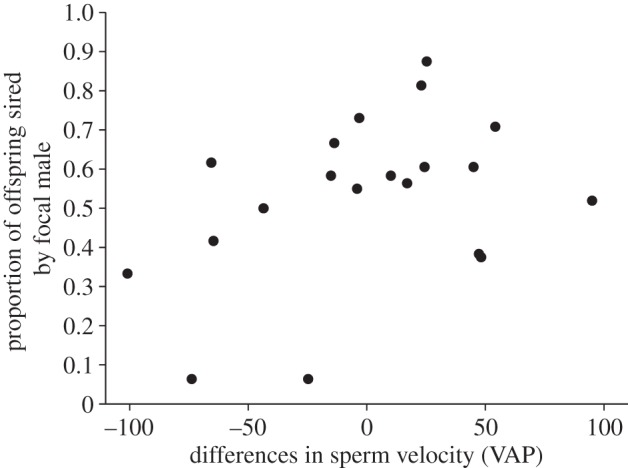
The relationship between differences in sperm velocity (average path velocity; VAP) and the proportion of offspring sired by focal males in each clutch. Note that the relationship depicted in this figure comes from a randomly assembled dataset of sperm competition trials involving unique focal males and OF donors (i.e. n = 2 crosses depicted in the diagonals of each block in figure 1).
Recent attempts to provide an empirical framework for disentangling the complex interactions underlying sperm competition have highlighted the potential advantages [17,18] and pitfalls [16] of using standardized competitors to evaluate the relative importance of male (intrinsic effects), female (cryptic female choice) and male-by-female (compatibility-based) interactions in sperm competition. The general framework offered by Garcia-Gonzalez & Evans [17], for example, recommends the use of standardized rival males when partitioning variation in fertilization success among focal sperm competitors, thus reducing sampling variation attributable to (stochastic) variation in the identity of (non-focal) rival males, and non-additive variation attributable to male-by-male or male-by-female interactions [49,54]. However, this approach has recently been criticized on the basis that estimates of sperm competitiveness under these restricted conditions cannot be generalized to other situations, precisely because male-by-male genotypic interactions generate biologically meaningful sources of variation in sperm competition [16]. We agree, but also add that except under restricted conditions (e.g. in the extreme case of semelparity), selection is rarely confined to specific bouts of sperm competition, and instead acts across multiple successive sperm competitive contests, where rival sperm competitive phenotypes are effectively ‘standardized’ to the mean population level. Within the quantitative genetic framework proposed by Garcia-Gonzalez & Evans [17], heritability estimates derived from the variance components from such designs would be uncontaminated by residual (unexplained) variance attributable to variation in non-focal males, and by non-additive (e.g. male-by-female) effects. In the present experiment, our use of standardized (non-focal) males and females was a necessary component of the experimental design to test whether OF mediates competitive fertilization success. Although our experiment failed to detect such effects, we see great value in extending the present design to other externally fertilizing species where egg-derived chemical ‘signals’ (e.g. OFs, chemoattractants; see [41,45]) are thought to play a role in mediating gamete choice.
Acknowledgements
We are grateful to the hatchery staff at Salmon Smolt NZ, and in particular Ben Divett, Karl French, Errol White, Tom Gough and Luke Price. We also thank Sara Ferreira, Cornelia Geßner and Sheri Johnson for assistance in the field. Finally, we thank Jim Briskie for access to computer-assisted sperm analyses software, Shinichi Nakagawa for statistical advice, and Paco Garcia-Gonzalez, Leigh Simmons, Craig Purchase and an anonymous reviewer for comments on earlier drafts of the manuscript. J.P.E. and P.R. contributed equally to this study. J.P.E. conceived the study and developed the broad experimental design, with contributions from N.J.G., P.R. and C.G.; P.R. and C.G. carried out spawning trials and collected phenotypic data; P.R. and N.J.G. performed molecular analyses; J.P.E. and P.R. analysed the data; J.P.E. wrote the first draft of the paper; all authors contributed towards subsequent drafts.
Funding statement
Funding came from a Research Collaboration Award (University of Western Australia) and a Marsden Grant (grant no. UOO913), which also supported salaries to P.R. and N.J.G.
References
- 1.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press [Google Scholar]
- 2.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534 (doi:10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 3.Pizzari T, Parker GA. 2009. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 207–245 Burlington, MA: Academic Press [Google Scholar]
- 4.Karr TL, Swanson WJ, Snook RR. 2009. The evolutionary significance of variation in sperm-egg interactions. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 305–365 Burlington, MA: Academic Press [Google Scholar]
- 5.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigricepts. Am. Nat. 122, 765–788 (doi:10.1086/284170) [Google Scholar]
- 6.Gillingham MAF, Richardson DS, Lovlie H, Moynihan A, Worley K, Pizzari T. 2009. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proc. R. Soc. B 276, 1083–1092 (doi:10.1098/rspb.2008.1549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretman A, Newcombe D, Tregenza T. 2009. Promiscuous females avoid inbreeding by controlling sperm storage. Mol. Ecol. 18, 3340–3345 (doi:10.1111/j.1365-294X.2009.04301.x) [DOI] [PubMed] [Google Scholar]
- 8.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501 (doi:10.1098/rspb.2008.1549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JP, Rutstein AN. 2008. Postcopulatory sexual selection favours intrinsically good sperm competitors. Behav. Ecol. Sociobiol. 62, 1167–1173 (doi:10.1007/s00265-008-0545-0) [Google Scholar]
- 10.Radwan J. 1998. Heritability of sperm competition success in the bulb mite, Rhizoglyphus robini. J. Evol. Biol. 11, 321–327 [DOI] [PubMed] [Google Scholar]
- 11.Tregenza T, Attia F, Bushaiba SS. 2009. Repeatability and heritability of sperm competition outcomes in males and females of Tribolium castaneum. Behav. Ecol. Sociobiol. 63, 817–823 (doi:10.1007/s00265-009-0716-7) [Google Scholar]
- 12.Edvardsson M, Arnqvist G. 2000. Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. Lond. B 267, 559–563 (doi:10.1098/rspb.2000.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark AG, Begun DJ, Prout T. 1999. Female x male interactions in Drosophila sperm competition. Science 283, 217–220 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Clark AG, Fiumera AC. 2013. Natural genetic variation in male reproductive genes contributes to nontransitivity of sperm competitive ability in Drosophila melanogaster. Mol. Ecol. 22, 1400–1415 (doi:10.1111/mec.12113) [DOI] [PubMed] [Google Scholar]
- 15.Bjork A, Starmer WT, Higginson DM, Rhodes CJ, Pitnick S. 2007. Complex interactions with females and rival males limit the evolution of sperm offence and defence. Proc. R. Soc. B 274, 1779–1788 (doi:10.1098/rspb.2007.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engqvist L. 2013. A general description of additive and nonadditive elements of sperm competitiveness and their relation to male fertilization success. Evolution 67, 1396–1405 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Gonzalez F, Evans JP. 2011. Fertilization success and the estimation of genetic variance in sperm competitiveness. Evolution 65, 746–756 (doi:10.1111/j.1558-5646.2010.01127.x) [DOI] [PubMed] [Google Scholar]
- 18.García-González F. 2008. The relative nature of fertilization success: implications for the study of post-copulatory sexual selection. BMC Evol. Biol. 8, 140 (doi:10.1186/1471-2148-8-140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentzen P, Olsen JB, McLean JE, Seamons TR, Quinn TP. 2001. Kinship analysis of Pacific salmon: insights into mating, homing, and timing of reproduction. J. Hered. 92, 127–136 (doi:10.1093/jhered/92.2.127) [DOI] [PubMed] [Google Scholar]
- 20.Turner E, Montgomerie R. 2002. Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 60, 1570–1579 (doi:10.1111/j.1095-8649.2002.tb02449.x) [Google Scholar]
- 21.Butts IAE, Johnson K, Wilson CC, Pitcher TE. 2012. Ovarian fluid enhances sperm velocity based on relatedness in lake trout, Salvelinus namaycush. Theriogenology 78, 2105–2109 (doi:10.1016/j.theriogenology.2012.06.031) [DOI] [PubMed] [Google Scholar]
- 22.Dietrich GJ, Wojtczak M, Slowinska M, Dobosz S, Kuzminski H, Ciereszko A. 2008. Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 24, 503–507 (doi:10.1111/j.1439-0426.2006.01130.x) [Google Scholar]
- 23.Rosengrave R, Gemmell NJ, Metcalf V, McBride K, Montgomerie R. 2008. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 19, 1179–1185 (doi:10.1093/beheco/arn089) [Google Scholar]
- 24.Urbach D, Folstad I, Rudolfsen G. 2005. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444 (doi:10.1007/s00265-004-0876-4) [Google Scholar]
- 25.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47 [PubMed] [Google Scholar]
- 26.Unwin MJ, Quinn TP, Kinnison MT, Boustead NC. 2000. Divergence in juvenile growth and life history in two recently colonized and partially isolated chinook salmon populations. J. Fish Biol. 57, 943–960 (doi:10.1111/j.1095-8649.2000.tb02203.x) [Google Scholar]
- 27.Walsh PS, Metzger DA, Higuchi R. 1991. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10, 506–513 [PubMed] [Google Scholar]
- 28.Condrey MJ, Bentzen P. 1998. Characterization of coastal cutthroat trout (Oncorhynchus clarki clarki) microsatellites and their conservation in other salmonids. Mol. Ecol. 7, 787–789 [PubMed] [Google Scholar]
- 29.Beacham TD, Candy JR, Le KD, Wetklo M. 2009. Population structure of chum salmon (Oncorhynchus keta) across the Pacific Rim, determined from microsatellite analysis. Fish. Bull. 107, 244–260 [Google Scholar]
- 30.Banks MA, Blouin MS, Baldwin BA, Rashbrook VK, Fitzgerald HA, Blankenship SM, Hedgecock D. 1999. Isolation and inheritance of novel microsatellites in chinook salmon (Oncorhynchus tschawytscha). J. Hered. 90, 281–288 (doi:10.1093/jhered/90.2.281) [Google Scholar]
- 31.Heath DD, Bryden CA, Shrimpton JM, Iwama GK, Kelly J, Heath JW. 2002. Relationships between heterozygosity, allelic distance (d2), and reproductive traits in chinook salmon, Oncorhynchus tshawytscha. Can. J. Fish. Aquat. Sci. 59, 77–84 (doi:10.1139/f01-192) [Google Scholar]
- 32.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 33.Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 34.Wang J. 2011. Coancestry: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Res. 11, 141–145 (doi:10.1111/j.1755-0998.2010.02885.x) [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956 [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team 2011. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 37.Bates D, Maechler M, Dai B.2008. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-37. See http://lme4.r-forge.r-project.org.
- 38.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 39.Blukacz EA, et al. 2010. Linking lake whitefish (Coregonus clupeaformis) condition with male gamete quality and quantity. J. Gt. Lakes Res. 36, 78–83 (doi:10.1016/j.jglr.2009.12.014) [Google Scholar]
- 40.Browne WJ, Subramanian SV, Jones K, Goldstein H. 2005. Variance partitioning in multilevel logistic models that exhibit overdispersion. J. R. Stat. Soc. A 168, 599–613 (doi:10.1111/j.1467-985X.2004.00365.x) [Google Scholar]
- 41.Evans JP, Garcia-Gonzalez F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 2855–2861 (doi:10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hood GM. 2009. PopTools version 3.1.1. See http://www.cse.csiro.au/poptools.
- 43.Galvano PM, Johnson K, Wilson CC, Pitcher TE, Butts IAE. 2013. Ovarian fluid influences sperm performance in lake trout, Salvelinus namaycush. Reprod. Biol. 13, 172–175. (doi:10.1016/j.repbio.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 44.Elofsson H, Van Look KJW, Sundell K, Sundh H, Borg B. 2006. Stickleback sperm saved by salt in ovarian fluid. J. Exp. Biol. 209, 4230–4237 (doi:10.1242/jeb.02481) [DOI] [PubMed] [Google Scholar]
- 45.Yeates SE, Diamond SE, Einum S, Emerson BC, Holt WV, Gage MJG. In press. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behavior. Evolution (doi:10.1111/evo.12208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126 (doi:10.1098/rspb.1990.0114) [Google Scholar]
- 47.Engqvist L. 2012. Genetic conflicts, intrinsic male fertility, and ejaculate investment. Evolution 66, 2685–2696 (doi:10.1111/j.1558-5646.2012.01641.x) [DOI] [PubMed] [Google Scholar]
- 48.Olsson M, Pagel M, Shine R, Madsen T. 1999. Sperm choice and sperm competition: suggestions for field and laboratory studies. Oikos 84, 172–175 (doi:10.2307/3546880) [Google Scholar]
- 49.Droge-Young EM, Manier MK, Lupold S, Belote JM, Pitnick S. 2012. Covariance among premating, post-copulatory and viability fitness components in Drosophila melanogaster and their influence on paternity measurement. J. Evol. Biol. 25, 1555–1563 (doi:10.1111/j.1420-9101.2012.02540.x) [DOI] [PubMed] [Google Scholar]
- 50.García-González F. 2008. Male genetic quality and the inequality between paternity success and fertilization success: consequences for studies of sperm competition and the evolution of polyandry. Evolution 62, 1653–1665 (doi:10.1111/j.1558-5646.2008.00362.x) [DOI] [PubMed] [Google Scholar]
- 51.Gilchrist AS, Partridge L. 1997. Heritability of pre-adult viability differences can explain apparent heritability of sperm displacement ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 264, 1271–1275 (doi:10.1098/rspb.1997.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasui Y. 1997. A ‘good-sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584 (doi:10.1086/286006) [Google Scholar]
- 53.Evans JP, Simmons LW. 2008. The genetic basis of traits regulating sperm competition and polyandry: can selection favour the evolution of good- and sexy-sperm? Genetica 134, 5–19 (doi:10.1007/s10709-007-9162-5) [DOI] [PubMed] [Google Scholar]
- 54.Fricke C, Martin OY, Bretman A, Bussiere LF, Chapman T. 2010. Sperm competitive ability and indices of lifetime reproductive success. Evolution 64, 2746–2757 (doi:10.1111/j.1558-5646.2010.01022.x) [DOI] [PubMed] [Google Scholar]


