Abstract
Silica is well known for its role as inducible defence mechanism countering herbivore attack, mainly through precipitation of opaline, biogenic silica (BSi) bodies (phytoliths) in plant epidermal tissues. Even though grazing strongly interacts with other element cycles, its impact on terrestrial silica cycling has never been thoroughly considered. Here, BSi content of ingested grass, hay and faeces of large herbivores was quantified by performing multiple chemical extraction procedures for BSi, allowing the assessment of chemical reactivity. Dissolution experiments with grass and faeces were carried out to measure direct availability of BSi for dissolution. Average BSi and readily soluble silica numbers were higher in faeces as compared with grass or hay, and differences between herbivores could be related to distinct digestive strategies. Reactivity and dissolvability of BSi increases after digestion, mainly due to degradation of organic matrices, resulting in higher silica turnover rates and mobilization potential from terrestrial to aquatic ecosystems in non-grazed versus grazed pasture systems (2 versus 20 kg Si ha−1 y−1). Our results suggest a crucial yet currently unexplored role of herbivores in determining silica export from land to ocean, where its availability is linked to eutrophication events and carbon sequestration through C–Si diatom interactions.
Keywords: herbivory, silica biogeochemistry, phytoliths, pastures, faeces deposition
1. Introduction
Silica (Si, the element silicon is commonly referred to as silica in biogeochemistry, as it always occurs associated with oxygen [O] in ecosystems) deposition in plants is an inducible defence mechanism countering herbivore grazing and pathogen attack, especially in grasses (Poaceae) [1,2]. Si is taken up by roots as monosilicic acid [Si(OH)4] from soil solution [3], which is usually referred to as dissolved Si (DSi). Some of the silica is then laid down in the plant as opaline biogenic silica (BSi) structures [SiO2.nH2O; BSi], commonly known as ‘phytoliths’ or ‘silica bodies’ [4,5], in fillings of cell walls, cell interiors (lumina) and intercellular spaces. Silica bodies can particularly be abundant in the epidermis of grasses, ranging from 1–3% BSi by dry mass in dryland grasses [6], up to 10–15% BSi in some wetland species [7]. As phytoliths are for a large part composed of durable, inorganic amorphous silica [8], this renders them hard to consume for herbivores: high BSi concentrations in grass leaves have been correlated with high levels of leaf abrasiveness [9,10]. Dietary silica is also able to physically erode insect mandibles [11,12] and teeth of vertebrate grazers [13,14]. Feeding on silica-rich diets negatively affects food digestibility [15], herbivore reproduction and growth rates [16], resulting in diet preference shifts [9] and reduced bite rates or grazing pressure [2,17]. This can have larger population scale consequences: plant-available Si concentrations and coupled Si-based defence mechanisms in grasses have been shown to trigger vole population dynamics [12], while plant Si availability can also impact the outcome of interspecific competition between grass species [18]. There is thus a growing consciousness that Si is a key player in plant–herbivore ecological interactions, acting on community structure of both herbivores and the foraged vegetation.
However, little research has approached the interaction between herbivores and plant BSi in the context of biogeochemical Si cycling. This can be attributable to the fact that plant Si is usually assumed to be chemically inert [19], with most of it passing through the digestive system of herbivores without any severe degradation or absorption, e.g. for phytoliths in sheep and cow faeces [4,6]. Our growing understanding of the importance of biological Si processing and the realization that even small shifts in terrestrial BSi reactivity could alter the balance between Si storage and export from ecosystems [20] challenges this paradigm. In the past decade, it has become clear that terrestrial ecosystems form filters between primary DSi mobilization from silicate weathering and its pathway towards coastal systems and the ocean, putting strong control on export fluxes of DSi and BSi to the coastal zone [20,21]. BSi fixation in terrestrial vegetation ranges worldwide from 60 to 200 Tmol yr−1. This number largely exceeds (10–40 times) the yearly flux of dissolved and suspended biogenic Si towards the ocean [20], where Si availability is linked to eutrophication events (especially in coastal zones [22]) and to diatom Si–C interactions and C burial in the ocean deeps [23]. Human transformations of the landscape are strongly impacting on ecosystem Si processing, yet the processes and underlying mechanisms are still poorly understood. Changes in riverine DSi fluxes along a temperate European watershed [24] as well as lowered BSi soil stocks under sustained agricultural conditions have already been observed [25–27]. Grasslands in particular play an important role in global Si cycling, showing a high capacity to store BSi as well as moving Si from relatively inert mineral silicate soil layers to biologically active soil horizons, by enhancing mineral weathering [28]. Changes in BSi reactivity and direct mobilization of BSi into DSi associated with digestion of grass biomass might therefore strongly alter the storage and recycling potential of BSi in the foraged grassland.
Considerable amounts of grass biomass yearly pass through herbivores' digestive tract systems in grassland systems worldwide. They typically account for 35–55% of the annual above-ground net primary production (ANPP), from which a substantial part (i.e. 30–40% of ANPP) is consumed by mammalian herbivores, with insects like grasshoppers using the remaining part (i.e. 5–15% of ANPP) [29]. Part of the ingested Si is dissolved in the digestive system fluids, from which only a negligible part is assimilated in the herbivore body (e.g. 0.01% for sheep [6]). Si recovery is thus expected to be near complete, e.g. for humans [30] and for animals [6,14]. Little or no research has, however, focused on the reactivity and the ecosystem redistribution of Si after passage through herbivores. We here hypothesize that the reactivity of BSi changes substantially upon this passage, both chemically and physically, analogous to the fate of diatom frustules in marine environments. Dissolvability of diatom BSi, in essence the same amorphous biogenic structure as phytoliths, is strongly enhanced after consumption by zooplankton and/or bacteria [31], mainly through the digestion of the organic matrix, frustule breakage and resulting increased surface area and bacterial encapsulation within faecal pellets [32]. This process drives the efficient recycling of ocean BSi: 97% of all annually produced BSi in the oceans dissolves again. This biological recycling mechanism is essential to sustain ocean diatom productivity and associated foodwebs. In addition, terrestrial herbivores may enhance dissolution through a temperature effect. As the dissolution of BSi is endothermic, temperature positively affects both its dissolution kinetics and solubility [33,34]. We thus hypothesize that grazing changes the distribution and dissolvability of BSi in grasslands, resulting in quick BSi turnover and increasing Si export capacity of grasslands.
To test this hypothesis, we analysed BSi in ingested grass, hay and faeces of four common grazers by performing multiple chemical extraction procedures for BSi that allow the assessment of chemical reactivity. In parallel, we performed dissolution experiments with grass and faeces to assess the direct availability of BSi for dissolution. We show that herbivory substantially increases reactivity of BSi in grasslands, resulting in a potentially strongly shifted balance of the sink versus source function of grasslands for Si.
2. Material and methods
(a). Sampling
In order to assess the impact of free-grazing versus stabled hay-fed animals on Si cycling, sampling was conducted in early summer (June 2011) and in winter (February 2012). In summer, fresh above-ground biomass (hand-cut grass in 0.25 m2 quadrants) and fresh faeces samples from ruminants Bos primigenius taurus B. (cow) and Ovis aries L. (sheep), and non-ruminant Equus africanus asinus L. (donkey) and Equus ferus caballus B. (horse) were collected in grazed pastures during dry weather conditions. Animals were grazing at different fields: pasture 1 (3 ha) was grazed by six cows and two horses, pasture 2 (1 ha) was grazed by four donkeys and four sheep. Both pastures were situated in northern Belgium and are representative for intensively grazed pastures, typically found in Western Europe, characterized by high grazing pressure in summer (i.e. two livestock units per hectare for cattle) and poor in plant species composition (i.e. more than 90% dominated by Lolium spp.). Twenty fresh faeces samples (i.e. five replicates for each animal) and 10 fresh biomass samples (i.e. five replicate litter samples in each pasture) were collected. Similarly, in winter, hay samples (i.e. five replicates) and fresh faeces materials (i.e. five replicates per animal) were sampled from stabled ruminants (cow and sheep) and a non-ruminant (horse). All animals were fed from the same hay stock. Livestock owners' permission was given for taking the samples.
(b). Chemical analysis
In order to assess the impact of herbivore digestion on Si concentration and reactivity, different chemical extraction procedures were conducted. Fresh grass litter (shoot and leaves) and hay samples were thoroughly rinsed with deionized water to remove all dust/soil contamination. Plant and faeces samples were dried at 70°C for 72 h, and wet and dry biomass were determined. Prior to Si extraction, dried plant samples were shredded by crushing with a mechanical mill (3–5 mm size).
(i). Total biogenic silica
Non-continuous extraction of BSi. Total BSi (mg g−1) was extracted twice from all samples (summer and winter campaign). The first extraction was done in 0.1 M Na2CO3
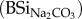 ; the second in 0.5 M NaOH (BSiNaOH). Both commonly used extracting agents were adopted here to increase comparability with other studies [35]. Dried material (30 mg) was incubated in 25 ml of the respective alkaline extracting agent in a water bath at 80°C [36]. After 4 h, the extracted fluid was passed through a 0.45 µm filter (Chromafil A45–25), and analysed spectrophotometrically for DSi on an inductive coupled plasma atomic emission spectrophotometer (ICP-AES, Thermo Scientific, ICAP 6000 Series).
; the second in 0.5 M NaOH (BSiNaOH). Both commonly used extracting agents were adopted here to increase comparability with other studies [35]. Dried material (30 mg) was incubated in 25 ml of the respective alkaline extracting agent in a water bath at 80°C [36]. After 4 h, the extracted fluid was passed through a 0.45 µm filter (Chromafil A45–25), and analysed spectrophotometrically for DSi on an inductive coupled plasma atomic emission spectrophotometer (ICP-AES, Thermo Scientific, ICAP 6000 Series).
Continuous extraction of BSi. Representative samples for all replicate plant and faeces samples (summer and winter campaign) were also analysed for total BSi using a continuous extraction procedure. This procedure was adopted to study reaction kinetics during the extraction and to assess differences in chemical reactivity. The dried sample (100 mg) was put in a stainless steel vessel with 180 ml of 0.5 M NaOH. The vessel was then put in a water bath at 85°C and a constant motor mixed the soil sample into the solution. The sample was then fed into the continuous analyser (Skalar) and analysed for DSi with the spectophotometric molybdate blue method. Extensive intercalibration between ICP (see earlier) and spectrometry ensured optimal reproducibility between both. Each extraction had a total duration of 35 min. Extracted Si values were obtained each 15 s. Results were fitted into a second-order model (equation (2.1)) assuming that there are one or two Si fractions, with different reactivity, reflected by the parameter k (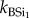 ,
, 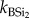 ).
).
| 2.1 |
where BSit, amount of extracted BSi at time t; BSi1, total amount of BSi in fraction 1; BSi2, total amount of BSi in fraction 2; 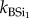 , reactivity of BSi in fraction 1;
, reactivity of BSi in fraction 1; 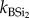 , reactivity of BSi in fraction 2; t, time.
, reactivity of BSi in fraction 2; t, time.
(ii). Readily soluble silica
Calcium chloride (CaCl2) is a weak extracting agent, assumed to only extract the readily soluble Si fraction [37], which can be used as an estimate of DSi availability for plants or as an equilibrium concentration for DSi in soil pore water [25]. All dried plant and faeces samples (2.0 g) from the summer campaign were shaken thoroughly for 16 h with 20 ml 0.01 M CaCl2 solution in a 35 ml tube at 20°C [25,38]. Extracted samples were then centrifuged at 4000 r.p.m. for 30 min and the supernatant was filtered over 0.45 µm pore size (Chromafil A45–25). Si concentration 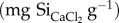 was analysed by means of ICP-AES.
was analysed by means of ICP-AES.
(c). Experimental set-up: dissolution experiment
To mimic in situ reactivity of Si after digestion, Si dissolution from fresh faeces and hay (winter campaign) was monitored over different time intervals in rain water. Fresh material (3 g) was put in 200 ml plastic boxes and spread out evenly over the bottom surface (ca 40 cm2). Dissolution experiments were carried out for 24 h, subsamples were taken at 11 time intervals (2, 5, 10 and 30 min, and 1, 2, 5, 10, 15, 18, 24 h) for (i) hay, (ii) cow faeces, (iii) sheep faeces and (iv) horse faeces, in a dark incubator at 20°C. In total, 66 boxes were prepared for hay, cow, sheep and horse faeces: five replicates and one blank per time interval. At the start of each experiment, all boxes were filled with 30 ml air-captured rain water (pH: 6.64, conductivity 68.7 µS cm−1 and DSi: 0.04 mg l−1). At the above specified time intervals, 10 ml of solution was sampled from a box and filtered over a filter with 0.45 µm pore size (Chromafil A45–25); the sampled box was not further used. Solutions were analysed for DSi using ICP-AES.
3. Results
Detailed results of all extractions and experiments are available in the electronic supplementary material.
(a). Chemical extractions
(i). Total biogenic silica
Non-continuous extraction of BSi. Overall, BSi (mg g−1) extracted in 0.5 M NaOH (BSiNaOH) showed higher values compared with samples extracted in 0.1 M Na2CO3
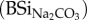 (table 1). BSi concentration (both BSiNaOH and
(table 1). BSi concentration (both BSiNaOH and 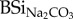 ; mg BSi g−1) of faeces samples were two to four times higher than for forage or hay (table 1). Except for stabled cow faeces,
; mg BSi g−1) of faeces samples were two to four times higher than for forage or hay (table 1). Except for stabled cow faeces, 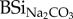 in faeces samples could be ordered based on their digestion system (ruminant versus non-ruminant): sheep < cow < horse < donkey.
in faeces samples could be ordered based on their digestion system (ruminant versus non-ruminant): sheep < cow < horse < donkey.
Table 1.
Results derived from different chemical extraction techniques. From left to right: (1) total BSi content extracted in 0.1 M Na2CO3
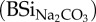 , (2) total BSi content extracted in 0.5 M NaOH (BSiNaOH), (3) DSi concentration after extraction in 0.01 M CaCl2
, (2) total BSi content extracted in 0.5 M NaOH (BSiNaOH), (3) DSi concentration after extraction in 0.01 M CaCl2
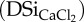 , (4) reaction order of the model where data were fit after continuous extraction of Si, (5) reactivity parameter k of fraction 1 (associated with certain percentage of BSi), (6) reactivity parameter of fraction 2 (if present) and (7) calculated differences in BSi between NaOH and Na2CO3 extraction (NCI-BSi) expressed as percentage relative to BSiNaOH. Columns 4 to 6 are derived from the continuous extraction of BSi. NV, no value.
, (4) reaction order of the model where data were fit after continuous extraction of Si, (5) reactivity parameter k of fraction 1 (associated with certain percentage of BSi), (6) reactivity parameter of fraction 2 (if present) and (7) calculated differences in BSi between NaOH and Na2CO3 extraction (NCI-BSi) expressed as percentage relative to BSiNaOH. Columns 4 to 6 are derived from the continuous extraction of BSi. NV, no value.
| sample |
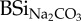 (mg Si g−1) (mg Si g−1) |
BSiNaOH (mg Si g−1) |
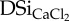 (mg Si g−1) (mg Si g−1) |
order | k1_c (min−1) | k2_c (min−1) | NCI-BSi (%) |
|---|---|---|---|---|---|---|---|
| pasture 1 | 4.0 (1.3)a | 4.2 (1.2)a | 0.19 (0.04)a | 1 | 0.35 | 1.8–3.9c | |
| pasture 2 | 2.9 (0.8)a | 3.2 (1.0)a | 0.16 (0.018)a | 1 | 0.40 | 2.8–17.3c | |
| cow faeces summer | 8.7 (1.1)a | 11.7 (1.4)a | 0.32 (0.12)a | 1 | 0.23 | 26.0–27.0c | |
| sheep faeces summer | 9.5 (0.6)a | 10.1 (0.5)a | 0.48 (0.03)a | 1 | 0.26 | 2.2–9.7c | |
| horse faeces summer | 10.8 (0.5)a | 11.3 (0.6)a | 0.07 (0.009)a | 2 | 0.29 (99)b | 1.61 (1)b | 2.6–10.9c |
| donkey faeces summer | 10.8 (0.7)a | 11.5 (0.4) | 0.33 (0.008)a | 2 | 0.24 (55)b | 0.41 (45)b | 3.1–7.4c |
| hay | 4.2 (0.2)a | 4.7 (0.4)a | NV | 2 | 0.35 (63)b | 0.83 (37)b | 5.5–18.0c |
| cow faeces winter | 14.6 (0.9)a | 16.3 (0.5)a | NV | 1 | 0.29 | 4.8–10.8c | |
| sheep faeces winter | 8.5 (0.5)a | 9.3 (0.3)a | NV | 1 | 0.23 | 7.6–14.6c | |
| horse faeces winter | 10.6 (0.3)a | 10.9 (0.6)a | NV | 1 | 0.43 | 0.2–7.9c |
aMean (s.d.).
bValues in parenthesis are % of BSi fraction associated with k1 and k2 reactivity parameter.
cRange (minimum–maximum values) NCI-BSi.
Continuous extraction of BSi and reactivity parameters k. Extraction curves for faeces samples from cow and sheep fitted to a first-order model, whereas donkey and horse samples fitted to a second-order model (i.e. with, respectively, only a k1 parameter and both a k1 and k2 reactivity parameter; table 1). Ruminant faeces yielded only one reactivity k parameter (k1) (k1 = 0.23, 0.23, 0.26 and 0.29 for cow summer, sheep winter, sheep summer and cow winter, respectively; table 1). A second higher reactive fraction of BSi was present in both donkey (k2 = 0.41) and horse faeces (k2 = 1.61); this fraction is large (44% of total BSiNaOH) in donkey, while negligible in horse (less than 1% of total BSiNaOH). The horse faeces sample occupied an intermediate position: it fitted to a first-order model, yet with a higher reactivity k1 parameter (k1 = 0.43) as compared with cow and sheep faeces. Grass samples collected during the summer campaign consisted of one BSi fraction, with k1 parameters (0.35–0.40) around 30% higher than the first-order reactivity of BSi in faeces samples (0.23–0.29). Opposite, hay comprised two BSiNaOH fractions. In the first fraction, 63% of BSiNaOH was associated with a low-reactivity parameter k1 of 0.35, which is comparable with grass, whereas in the second fraction 37% of BSiNaOH was highly reactive, with a reactivity parameter k2 = 0.83.
(ii). Readily soluble silica
In general, readily soluble silica concentrations 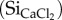 were higher and display more variation for faeces samples (0.06–0.50 mg
were higher and display more variation for faeces samples (0.06–0.50 mg  ) when compared with pasture forage (0.14–0.27 mg
) when compared with pasture forage (0.14–0.27 mg 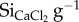 ; table 1). Cow faeces showed the highest variation for
; table 1). Cow faeces showed the highest variation for 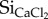 (0.16–0.43 mg
(0.16–0.43 mg  ), readily soluble Si concentrations for horse faeces are very low yet consistent (0.061–0.082 mg
), readily soluble Si concentrations for horse faeces are very low yet consistent (0.061–0.082 mg  ).
).
(b). Dissolution experiment
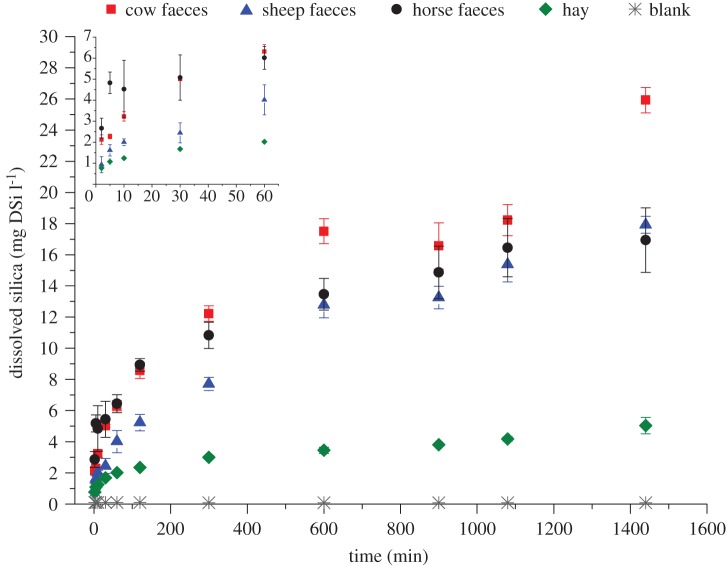
Overall, leaching from faeces went three to five times faster as compared with hay (figure 1): after 24 h DSi from cow, sheep and horse faeces were, respectively, 5.1, 3.6 and 3.4 times higher as compared with DSi from hay. In a similar way as initial 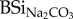 concentrations of faeces and hay, highest concentrations of DSirain were found in cow faeces, followed by horse and sheep, whereas hay showed lowest values (table 1). When expressed relatively to initial
concentrations of faeces and hay, highest concentrations of DSirain were found in cow faeces, followed by horse and sheep, whereas hay showed lowest values (table 1). When expressed relatively to initial 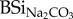 , the percentage of mobilized Si after 24 h exposure to rain water in cow, horse and sheep faeces was, respectively, 60%, 16% and 8% of total
, the percentage of mobilized Si after 24 h exposure to rain water in cow, horse and sheep faeces was, respectively, 60%, 16% and 8% of total 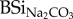 , whereas this was only 4% in hay.
, whereas this was only 4% in hay.
Figure 1.
Dissolution of BSi in grass and faeces after exposure to rain water. Results, expressed as mg DSi l−1, are plotted as mean values (symbols) and standard deviations (bars; n = 5) for different series (cow faeces, sheep faeces, horse faeces and hay) during 24 h (1440 min). Blank rain water samples were also incubated and sampled per time step (n = 1). The first 60 min of the dissolution experiment are visualized in detail in a smaller inset graph.
4. Discussion
Herbivores prove to be crucial in increasing the dissolution potential of BSi in grasslands, acting on Si turnover rates and mobilization potential on the ecosystem scale. This opposes the traditional viewpoint that BSi is largely unaffected by herbivore digestion, owing to its chemical inertness and passive transfer through the herbivore gut. More than 50% of the continents is nowadays grazed by either domestic or wild herbivores [39], and our results point to herbivore stimulation of continental Si fluxes and coupled C sequestration mechanisms.
(a). Grazers: biocatalysts of Si cycling
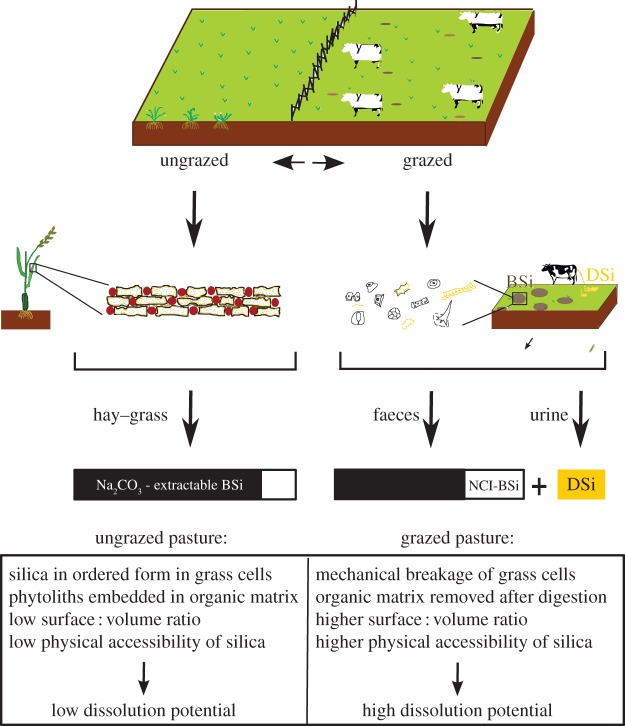
Cows are arguably the most important cultivated grazers: world's cattle population is estimated to be about 1.5 billion [40]. Enrichment of BSi in cow faeces samples implies that cows' faeces are potential BSi dissolution and mobilization hotspots: higher amounts of DSi are dissolving from the faeces in both CaCl2 and rainwater compared with grass litter/hay. One could easily think that faeces samples are leaching higher amounts of DSi because of higher initial BSi concentrations as compared with forage. Yet, it is clear from our results that BSi transport through herbivore guts magnifies the reactivity of the BSi fractions compared with grasses. We hypothesize that this is related to the physical accessibility of BSi for dissolution and/or mobilization. Alkaline saliva production (pH around 8.3) and repeated chewing in cattle favours solubility of plant BSi [41]. Digestion removes a major fraction of organic matter in the grasses: the creation of hotspots of BSi thus coincides with increasing BSi directly available for dissolution (figure 2). This is strongly reminiscent of dissolution of diatom frustules in the ocean, where organic matrix attack by zooplankton and/or bacteria strongly increases BSi turnover rates [31]. At first sight contradictory, the digestion processes lowered the total reactivity parameter k of BSi in faeces with respect to forage or hay. However, it should be emphasized that this reactivity k reflects the ‘full’ intrinsic chemical reactivity of a ground sample. During chemical extraction under high temperature and strong alkaline conditions, all BSi in the ground sample is directly available for dissolution regardless of surrounding matrices. Part of the BSi is likely already mobilized in the urine after dissolution in cows. This will preferably be the most reactive BSi fractions, and this is reflected in the intrinsic chemical properties of the faeces, where the most reactive BSi has already been removed compared with the hay and grass.
Figure 2.
Schematic of the different BSi fractions in grass and the relative change in BSi fractions after digestion by herbivores. Non-extractable Na2CO3 fraction (NCI-BSi) is depicted as a white bar, Na2CO3 extractable fraction as a black bar and mobilization of DSi in urine as a yellow bar. As part of plant ingested Si in herbivores is released via urine, the relative portion of NCI-BSi is larger in faeces as compared with hay or grass.
The idea of herbivore-mediated BSi dissolution is supported by differences in digestive systems, representing (i) ruminants (cow and sheep) and (ii) hindgut fermenters (donkey and horse). Digestion efficiency increases with food particles' mean retention time (MRT). The latter is influenced by digestion type and is higher in ruminants compared with hindgut fermenters [42]. Ruminants are characterized by a long MRT, low food intake and a relatively high digestibility of organic matter and fibre (=cellulose + lignin), particularly at high fibre content, whereas opposite strategies are observed in hindgut fermenters rendering lower digestibility rates [42]. Indeed, donkey and horse faeces showed, consistently through the dataset, k-parameters which were reminiscent of the forage grass or hay, whereas for sheep and cow a larger decrease in chemical reactivity was observed (except for one negligible high-reactivity fraction in donkey). This supports our earlier hypothesis that intrinsic chemical reactivity of forage should become lower in faeces with longer residence times in the herbivore, as part becomes mobilized in the urine. Reactivity parameters are also different for hay and in situ sampled grass, where hay has a fraction not present in the field sampled grass, with a high intrinsic reactivity. Results from an earlier dissolution experiment with forage in the rumen of a cow showed that more DSi dissolves from old grass already impacted by some decomposition as compared with fresh grass and a change in the ratio between different Si fractions was observed [4]. Also microscopic evidence suggested that old grass leaves show more signs of internal mechanical damage which can explain the higher reactivity of BSi in hay.
In general, we conclude that the relative field availability of BSi for dissolution, compared with grass litter BSi, is strongly enhanced by herbivores, and that this is likely due to degradation of surrounding matrices during gut passage. The impact on these matrices should increase with increasing gut residence time. Yet also the chemical reactivity of BSi changes during gut passage resulting in different processes, including the preferential in-herbivore dissolution of high-reactivity phases, low-reactivity phases becoming more reactive during gut passage, as well as mobilization of part of the BSi in urine.
Urine production is thus a second way in which herbivores are directly mobilizing DSi from BSi stocks in grasslands. As it is true that ‘what comes in, must go out’ [6], one can assume that BSi excreted in the faeces is equal in amount to initially ingested grass BSi. If part of the ingested BSi is directly mobilized as urine, this should be reflected in reactivity of faeces BSi. Differences between BSiNaOH and 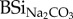 for all samples indicate that a Na2CO3-inextractable BSi (NCI-BSi) phase exists (table 1), with Na2CO3 as the weakest of both extractants. Changes in % NCI-BSi (i.e. expressed as a fraction of total NaOH extracted BSi) between both foraged grass and faeces could indicate partial mobilization of the Na2CO3 extractable fraction via urine. Overall faeces from non-stabled animals show higher NCI-BSi percentages as compared with foraged grass (table 1). Cows here have largest NCI-BSi percentages, again pointing to cows as most efficient mobilizers of BSi. Increases in the percentage of % NCI-BSi between grass and faeces (0–20%) are comparable with other studies pointing to 1–10% of total ingested BSi mobilized in urine [4,6]. For stabled animals, hay displays equal fractions of NCI-BSi percentages with respect to faeces. This is likely due to the fact that the most readily soluble Si has already been released from the hay during storage.
for all samples indicate that a Na2CO3-inextractable BSi (NCI-BSi) phase exists (table 1), with Na2CO3 as the weakest of both extractants. Changes in % NCI-BSi (i.e. expressed as a fraction of total NaOH extracted BSi) between both foraged grass and faeces could indicate partial mobilization of the Na2CO3 extractable fraction via urine. Overall faeces from non-stabled animals show higher NCI-BSi percentages as compared with foraged grass (table 1). Cows here have largest NCI-BSi percentages, again pointing to cows as most efficient mobilizers of BSi. Increases in the percentage of % NCI-BSi between grass and faeces (0–20%) are comparable with other studies pointing to 1–10% of total ingested BSi mobilized in urine [4,6]. For stabled animals, hay displays equal fractions of NCI-BSi percentages with respect to faeces. This is likely due to the fact that the most readily soluble Si has already been released from the hay during storage.
(b). Outlook: grass, grazers and their interaction and impact on the environment
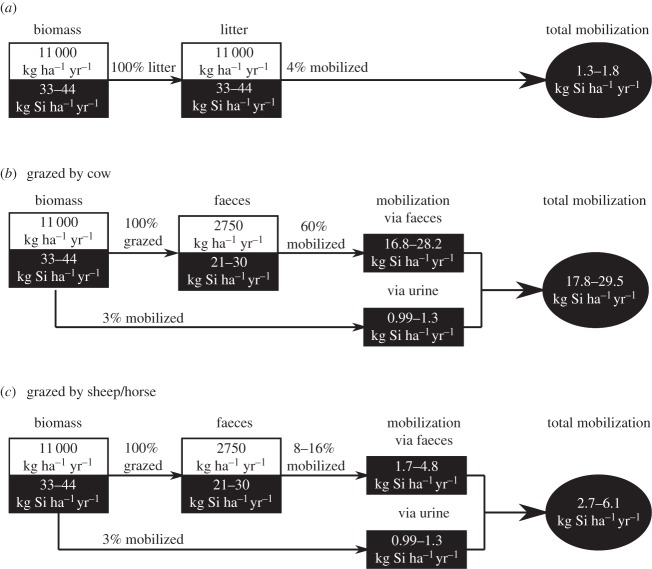
It is clear that grazing will strongly increase the potential turnover of BSi, and thus also the potential DSi leaching from grasslands. We calculated total amounts of BSi dissolved for a grazed pasture system, respectively, for a cow-based and a non-cow-based system (i.e. horse/sheep), and compared this with an ungrazed pasture (figure 3). We simplified by assuming that all standing vegetation biomass in ungrazed systems will become litter (which is an overestimation) and that herbivores consume all ANPP. A conservative estimation of ANPP in temperate humid grasslands of 11 000 kg ha−1 yr−1 is used [43]. Owing to constraints in measuring ANPP in grazed/ungrazed systems and doubts whether or not grazing can enhance primary production [39,44], we omitted any potential (positive) effect of grazing on biomass production in pastures. For all herbivores, a biomass conversion ratio of 1 : 4 was used in the calculation, congruent with a digestibility of 70–75%; which is the expected value for cattle in our study area [45]. DSi mobilization in urine was based on our results and literature [4,6] conservatively put at 3%. Our estimate assumes that the 24 h dissolution potential in the dissolution experiment is indicative for the BSi mobilization potential under field conditions. This should not be considered as an exact number for mobilization rates of DSi from in situ grasslands. Field mobility strongly depends on factors such as rain intensity and frequency, water table and hydrology. However, our results do show the high potential of grazed pasture compared with ungrazed pasture to enhance DSi exports to adjacent rivers, owing to the higher turnover rate and leaching potential. In cow-based pastures, BSi mobilization ranges from 18 to 28 kg Si ha−1 yr−1, which is 15 times higher than an ungrazed pasture (1.3–1.8 kg Si ha−1 yr−1). The increased mobilization is lower for sheep and horse, but still amounts up to two and four times higher than an ungrazed pasture.
Figure 3.
Schematic of hypothetical ungrazed (a) and grazed grassland, dominated by (b) cows and (c) horses or sheep. Annual net above-ground primary production (ANPP) is assumed to be the same for both systems (11 000 kg ha−1 yr−1). All biomass is converted to litter, or all biomass is consumed by the grazers in the grazed systems. A biomass conversion ratio of 1 : 4 for grass versus faeces is suggested for all herbivores. Mobilization percentages for faeces are based on ranges (minimum–maximum values) obtained in the dissolution experiment after 24 h in rain water; for urine, a conservative estimation of 3% mobilization was used. White boxes represent annual biomass production (dry weight, expressed as kg ha−1 yr−1), black boxes and circles represent annual Si fluxes (expressed as kg Si−1 ha yr−1).
DSi export fluxes from grasslands range between 0.2 and 11 kg Si ha−1 yr−1 for dry and wet grasslands, respectively [28], and our extrapolation results fall in the same order of magnitude for both grazed and ungrazed systems. Importantly, intensive grazing drives potential export fluxes to the higher value ranges.
The above calculation and reasoning—although preliminary and entirely based on chemical analysis of BSi reactivity—show that grazing can have a tremendous impact on phytolith burial potential. Nevertheless, a thorough understanding of the exact mechanisms and processes occurring during Si-uptake and precipitation in plants and the impact of grazing and herbivore digestion is needed. Work on the functional role of Si in plants and its resulting impact on Si export fluxes is still in its infancy and a coordinated research effort of scientists from different disciplines is required (biogeochemistry, plant physiology, community ecology, etc.) [46]. Different morphologies of phytoliths, for example, have been assumed to impact dissolution with in general grass phytoliths dissolving more slowly as compared with forest silica bodies [47], making forests potentially larger exporters for DSi. Within a plant, Si can be precipitated as infillings of cells, with only small amounts of occluded organic matter, or Si can be deposited in the cell wall where it is laid down directly on carbohydrates [5], and may form organosilicon complexes [48]. It could be expected that the reactivity and accessibility of both types of phytoliths differs as cell wall silica exposes a larger surface area after breakdown and thus digestion. Detailed microscopic analysis is needed to further elucidate the impact of digestion on Si reactivity on the level of the plant cell. More specifically, the role of carbon occluded or associated with phytoliths needs to be addressed further [49], given that plant-available Si concentration has been shown to influence decomposition rate and turnover of C in reed litter [50].
(c). Implications
Grazing is an important yet currently overlooked component of the Si cycle in grassland ecosystems, potentially increasing Si mobilization from grasslands by an order of magnitude, due to both changes in the physical properties of organic matrices (cf. zooplankton/bacteria in the ocean and diatom frustules) and changes in the intrinsic chemical reactivity of the BSi. Land cultivation and crop harvest were only recently acknowledged as an important driver for continental Si fluxes [24], with deforestation and land cultivation leading to strongly reduced continental Si export fluxes on timescales longer than 250 years. This study implies that also grazing can impact on continental Si mobilization. Whether or not the high reactivity and leaching potential of Si in a herbivore's excretion products can counteract to some extent decreased Si export fluxes from (cultivated) soils will be highly dependent on the way animal faeces is managed. Worldwide, (native) grasslands are converted to other land use types, mostly urban areas or agricultural land. Western European permanent pastures are scarce as they are often used in a crop-rotation system. Manure from (stabled) livestock might be restored to croplands, whereby it is spread evenly on land, becoming readily available for plant uptake from soil solution. Government environmental programmes, however, strictly limit the use of animal manure for reuse on arable land, and surplus manure is used in other applications preventing its return in the aquatic system (e.g. biofuels, sewage systems, etc.). It is clear that our new findings on Si reactivity in grazed systems could have far reaching consequences regarding management of pasture systems.
Interestingly, new light is shed on the coevolution of grasses, herbivores and diatoms. Expansion of grasses in the Early Miocene coincided with the origin of high-crowned teeth in grazing ungulates, which is assumed to be driven by the consumption of abrasive, silica-rich phytoliths in their grass forage [51]. Massive accumulation of phytoliths in grassland systems has been considered to act as a key player to drive Miocene diatomite abundance and diversity in non-marine [51] and even marine sediment records [52]. It was assumed that phytoliths were mobilized from grassland soils through wind, fire, run-off and/or dissolution to groundwater. Yet, solid causal evidence for the correlation between grass–diatom coevolution is lacking. We raise the hypothesis that enhanced reactivity of BSi owing to grazing is probably an important trigger for the biogeochemical link between grasses and diatoms during evolution, while the intensity of the herbivore effect will depend on factors such as temperature and wind conditions, hydrology, herbivore type, etc. Given the catalysing effect of herbivores on Si reactivity and leaching potential, we conclude that herbivores play a major role in Si delivery towards the aquatic system.
Acknowledgements
We thank two anonymous reviewers for their constructive comments and criticisms. F.V. wrote the first drafts of the manuscript, A.B. performed laboratory work on continuous extraction, F.V., J.S. and N.R. collected and analysed data and, E.S. and S.V.D. were involved in the manuscript and concept development from the start, all authors contributed substantially to discussion.
Funding statement
F.V. thanks Special Research Funding of the University of Antwerp (BOF-UA) for PhD fellowship funding, and T. Van der Spiet and A.Cools for laboratory analysis. A.S. and E.S. thank FWO (Research Foundation Flanders) for, respectively, PhD fellowship and postdoctoral research funding, J.S. thanks Agency for Innovation by Science and Technology (IWT) for personal research funding and FWO for funding the project ‘Tracking the biological control on Si mobilization in upland ecosystems’ (project no. G014609N). We thank BELSPO (Belgian Science Policy) for funding SOGLO (soils and global change).
References
- 1.McNaughton SJ, Tarrants JL, McNaughton MM, Davis RH. 1985. Silica as a defense against herbivory and a growth promotor in African grasses. Ecology 66, 528–535 (doi:10.2307/1940401) [Google Scholar]
- 2.Massey FP, Massey K, Ennos AR, Hartley SE. 2009. Impacts of silica-based defences in grasses on the feeding preferences of sheep. Basic Appl. Ecol. 10, 622–630 (doi:10.1016/j.baae.2009.04.004) [Google Scholar]
- 3.Ma JF, Yamaji N. 2006. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397 (doi:10.1016/j.tplants.2006.06.007) [DOI] [PubMed] [Google Scholar]
- 4.Blackman E, Bailey CB. 1971. Dissolution of silica from dried grass in nylon bags placed in the rumen of a cow. Can. J. Anim. Sci. 51, 327–332 (doi:10.4141/cjas71-045) [Google Scholar]
- 5.Piperno D. 2006. Phytoliths: a comprehensive guide for archaelogists and paleoecologists. Lanham, MD: Altamira press; (A division of Rowman and Littlefield Publishers Inc., 238 p). [Google Scholar]
- 6.Jones LHP, Handreck KA. 1965. Relation between silica content of diet and excretion of silica by sheep. J. Agr. Sci. 65, 129–134 (doi:10.1017/S0021859600085439) [Google Scholar]
- 7.Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E. 2010. Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytol. 186, 385–391 (doi:10.1111/j.1469-8137.2009.03176.x) [DOI] [PubMed] [Google Scholar]
- 8.Fraysse F, Cantais F, Pokrovsky OS, Schott J, Meunier JD. 2006. Aqueous reactivity of phytoliths and plant litter: physico-chemical constraints on terrestrial biogeochemical cycle of silicon. J. Geochem. Explor. 88, 202–205 (doi:10.1016/j.gexplo.2005.08.039) [Google Scholar]
- 9.Massey FP, Hartley SE. 2006. Experimental demonstration of the antiherbivore effects of silica in grasses: impacts on foliage digestibility and vole growth rates. Proc. R. Soc. B 273, 2299–2304 (doi:10.1098/rspb.2006.3586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massey FP, Ennos AR, Hartley SE. 2007. Grasses and the resource availability hypothesis: the importance of silica-based defences. J. Ecol. 95, 414–424 (doi:10.1111/j.1365-2745.2007.01223.x) [Google Scholar]
- 11.Kvedaras OL, Byrne MJ, Coombes NE, Keeping MG. 2009. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agr. Forest Entomol. 11, 301–306 (doi:10.1111/j.1461-9563.2009.00430.x) [Google Scholar]
- 12.Massey FP, Hartley SE. 2009. Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 78, 281–291 (doi:10.1111/j.1365-2656.2008.01472.x) [DOI] [PubMed] [Google Scholar]
- 13.Baker G, Jones LHP, Wardrop ID. 1959. Cause of wear in sheep's teeth. Nature 184, 1583–1584 (doi:10.1038/1841583b0) [DOI] [PubMed] [Google Scholar]
- 14.Hummel J, Findeisen E, Suedekum K-H, Ruf I, Kaiser TM, Bucher M, Clauss M, Codron D. 2011. Another one bites the dust: faecal silica levels in large herbivores correlate with high-crowned teeth. Proc. R. Soc. B 278, 1742–1747 (doi:10.1098/rspb.2010.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt JW, Dean AP, Webster RE, Johnson GN, Ennos AR. 2008. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 102, 653–656 (doi:10.1093/aob/mcn130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basagli MAB, Moraes JC, Carvalho GA, Ecole CC, Gonçalves-Gervásio RdCR. 2003. Effect of sodium silicate application on the resistance of wheat plants to the green-aphids Schizaphis graminum (Rond.) (Hemiptera: Aphididae). Neotrop. Entomol. 32, 659–663 (doi:10.1590/S1519-566X2003000400017) [Google Scholar]
- 17.Cotterill JV, Watkins RW, Brennon CB, Cowan DP. 2007. Boosting silica levels in wheat leaves reduces grazing by rabbits. Pest Manag. Sci. 63, 247–253 (doi:10.1002/ps.1302) [DOI] [PubMed] [Google Scholar]
- 18.Garbuzov M, Reidinger S, Hartley S. 2011. Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Ann. Bot. 108, 1355–1363 (doi:10.1093/aob/mcr230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iller RK. 1955. The colloidal chemistry of silica and silicates. Ithaca, NY: Cornell University Press [Google Scholar]
- 20.Struyf E, Conley D. 2012. Emerging understanding of the ecosystem silica filter. Biogeochemistry 107, 9–18 (doi:10.1007/s10533-011-9590-2) [Google Scholar]
- 21.Derry LA, Kurtz AC, Ziegler K, Chadwick OA. 2005. Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 433, 728–731 (doi:10.1038/nature03299) [DOI] [PubMed] [Google Scholar]
- 22.Cloern JE. 2001. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 210, 223–253 (doi:10.3354/meps210223) [Google Scholar]
- 23.Ragueneau O, Schultes S, Bidle K, Claquin P, Moriceau B. 2006. Si and C interactions in the world ocean: importance of ecological processes and implications for the role of diatoms in the biological pump. Glob. Biogeochem. Cycle 20, GB4S02 (doi:10.1029/2006gb002688) [Google Scholar]
- 24.Struyf E, et al. 2010. Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 1, 129 (doi:10.1038/ncomms1128) [DOI] [PubMed] [Google Scholar]
- 25.Clymans W, Struyf E, Govers G, Vandevenne F, Conley DJ. 2011. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 8, 2281–2293 (doi:10.5194/bg-8-2281-2011). [Google Scholar]
- 26.Guntzer F, Keller C, Poulton P, McGrath S, Meunier J-D. 2011. Long-term removal of wheat straw decreases soil amorphous silica at Broadbalk, Rothamsted. Plant Soil 352, 173–184 (doi:10.1007/s11104-011-0987-4) [Google Scholar]
- 27.Vandevenne F, Struyf E, Clymans W, Meire P. 2012. Agricultural silica harvest: have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 10, 243–248 (doi:10.1890/110046) [Google Scholar]
- 28.Blecker SW, McCulley RL, Chadwick OA, Kelly EF. 2006. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycle 20, Gb3023 (doi:10.1029/2006gb002690) [Google Scholar]
- 29.Detling JK. 1988. Grasslands and savanna: regulation of energy flow and nutrient cycling by herbivores. Ecol. Stud. 67, 131–147 (doi:10.1007/978-1-4612-3842-3_7) [Google Scholar]
- 30.Robberecht H, Van Cauwenbergh R, Van Vlaslaer V, Hermans N. 2009. Dietary silicon intake in Belgium: sources, availability from foods, and human serum levels. Sci. Total Environ. 407, 4777–4782 (doi:10.1016/j.scitotenv.2009.05.019) [DOI] [PubMed] [Google Scholar]
- 31.Bidle KD, Azam F. 1999. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 397, 508 (doi:10.1038/17351) [Google Scholar]
- 32.Loucaides S. 2009. Dissolution of biogenic silica: roles of pH, salinity, pressure, electrical charging and reverse weathering. Utrecht, The Netherlands: University of Utrecht [Google Scholar]
- 33.Van Cappellen P, Qiu L. 1997. Biogenic silica dissolution in sediments of the Southern Ocean. I. Solubility. Deep Sea Res. Part II 44, 1109–1128 (doi:10.1016/s0967-0645(96)00113-0) [Google Scholar]
- 34.Van Cappellen P, Qiu L. 1997. Biogenic silica dissolution in sediments of the Southern Ocean. II. Kinetics. Deep Sea Res. Part II 44, 1129–1149 (doi:10.1016/s0967-0645(96)00112-9) [Google Scholar]
- 35.Saccone L, Conley DJ, Koning E, Sauer D, Sommer M, Kaczorek D, Blecker SW, Kelly EF. 2007. Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. Eur. J. Soil Sci. 58, 1446–1459 (doi:10.1111/j.1365-2389.2007.00949.x) [Google Scholar]
- 36.DeMaster DJ. 1981. The supply and accumulation of silica in the marine environment. Geochim. Cosmochim. Acta 45, 1715–1732 (doi:10.1016/0016-7037(81)90006-5) [Google Scholar]
- 37.Berthelsen S, Noble A, Garside A. 2001. Silicon research down under: past, present, and future. In Silicon in agriculture (eds Datnoff LE, Snyder G, Korndörfer G.), pp. 241–256 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 38.Höhn A, Sommer M, Kaczorek D, Schalitz G, Breuer J. 2008. Silicon fractions in histosols and gleysols of a temperate grassland site. J. Plant Nutr. Soil Sci. 171, 409–418 (doi:10.1002/jpln.200625231) [Google Scholar]
- 39.Frank DA, Kuns MM, Guido DR. 2002. Consumer control of grassland plant production. Ecology 83, 602–606 (doi:10.2307/3071865). [Google Scholar]
- 40.Thornton PK. 2010. Livestock production: recent trends, future prospects. Phil. Trans. R. Soc. B 365, 2853–2867 (doi:10.1098/rstb.2010.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey CB, Balch CC. 1961. Saliva secretion and its relation to feeding in cattle. Brit. J. Nutr. 15, 383–402 (doi:10.1079/BJN19610048) [DOI] [PubMed] [Google Scholar]
- 42.Steuer P, Sudekum KH, Muller DWH, Franz R, Kaandorp J, Clauss M, Hummel J. 2011. Is there an influence of body mass on digesta mean retention time in herbivores? A comparative study on ungulates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160, 355–364 (doi:10.1016/j.cbpa.2011.07.005) [DOI] [PubMed] [Google Scholar]
- 43.Scurlock JMO, Johnson K, Olson RJ. 2002. Estimating net primary productivity from grassland biomass dynamics measurements. Glob. Change Biol. 8, 736–753 (doi:10.1046/j.1365-2486.2002.00512.x). [Google Scholar]
- 44.McNaughton SJ, Milchunas DG, Frank DA. 1996. How can net primary productivity be measured in grazing ecosystems? Ecology 77, 974–977 (doi:10.2307/2265518) [Google Scholar]
- 45.Tamminga S, Aarts F, Bannink A, Oenema O, Monteny GJ. 2004. Actualisering van geschatte N en P excreties door rundvee. Wageningen, The Netherlands: Milieu en Landelijk gebied; 25; 48 p [Google Scholar]
- 46.Schoelynck J, Müller F, Vandevenne F, Bal K, Barão L, Smis A, Opdekamp W, Meire P, Struyf E. 2013. Silicon–vegetation interaction in multiple ecosystems: a review. J. Veg. Sci. (doi:10.1111/jvs.12055) [Google Scholar]
- 47.Cornelis JT, Delvaux B, Georg RB, Lucas Y, Ranger J, Opfergelt S. 2011. Tracing the origin of dissolved silicon transferred from various soil–plant systems towards rivers: a review. Biogeosciences 8, 89–112 (doi:10.5194/bg-8-89-2011) [Google Scholar]
- 48.Hi C, Wang L, Liu J, Liu X, Li X, Ma J, Lin Y, Xu F. 2013. Evidence for ‘silicon’ within the cell walls of suspension-cultured rice cells. New Phytol. (doi:10.1111/nph.12401) [DOI] [PubMed] [Google Scholar]
- 49.Parr J, Sullivan L, Chen BH, Ye GF, Zheng WP. 2010. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Change Biol. 16, 2661–2667 (doi:10.1111/j.1365-2486.2009.02118.x) [Google Scholar]
- 50.Schaller J, Struyf E. 2013. Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 709, 201–212 (doi:10.1007/s10750-013-1449-1) [Google Scholar]
- 51.Kidder DL, Gierlowski-Kordesch EH. 2005. Impact of grassland radiation on the nonmarine silica cycle and miocene diatomite. PALAIOS 20, 198–206 (doi:10.2110/palo.2003.p03-108) [Google Scholar]
- 52.Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR. 2004. The evolution of modern eukaryotic phytoplankton. Science 305, 354–360 (doi:10.1126/science.1095964) [DOI] [PubMed] [Google Scholar]