Abstract
Coastal ecosystems that are characterized by kelp forests encounter daily pH fluctuations, driven by photosynthesis and respiration, which are larger than pH changes owing to ocean acidification (OA) projected for surface ocean waters by 2100. We investigated whether mimicry of biologically mediated diurnal shifts in pH—based for the first time on pH time-series measurements within a kelp forest—would offset or amplify the negative effects of OA on calcifiers. In a 40-day laboratory experiment, the calcifying coralline macroalga, Arthrocardia corymbosa, was exposed to two mean pH treatments (8.05 or 7.65). For each mean, two experimental pH manipulations were applied. In one treatment, pH was held constant. In the second treatment, pH was manipulated around the mean (as a step-function), 0.4 pH units higher during daylight and 0.4 units lower during darkness to approximate diurnal fluctuations in a kelp forest. In all cases, growth rates were lower at a reduced mean pH, and fluctuations in pH acted additively to further reduce growth. Photosynthesis, recruitment and elemental composition did not change with pH, but δ13C increased at lower mean pH. Including environmental heterogeneity in experimental design will assist with a more accurate assessment of the responses of calcifiers to OA.
Keywords: ocean acidification, natural pH fluctuations, coralline macroalgae, environmental heterogeneity, temperate rocky reefs
1. Introduction
Ocean acidification (OA), the process of sustained absorption of anthropogenically derived atmospheric CO2 by the world's oceans [1], is predicted to cause large-scale changes in many marine ecosystems [2]. This absorption of CO2 has already led to significant changes to the seawater carbonate system and is predicted to cause a decrease in pH of 0.3–0.5 units by the end of the century [3]. If pH is reduced by 0.4 units, these changes are expected to reduce [CO32−] by 30%, increase [HCO3−] by 9% and increase [H+] by approximately 200% [3,4]. OA is also predicted to decrease the net calcification and/or increase dissolution of many organisms that build CaCO3 structures [5].
In the open ocean, pH does not vary greatly in time and space, making laboratory simulations of future pH levels relatively straight forward [6]. By contrast, near-shore marine organisms live in a highly variable pH environment where daily pH fluctuations owing to biological activity can exceed 1 unit [6–16]. These changes are often driven by primary producers increasing pH in the surrounding seawater during the day via photosynthesis, and decreasing pH at night owing to respiration [15]. In some regions, night-time decreases in pH (to less than 7.4; [17]) exceed those predicted owing to OA over the next 100 years (pH ∼ 7.65; [3]).
Currently, it is not known how daily shifts in pH within near-shore ecosystems influence the physiology or the ecology of calcifying organisms, nor is it understood how these pH fluctuations could interact with the effects of OA. It is difficult to reproduce the environmental heterogeneity that occurs in the field within a laboratory setting. For example, experimental manipulations of light and temperature in experiments with marine species usually use tightly controlled continuous levels, even though these environmental factors are much more variable in the field [18,19]. To date, only one study has manipulated pH over a diurnal cycle mimicking ecologically relevant pH shifts (daytime pH = 8.00, night-time pH = 7.77) to examine short-term (3–6 day) effects on coral recruits [20]. Although this study [20] did not report the pH variability occurring naturally in the organism's habitat, they found that in some instances coral recruits responded positively to both daily fluctuations in pH and to OA. Incorporating such daily fluctuations in pH/CO2 into OA manipulation studies is the next step in better identification of the responses of near-shore species to climate change-mediated alteration of pH conditions.
Periods of high pH/low CO2 could potentially ameliorate some of the negative effects of OA on calcifying organisms [12,15,20], by providing them with a period of respite where they can calcify at much higher rates [9]. This study investigated the interactive effects of daily fluctuations in pH that simulate biological activity, and the long-term decline in seawater pH predicted owing to OA on the growth and physiology of the coralline macroalga Arthrocardia corymbosa, a member of a genus that is found in temperate regions of both the Northern and Southern Hemispheres [21,22]. Arthrocardia corymbosa was incubated in pH treatments representative of typical surface seawater today and seawater pH predicted for 2100 in static conditions that are characteristic of oceanic waters. Both of these treatments also included diurnal fluctuations in pH that more accurately mimicked the amplitude of pH change typical of coastal waters dominated by macroalgae than previous research investigating the effects of OA on marine organisms. The magnitude of pH change within incubations was based on pH changes measured in situ in a kelp forest (from where A. corymbosa was collected) and from published oceanic measurements [6,23]. The aim of the laboratory incubations was to determine whether elevated daytime pH (similar to that observed in regions influenced by macroalgal photosynthesis) could provide a period where high calcification rates could compensate for the overall decline predicted to occur because of lowering seawater pH owing to OA.
2. Material and methods
(a). Monitoring in situ pH fluctuations
An ENVCO pHTempion combined pH and temperature logger and was placed within a Macrocystis pyrifera forest (1–2 m depth) at Karitane, near Dunedin, South Island, New Zealand (45°38'20" S, 170°40'15" E) for 4–5 days in each season between 4 April 2010 and 27 May 2011. For a description of the macroalgal community composition and underwater irradiance at the study site, see Hepburn et al. [24]. pH was measured on the total scale (pHT).
(b). Macroalgal collection
Thirty assemblages of the articulate coralline alga A. corymbosa were collected on 13 March 2011 from the same M. pyrifera forest in which the coastal pH data were recorded. Each assemblage contained 10 individual A. corymbosa (40 mm high) on a small base of crustose coralline algae. Six of these assemblages were sacrificed to assess the physiological status of the macroalgae at the start of the experiment (hereafter, ‘initial’ samples).
(c). Experimental design and seawater carbonate chemistry
The remaining 24 assemblages were placed into one of four pH treatments for 40 days. pH treatment levels were multifactorial with two mean pH levels measured on the total scale (pHT 8.05 and 7.65). For each level of mean pH, there were two levels of pH variability. In one treatment, pH was held constant. In a second treatment, pH fluctuated around the mean and was 0.4 pH units higher than the mean during the day and 0.4 units lower than the mean during the night. The target mean pH within each treatment was achieved using a modified version of the pH-controlled automated culture system described by McGraw et al. [25], in which pH is measured spectrophotometrically to ±0.01 units accuracy and controlled within 0.03 units. The mean pH in the ambient seawater treatments (8.05) was selected to represent unmodified seawater from an oceanic site 68.5 km offshore (8.05) [23], a value that is very similar to that considered representative of the global mean pH of surface waters in the best practices guide (8.065) [3].
Seawater (salinity 34 SA and pH 8.05) was collected from Otago Harbour, South Island, New Zealand (45°52.51’ S, 170°30.9’ E) every 6 days and stored in a 1000 l tank. Mean nitrate and phosphate concentrations were 1.16 ± 0.07 and 0.24 ± 0.01 μmol l−1, respectively, throughout the experiment (see the electronic supplementary materials for methodological details). Twice a day, a 150 l storage tank was filled with seawater filtered using Filter Pure polypropylene spun melt (0.5 µm pore size) and ultraviolet sterilized with an Aquastep 25 W Ultraviolet Sterilizer. The pH of the seawater in the 150 l storage tank was increased to pHT 8.45 using NaOH additions. This was necessary because the pH of seawater from the seawater collection site was 8.05, but seawater with a higher pH was needed to achieve the daytime pH values in the fluctuating mean pH 8.05 treatment (pH 8.45). All other treatments were subsequently achieved by reducing the pH using the methods detailed below. This system was housed in a walk-in temperature controlled room at 10.8°C and under a mean irradiance of 18 μmol m−2 s−1 photon flux density 12 L : 12 D ratio.
Each of the 24 A. corymbosa assemblages was cultured separately in a 650 ml Perspex flow-through culture chamber. Each chamber was attached to the outflow of an individual 1 l Perspex header tank. The pH in each header tank was controlled by automatically refilling the tank with seawater from a 1 l mixing tank. Target pHT levels were achieved in the mixing tank by adding exactly equal amounts of HCl and NaHCO3 (a process chemically identical to adding CO2) [26] to 1 l of seawater that was pumped from the 150 l storage tank. Before the newly mixed seawater was transferred to the appropriate header tank, pHT was measured at 10.8°C using the automated spectrophotometric system. If the measured pH was within 0.03 units of the target pHT, the seawater was transferred to the appropriate 1 l header tank. If the pH varied more than 0.03 pH units from the target value, the seawater in the mixing tank was sent to waste and the process repeated until the correct pH was achieved. Using this method, the automated system delivered new seawater at the target pH to each of the 24 header tanks approximately every 4.4 h. The order in which the seawater was refreshed in each of the 24 treatment chambers was randomly allocated at the beginning to avoid potential artefacts.
Arthrocardia corymbosa assemblages were individually tied with nylon to circular Perspex plates, each of which had six holes (1 cm diameter) to allow seawater to flow around the macroalgae. These plates were located 5 mm above the bottom of the culture tank. To minimize the thickness of diffusion boundary layers that can form at the surface of macroalgae and cause large differences in pH between an organism's surface and the bulk seawater [8], culture chambers were individually placed on a magnetic stirrer (Ika Squid, Global Science) at 450 r.p.m. and a 25 mm long stirrer bar was placed into each of the culture chambers. In order to monitor changes in seawater pH owing to biological activity within the culture tank, additional spectrophotometric pH measurements were made within each culture chamber at 06.00 (night) and 18.00 h (day) on day 1, and every 5 days thereafter. On days 1 and 40, 500 ml samples were taken from each culture chamber at 06.00 and 18.00 h and used to determine AT (see the electronic supplementary material for details).
(d). Biotic responses
Macroalgal growth was measured by weighing algae at the beginning of the experiment and again after 40 days, and then converting this into growth rate relative to the initial blotted wet weight [27]. On day 11 of the experiment, recruitment of juvenile A. corymbosa was visible. Recruitment onto the Perspex plates that were located under the algal assemblages was measured on day 40 by photographing the plates and sub-sampling a random 10 × 10 mm2 in which the number of individuals was counted using the software ImageJ 1.42q [28].
The rates of gross photosynthesis and respiration of the A. corymbosa assemblages in each of the culture chambers were measured non-invasively between 16.00 and 18.00 h in light and between 06.00 and 08.00 h in the darkness on days 1, 10, 30 and 40. A Presens 50 µm oxygen microoptode measured linear changes in oxygen concentrations over time through a purpose-built aperture. Seawater flow into and out of the cell was halted for 5 min, while oxygen evolution or consumption was recorded. Gross photosynthetic rates were calculated using linear regression then standardized to algal wet weight, and net rates were determined by deducting respiration. Algal wet weight after day 1 and before day 40 was estimated using a linear regression between the two time points for the purposes of the calculations.
The ratio of variable (Fv) to maximal (Fm) quantum yield of photosystem II (Fv/Fm) of all mature, experimental A. corymbosa was measured on days 1 and 40 using a Pulse Amplitude Modulated (PAM) chlorophyll fluorescence meter (Diving PAM, Walz, Germany). Fv/Fm was measured on A. corymbosa individuals that had been dark adapted for 15 min. This model PAM has a red-light-emitting diode, and both gain and dampening were set to 2. On all occasions, Fo was greater than 130 before measurements were made.
Pigment concentrations were determined for the six initial A. corymbosa on day 1 and for each experimental macroalga on day 40 following the exact methods of Sampath-Wiley & Neefus [29] for phycobillins (phycocyanin, PC and phycoerythrin, PE), and Richie [30] using ethanol extraction for chlorophyll (Chl) a.
%C, %N, δ13C and δ15N were analysed from the organic tissue of the initial and experimental macroalgae by removing all inorganic tissue in 1 M HCl then drying at 80°C and grinding samples in a mortar and pestle. Sub-samples were then combusted in a CE NA1500 Elemental Analyzer (Carlo-Erba instruments) interfaced to a Europa Scientific 20–20 update continuous flow mass spectrometer. Corrections for drift were made automatically every five samples from an EDTA standard with a known isotope ratio. Inorganic δ13C was also sampled in the same way, but the organic tissue was first removed with bleach [31]. The initial and treatment A. corymbosa were also analysed for Ca, Mg, Sr and Mn content. Dried samples were dissolved in concentrated HNO3 in Savillex vessels. The analytes were quantified against multi-element standards on an Agilent 7500ce ICP-MS using a helium collision cell following the manufacturer's recommendations.
(e). Statistical analyses
Relative growth rates, recruitment, pigments (PE, PC and Chl a) and %Ca, %Mg, %MgCO3, %C, %N, δ13C and δ15N were analysed using a two-way analysis of variance (ANOVA) with pH mean and pH variability classed as factors in the model, each with two levels (pH 8.05, pH 7.65; and static, fluctuating, respectively). The interaction between the two factors was also included in the model. Fv/Fm was analysed as a repeated measures ANOVA, with time as the random factor and pH mean and pH variability as the fixed factors along with the interaction terms. Photosynthetic rates were analysed in the same way. All data used in univariate analyses were analysed for homoscedasticity and normality. Recruitment data failed this assumption and were log (X + 1) transformed. When p-values under 0.05 were detected, Tukey honestly significant difference (HSD) post hoc tests were used to determine differences between treatments. All statistical analyses were performed in R v. 2.7.0 [32].
3. Results
(a). In situ and experimental pH
pH variability within the M. pyrifera kelp bed ranged by 0.94 units (7.92–8.86) over 5 days in the austral summer (figure 1). The average pH within the kelp bed was highest in the summer (8.43), lowest in the winter (7.93) and was 8.32 on average. Clear diurnal fluctuations in kelp bed pH were evident with values decreasing at night and increasing to a peak around noon (figures 1 and 2). pH within the culture tanks that housed A. corymbosa were close (within 0.05 units) to the target pH means (means and standard error shown in table 1, for a visualization, see figure 2a,b) during the night and the day. These values corresponded to pCO2 concentrations of 145 μatm for pH 8.45, 415 μatm for pH 8.05, 1130–1150 µatm for pH 7.65 and 2960 µatm for pH 7.25. As expected, AT remained relatively constant across pH treatments. For other carbonate parameters, see table 1.
Figure 1.
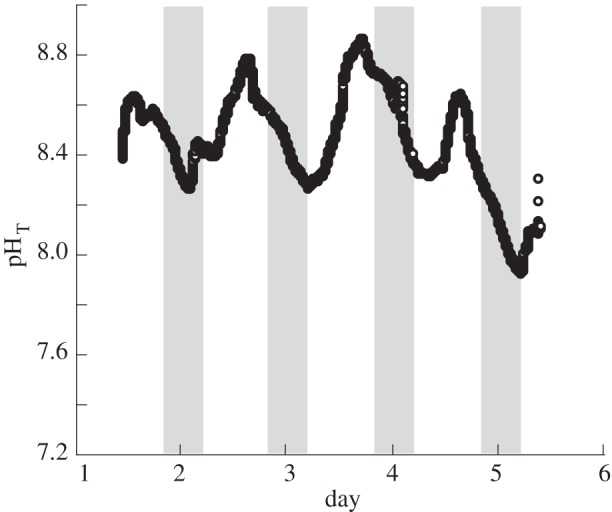
Summertime pHT measurements over 5 days within a kelp bed located in 1–2 m water depth. Grey bars indicate periods of darkness.
Figure 2.
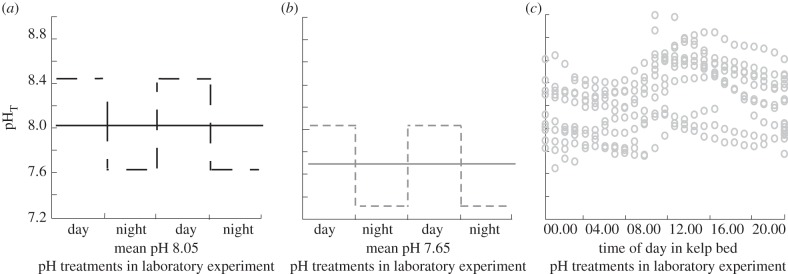
Mean daytime and night-time pH within the mixing tanks in the laboratory experiment showing the pH treatments contrasted against the conditions in the field. (a) Mean seawater pHT supplied to the ambient pH 8.05 static (±0.01) (solid line) and fluctuating (±0.40) (dotted line) treatments during the day and the night. (b) Mean seawater pHT supplied to the OA scenario pH 7.65 static (±0.01) and fluctuating (±0.40) treatments during the day and the night. The standard error for each mean = 0.01. (c) pH values within the kelp bed habitat (1–2 m depth) over 20 days between April 2010 and May 2011.
Table 1.
Carbonate parameters (mean±s.e.) during the day and night in the 650 ml culture tanks containing individuals of A. corymbosa in each of the four pH treatments. AT was measured in water taken from the culture tank (n = 12), and pHT was measured in both the mixing tank (n = 48) and culture tank (n = 528). Remaining parameters were calculated from pH and AT from the culture tank at a temperature of 10.8°C and salinity of 34.3 using the Mehrbach equilibrium constants as refit by Dickson & Millero [33]. S indicates static pH treatments, while F indicates fluctuating pH treatments.
| time |
day |
night |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| carbonate chemistry | pHT-mixing tank | pHT-culture tank | AT (μmol kg−1) | DIC (μmol kg−1) | pCO2 (μatm) | CO3 2− (μmol kg−1) | HCO3 − (μmol kg−1) | pHT-mixing tank | pHT-culture tank | AT (μmol kg−1) | DIC (μmol kg−1) | pCO2 (μatm) | CO3 2− (μmol kg−1) | HCO3 − (μmol kg−1) |
| pH 8.05 S | 8.05±0.01 | 8.04±0.01 | 2420±6 | 2220±2 | 415±0.3 | 143±0.1 | 2060±2 | 8.04±0.01 | 8.00±0.01 | 2420±6 | 2230±1 | 422±0.2 | 142±0.1 | 2070±1 |
| pH 8.05 F | 8.43±0.01 | 8.39±0.01 | 2440±5 | 2030±2 | 145±0.1 | 289±0.3 | 1730±2 | 7.65±0.01 | 7.65±0.01 | 2400±5 | 2360±2 | 1150±0.8 | 62±0.1 | 2250±2 |
| pH 7.65 S | 7.65±0.01 | 7.68±0.01 | 2400±6 | 2360±2 | 1130±1 | 63±0.1 | 2250±2 | 7.65±0.01 | 7.64±0.01 | 2400±6 | 2360±2 | 1130±1 | 62±0.1 | 2240±2 |
| pH 7.65 F | 8.05±0.01 | 8.02±0.01 | 2420±1 | 2220±1 | 410±0.3 | 144±0.1 | 2060±1 | 7.25±0.01 | 7.32±0.02 | 2380±1 | 2470±1 | 2960±2 | 25±0.1 | 2320±2 |
(b). Effects of pH on growth rates
Decreasing the mean pH and increasing the variability in pH both resulted in reduced growth rates for A. corymbosa over the 40 day experiment (pH mean F1,20 = 9.24, p = 0.006 and pH variability F1,20 = 9.82, p = 0.005; figure 3a). The highest growth rates occurred in the pH 8.05 static treatment and the lowest rates in the pH 7.65 fluctuating treatment. Compared with macroalgae in the pH 8.05 static treatment, growth rates were 62% lower in the pH 7.65 static treatment, 64% lower in the pH 8.05 fluctuating treatment and 98% lower in the pH 7.65 fluctuating treatment. There was no interaction between the effects of daily fluctuations in pH and the mean pH levels used (F1,20 = 0.50, p = 0.49; figure 3a).
Figure 3.
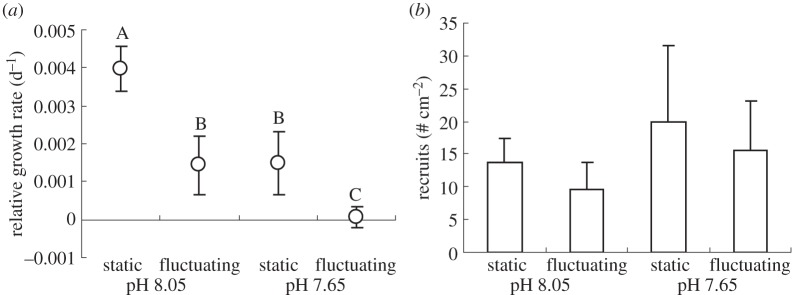
Biotic responses of the articulated coralline macroalga A. corymbosa exposed to one of four pH treatments for 40 days (see table 1 and figure 3 for pH conditions). (a) Relative growth rates and (b) recruitment of A. corymbosa. Treatments sharing the same letter in (a) are not statistically different (Tukey's HSD p ≤ 0.05), whereas in (b) there were no treatment effects. Error bars represent standard error, n = 6.
(c). Recruitment of juvenile coralline algae
Juveniles recruited onto the Perspex plates beneath the adults throughout the experiment, and at day 40 the number of visible recruits was counted. There were between 10 and 20 recruits cm−2 and mean pH treatment, level of pH variability, and the interaction between the two factors did not influence the number of recruits (figure 3b; F1,20 < 0.21, p > 0.65 on all occasions).
(d). Effects of pH on other biotic responses
There was no effect of decreased mean pH or increased variability in pH on rates of photosynthesis, Fv/Fm, pigment concentrations, nor any other measured physiological response of A. corymbosa. Net photosynthetic rates were not different between treatments at any given time point, nor was there any difference among treatments over time (Tukey's HSD all p > 0.11; table 2). Mean Fv/Fm was greater than 0.57 (indicative of photosynthetically healthy coralline algae) [34] both before and after the 40 day experiment in all treatments (see electronic supplementary material, table S1). Inorganic material accounted for 59–64% of the total dry weight and there was no effect of mean pH, the level of pH variability or the interaction between the two factors on the %Ca (F1,20 < 0.48, p > 0.50), %Mg (F1,20 < 0.26, p > 0.62), the %MgCO3 (F1,18 < 3.93, p > 0.06) nor in the inorganic δ13C between treatments (F1,20 < 2.04 p > 0.17) (see electronic supplementary material, table S2). Pigment concentrations, C : N ratio, and the δ15N of the organic tissue did not vary with mean pH treatment, nor the level of pH variability (p > 0.18 on all occasions, see electronic supplementary material, tables S3 and S4). There was a statistically significant decrease (2‰) in organic δ13C signatures between pH 8.05 and 7.65 treatments (see electronic supplementary material, table S4).
Table 2.
Gross photosynthetic rates (μmol O2 g−1 s−1) measured at 14.00, on four days during the experiment. S indicates static pH treatments, while F indicates fluctuating pH treatments. n = 6, mean ± s.e.
| time | day 1 | day 10 | day 30 | day 40 |
|---|---|---|---|---|
| pH 8.05 S | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.05 ± 0.02 |
| pH 8.05 F | 0.03 ± 0.01 | 0.02 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| pH 7.65 S | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.02 |
| pH 7.65 F | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 |
4. Discussion
This study demonstrates that diurnal variability in pH, similar to that occurring within coastal systems, is an important factor controlling the growth rates of calcifying organisms in today's ocean and may have significant implications for predicting the responses of coastal calcifiers to OA. Coralline macroalgae grown in seawater with diurnally fluctuating pH (with pH higher during the day and lower at night) had significantly lower growth rates than the equivalent treatments with constant pH. The response of coralline macroalgae to OA under the fluctuating treatment was stronger than would be predicted under static conditions alone, as the absolute growth rates were even further reduced by the additive negative effects of diurnal fluctuations in pH. In addition, no other diagnostic that provides a measure of organism fitness was influenced by pH treatment, except organic δC13, which increased under both lower mean pH treatments, indicating an increase in the use of diffusive CO2 [35,36]. Increased variability in pH did not act to ameliorate the longer term effects of OA (at least over 40 days) as was hypothesized, but amplified OA's negative influence on growth. This reduction in growth was most probably owing to dissolution of calcareous structures during exposure to low pH during the night (discussed below).
The response of A. corymbosa in this study indicates that exposure to the extremes of naturally fluctuating pH is potentially as important as mean decreases in pH owing to OA. The negative response of A. corymbosa to fluctuating pH is opposite to our initial predictions, and different to the response of coral recruits to fluctuations in pH [20]. The growth rate of Seriatopora caliendrum recruits over 3–6 days was higher under a diurnally fluctuating pH treatment (pH 8.00 during the day and pH 7.77 at night) and a static low pH of 7.77, compared with a static pH 8.00 treatment [20]. Differential responses of marine organisms to diurnal fluctuations in other environmental factors have also been reported. For example, some adult corals (Pocillopora meandrina and Porites rus) respond negatively to fluctuations in temperature [37], whereas the adult corals Pocillopora damicornis and Seriatopora hystrix showed no response to fluctuating temperature, but their larvae responded positively [18,37]. Taken together, these studies indicate that responses to short-term changes in environmental variables can be complex and the direction and magnitude of these effects may be species- (or even life-stage) specific. Short-term local variability in environmental factors driven by natural processes, such as the diurnal fluctuations in primary production highlighted in our study, may play a more important role than the long-term global changes predicted to occur owing to anthropogenic processes [38].
Arthrocardia corymbosa showed little response to the effects of OA compared with the responses of tropical and subtropical coralline macroalgae, where static low pH had an adverse effect on coralline macroalgal pigments, photosynthetic rates [39,40] and recruitment [41,42]. The lack of negative physiological responses to pH shown here (apart from growth) could be owing to the lower temperature (10.8°C) employed in our experiment compared with other studies. Higher temperatures exacerbate the negative impacts of OA [39,43], and experiments where widespread bleaching (i.e. mortality) or lower recruitment are reported were conducted at temperatures more than or equal to 19°C [39,41,43,44]. Another explanation for the lack of physiological responses observed in this study is that the negative responses attributed to OA by previous research could be an artefact of inappropriate methods of pH manipulation (e.g. HCl [26]), or inappropriate of control of pH (i.e. high pH variability and/or no measurement of pH within the culture tank) [40,41]. Measured pH within culture tanks can be very different to that of the inflowing seawater encountered by experimental macroalgae, as photosynthesis and respiration can alter pH both within the mainstream seawater in culture tanks [45,46] and within the diffusion boundary layer around macroalgae [8,15]. We recommend that studies need to measure pH within culture tanks, provide adequate mixing/exchange of seawater and report these details sufficiently.
An alternate explanation of the lack of physiological responses of A. corymbosa—and potentially other species inhabiting similar coastal habitats [47,48]—to OA is that they may be tolerant of the effects of changes in pH because they regularly encounter daily shifts in pH in the field. Populations of organisms that contend with regular fluctuations in an environmental variable are often more able to adapt to permanent changes in that variable [49], owing to increased retention of phenotypic plasticity that is sometimes lost by organisms residing in relatively static environmental conditions [50,51]. By regularly encountering variable pH conditions, organisms from coastal systems may be more tolerant to the effects of continual periods of low pH caused by OA [15,52].
Our in situ pH measurements indicate that metabolic activity not only causes increased variability in pH within kelp forest habitats, but it may also lead to an increase in the mean pH and a decrease in the mean pCO2 during periods of high light and primary productivity (i.e. summer). Our pH treatment of 8.05 was selected to represent the current mean pH of the world's oceans, as recommended by the best practices guide; in future experiments, a mean pH which is representative of that within kelp beds could be used (e.g. with a higher mean pH). Furthermore, while the fluctuations in pH used here more accurately mimicked the largest amplitude of pH change occurring in the field (±0.89 units over 24 h) than in previous studies, in a future high CO2 ocean, the night-time reductions in pH owing to respiration may be less than that used here (pH 7.25). Measurements of pH at the surface of macroalgae indicate that respiration-driven reductions in pH in the dark are lower in seawater simulating an OA scenario (approx. 0.10 units) than they are in ambient seawater (approx. 0.25–0.50 units), most probably because more CO2 is needed to further lower pH as the concentrations increase [8]. This means that future pH fluctuations encountered by macroalgae may not be symmetrical around the daily mean, as in the treatments used here, and that macroalgal metabolism is even more likely to raise mean pH during periods of high irradiance under a future OA scenario. The investigation of these phenomena was outside the scope of this study, but could be an important focus for future investigations examining the buffering capacity of biologically active coastal ecosystems against pH change owing to OA.
Further investigations examining the role that OA will play in influencing ecological and physiological processes will be strengthened if they acknowledge that natural variability in pH (both within the habitat and at the surface of the organism, i.e. within the boundary layer) could influence these processes to the same extent as OA. The variability in pH at our coastal site is an order of magnitude larger that recorded at open ocean sites where pH varied by 0.025 units over 30 days [6] and pH derived from pCO2 varied by 0.08 units over 13 years [23]. This is consistent with pH measurements made in other coastal environments globally [6,10]. We demonstrate that these daily fluctuations in pH can influence the growth of calcifiers and suggest that realistically fluctuating pH levels during OA experiments on coastal species is important for future research. While this study is the first to attempt to mimic in situ observed changes in the magnitude of pH change, and the duration of exposure to high/low pH, this approach will be refined in future experiments. This will be achieved by allowing ecologically realistic assemblages of macroalgae to alter pH within the experimental set-up themselves, or by using culture systems such as ours [25] that are capable of altering pH on an hourly basis to accurately simulate conditions measured in the field.
To make accurate predictions regarding how climate change will influence marine organisms, we need to first understand how variability in local environmental conditions influence species in today's seas at both local scales and larger, regional scales [53]. Using coastal species as an example, the pH variability that an individual is exposed to will depend on a variety of environmental factors that each vary on different scales. At small scales (millimetres to metres), the organism's light environment or flow conditions will influence the pH encountered [15]. At larger scales (hundreds of metres to kilometres), the distance from inputs of low pH freshwater, or the degree of upwelling encountered at a particular locality, will influence the regional pH conditions [54,55]. Studies that detail pH variability at the level of the organism, and then determine how frequently such variability is encountered across larger geographical scales [56], will help broaden our understanding of the present-day effects of pH variability on specific marine organisms. Our demonstration of the fundamental role that pH variability can play in an organism's response to OA illustrates that an understanding of in situ pH variability and its biological consequences is required to reasonably predict how near-shore ecosystems will respond to longer term changes in oceanic properties.
Acknowledgements
We thank Peter Dillingham for statistical advice; Rochelle Dewdney, Aimee Pritchard and Stewart Bell for assistance in the field; and Malcolm Reid, Christine Davies and Abby Smith for laboratory assistance. We also thank Sean Connell, David Schiel and an anonymous reviewer for comments on previous versions of this manuscript.
Funding statement
C.E.C. was supported by a University of Otago Doctoral Scholarship and a University of Otago Postgraduate Publishing Bursary; C.L.H. was funded by a Royal Society of New Zealand Marsden grant (UOO0914), a Performance Based Research Fund from the Department of Botany, University of Otago and a University of Otago Research Grant.
References
- 1.Caldeira K, Wickett ME. 2003. Anthropogenic carbon and ocean pH. Nature 425, 365 (doi:10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 2.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 3.Riebesell U, Fabry VJ, Hansson L, Gattuso J-P. 2010. Guide to best practices for ocean acidification research and data reporting, p. 260 Luxembourg: Publications Office of the European Union [Google Scholar]
- 4.Orr JC, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 5.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (doi:10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 6.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 (doi:10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleboe AL, Hansen PJ. 2007. High pH in shallow-water macroalgal habitats. Mar. Ecol. Prog. Ser. 338, 107–117 (doi:10.3354/meps338107) [Google Scholar]
- 8.Cornwall CE, Hepburn CD, Pilditch CA, Hurd CL. 2013. Concentration boundary layers around complex assemblages of macroalgae: implications for the effects of ocean acidification on understorey coralline algae. Limnol. Oceanogr. 58, 121–130 [Google Scholar]
- 9.Semesi IS, Beer S, Björk M. 2009. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 382, 41–47 (doi:10.3354/meps07973) [Google Scholar]
- 10.Pelejero C, Calvo E, Hoegh-Guldberg O. 2010. Paleo-perspectives on ocean acidification. Trends Ecol. Evol. 25, 332–344 (doi:10.1016/j.tree.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 11.Delille B, Borges AV, Delille D. 2009. Influence of giant kelp beds (Macrocystis pyrifera) on diel cycles of pCO2 and DIC in the Sub-Antarctic coastal area. Estuar. Coast Shelf Sci. 81, 114–122 (doi:10.1016/j.ecss.2008.10.004) [Google Scholar]
- 12.Anthony KRN, Kleypas JA, Gattuso J-P. 2011. Coral reefs modify their seawater carbon chemistry—implications for impacts of ocean acidification. Glob. Change Biol. 17, 3655–3666 (doi:10.1111/j.1365-2486.2011.02510.x) [Google Scholar]
- 13.Kleypas JA, Anthony KRN, Gattuso J-P. 2011. Coral reef modify their seawater carbon chemistry—case study from a barrier reef (Moorea, French Polynesia). Glob. Change Biol. 17, 3667–3678 (doi:10.1111/j.1365-2486.2011.02530.x) [Google Scholar]
- 14.de Beer D, Larkum AWD. 2001. Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant Cell Environ. 24, 1209–1217 (doi:10.1046/j.1365-3040.2001.00772.x) [Google Scholar]
- 15.Hurd CL, Cornwall CE, Currie KI, Hepburn CD, McGraw CM, Hunter KA, Boyd P. 2011. Metabolically-induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Glob. Change Biol. 17, 3254–3262 [Google Scholar]
- 16.Morris S, Taylor AC. 1983. Diurnal and seasonal variation in physico-chemical conditions within intertidal rock pools. Estuar. Coast Shelf Sci. 17, 339–355 (doi:10.1016/0272-7714(83)90026-4) [Google Scholar]
- 17.Wootton JT, Pfister CA, Forester JD. 2008. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl Acad. Sci. USA 105, 18 848–18 853 (doi:10.1073/pnas.0810079105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnam HM, Edmunds PJ. 2011. The physiological response of reef corals to diel fluctuations in seawater temperature. J. Exp. Mar. Biol. Ecol. 396, 216–223 (doi:10.1016/j.jembe.2010.10.026) [Google Scholar]
- 19.van de Poll WH, Visser RJW, Buma AGJ. 2007. Acclimation to a dynamic irradiance regime changes excessive irradiance sensitivity of Emiliania huxleyi and Thalassiosira weissflogii. Limnol. Oceanogr. 52, 1430–1438 (doi:10.4319/lo.2007.52.4.1430) [Google Scholar]
- 20.Dufault AM, Cumbo VR, Fan T-Y, Edmunds PJ. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B 279, 2951–2958 (doi:10.1098/rspb.2011.2545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martone PT, Lindstrom SC, Miller KA, Gabrielson PW. 2012. Chiharaea and Yamadaia (corallinales, rhodophyta) represent reduced and recently derived articulated coralline morphologies. J. Phycol. 48, 859–868 [DOI] [PubMed] [Google Scholar]
- 22.Dye AH. 1993. Recolinization of intertidal macroalgae in relation to gap size and molluscan herbivory on a rocky shore on the east coast of southern Africa. Mar. Ecol. Prog. Ser. 95, 263–271 (doi:10.3354/meps095263) [Google Scholar]
- 23.Currie KI, Reid MR, Hunter KA. 2011. Interannual variability of carbon dioxide drawdown by subantarctic surface water near New Zealand. Biogeochemistry 104, 23–34 (doi:10.1007/s10533-009-9355-3) [Google Scholar]
- 24.Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob. Change Biol. 17, 2488–2497 (doi:10.1111/j.1365-2486.2011.02411.x) [Google Scholar]
- 25.McGraw CM, Cornwall CE, Reid MR, Currie KI, Hepburn CD, Boyd P, Hurd CL, Hunter KA. 2010. An automated pH-controlled culture system for laboratory-based ocean acidification experiments. Limnol. Oceanogr. Methods 8, 686–694 (doi:10.4319/lom.2010.8.686) [Google Scholar]
- 26.Gattuso J-P, Gao K, Lee K, Rost B, Schulz KG. 2010. Approaches and tools to manipulate the carbonate chemistry. In Guide to best practices for ocean acidification research and data reporting (eds Riebesell U, Fabry VJ, Hansson L, Gattuso J-P.), pp. 41–51 Luxembourg: Publications Office of the European Union [Google Scholar]
- 27.Han T, Kain JM. 1996. Effect of photon irradiance and photoperiod on young sporophytes of four species of the Laminariales. Eur. J. Phycol. 31, 233–240 (doi:10.1080/09670269600651431) [Google Scholar]
- 28.Rasband WS. 1997. ImageJ. Bethesda, MD: National Institutes of Health [Google Scholar]
- 29.Sampath-Wiley P, Neefus CD. 2007. An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J. Appl. Phycol. 19, 123–129 (doi:10.1007/s10811-006-9118-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie RJ. 2008. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46, 115–126 (doi:10.1007/s11099-008-0019-7) [Google Scholar]
- 31.Lee D, Carpenter SJ. 2001. Isotopic disequilibrium in marine calcareous algae. Chem. Geol. 172, 307–329 (doi:10.1016/S0009-2541(00)00258-8) [Google Scholar]
- 32.R Core Development Team 2008. R: a language and environment for statistical computing. 2.7.0 edn. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 33.Dickson AG, Millero FJ. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (doi:10.1016/0198-0149(87)90021-5) [Google Scholar]
- 34.Harrington L, Fabricius K, Eaglesham G, Negri A. 2005. Synergistic effects of diuron and sedimentation on photosynthesis and survival of crustose coralline algae. Mar. Pol. Bull 51, 415–427 (doi:10.1016/j.marpolbul.2004.10.042) [DOI] [PubMed] [Google Scholar]
- 35.Raven JA, et al. 2002. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct. Plant Biol. 29, 355–378 (doi:10.1071/PP01201) [DOI] [PubMed] [Google Scholar]
- 36.Cornelisen CD, Wing SR, Clark KL, Bowman MH, Frew RD, Hurd CL. 2007. Patterns in the δ13C and δ15N signature of Ulva pertusa: interaction between physical gradients and nutrient source pools. Limnol. Oceanogr. 52, 820–832 (doi:10.4319/lo.2007.52.2.0820) [Google Scholar]
- 37.Putnam HM, Edmunds PJ. 2008. Responses of coral hosts and their algal symbionts to thermal heterogeneity. In Proc. 11th International coral Reef Symposium, pp. 393–397 Fort Lauderdale, FL [Google Scholar]
- 38.Joint I, Doney SC, Karl DM. 2011. Will ocean acidification affect marine microbes? ISME 5, 1–7 (doi:10.1038/ismej.2010.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105 17 442–17 446 (doi:10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao K, Zheng Y. 2010. Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis. Glob. Change Biol. 16, 2388–2398 (doi:10.1111/j.1365-2486.2009.02113.x) [Google Scholar]
- 41.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, MacKenzie FT. 2008. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117 (doi:10.1038/ngeo100) [Google Scholar]
- 42.Russell BD, Thompson JI, Falkenberg LJ, Connell SD. 2009. Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Glob. Change Biol. 15, 2153–2162 (doi:10.1111/j.1365-2486.2009.01886.x) [Google Scholar]
- 43.Martin S, Gattuso J-P. 2009. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Change Biol. 15, 2089–2100 (doi:10.1111/j.1365-2486.2009.01874.x) [Google Scholar]
- 44.Russell BD, Passarelli CA, Connell SD. 2011. Forecasted CO2 modified the influence of light in shaping subtidal habitat. J. Phycol. 47, 744–752 (doi:10.1111/j.1529-8817.2011.01002.x) [DOI] [PubMed] [Google Scholar]
- 45.Rost B, Zondervan I, Wolf-Gladrow D. 2008. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar. Ecol. Prog. Ser. 373, 227–237 (doi:10.3354/meps07776) [Google Scholar]
- 46.Cornwall CE, Hepburn CD, Pritchard DW, McGraw CM, Currie KI, Hunter KA, Hurd CL. 2012. Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. J. Phycol. 48, 137–144 (doi:10.1111/j.1529-8817.2011.01085.x) [DOI] [PubMed] [Google Scholar]
- 47.Egilsdottir H, Noisette F, Noël LM-LJ, Olafsson J, Martin S. 2013. Effects of pCO2 on physiology and skeletal mineralogy in a tide pool coralline alga Corallina elongata. Mar. Biol. 160, 2103–2112 (doi:10.1007/s00227-012-2090-7) [Google Scholar]
- 48.Kelly MW, Padilla-Gamińo JL, Hofmann GE. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Change Biol. 19, 2536–2546 (doi:10.1111/gcb.12251) [DOI] [PubMed] [Google Scholar]
- 49.Kussell E, Leiber S. 2005. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078 (doi:10.1126/science.1114383) [DOI] [PubMed] [Google Scholar]
- 50.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (doi:10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 51.Masel J, King OD, Maughan H. 2007. The loss of adaptive plasticity during long periods of environmental stasis. Am. Nat. 169, 38–46 (doi:10.1086/510212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eshel I, Matessi C. 1998. Canalization, genetic assimilation and preadaptation: a quantitative genetic model. Genetics 149, 2119–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reusch TBH, Boyd PW. 2013. Experimental evolution meets marine phytoplankton. Evolution 67, 1849–1859 (doi:10.1111/evo.12035) [DOI] [PubMed] [Google Scholar]
- 54.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in and carbonate saturation in an urbanized estuary. Estuar. Coast Shelf Sci. 88, 442–449 (doi:10.1016/j.ecss.2010.05.004) [Google Scholar]
- 55.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. 2008. Evidence for upwelling of corrosive ‘acidified’ water onto the coastal shelf. Science 320, 1490–1492 (doi:10.1126/science.1155676) [DOI] [PubMed] [Google Scholar]
- 56.Hofmann GE, et al. 2013. Exploring local adaptation and the ocean acidification seascape—studies in the California Current Large Marine Ecosystem. Biogeosci. Discuss. 10, 11 825–11 856 (doi:10.5194/bgd-10-11825-2013) [Google Scholar]


