Abstract
Cooperatively breeding animals live in social groups in which some individuals help to raise the offspring of others, often at the expense of their own reproduction. Kin selection—when individuals increase their inclusive fitness by aiding genetic relatives—is a powerful explanation for the evolution of cooperative breeding, particularly because most groups consist of family members. However, recent molecular studies have revealed that many cooperative groups also contain unrelated immigrants, and the processes responsible for the formation and maintenance of non-kin coalitions are receiving increasing attention. Here, I provide the first systematic review of group structure for all 213 species of cooperatively breeding birds for which data are available. Although the majority of species (55%) nest in nuclear family groups, cooperative breeding by unrelated individuals is more common than previously recognized: 30% nest in mixed groups of relatives and non-relatives, and 15% nest primarily with non-relatives. Obligate cooperative breeders are far more likely to breed with non-kin than are facultative cooperators, indicating that when constraints on independent breeding are sufficiently severe, the direct benefits of group membership can substitute for potential kin-selected benefits. I review three patterns of dispersal that give rise to social groups with low genetic relatedness, and I discuss the selective pressures that favour the formation of such groups. Although kin selection has undoubtedly been crucial to the origin of most avian social systems, direct benefits have subsequently come to play a predominant role in some societies, allowing cooperation to persist despite low genetic relatedness.
Keywords: cooperative breeding, parasitism, mating systems, unrelated, direct benefits
1. Introduction
Approximately 9% of the world's bird species breed in cooperative groups in which several individuals provide parental care to a single clutch of offspring [1]. In the majority of these cases, cooperative groups form when offspring from one brood remain on their natal territory to help raise younger relatives. Nesting groups are therefore primarily composed of family members, and non-breeding ‘helpers’ gain indirect fitness benefits by caring for non-descendent kin. In combination with ecological constraints on independent breeding, such as scarce mates or nesting sites, kin selection provides a powerful explanation as to why cooperatively breeding groups are typically composed of relatives (reviewed in [2,3]).
Social systems composed of unrelated individuals are rarer and more difficult to explain because kin selection cannot maintain their cooperative interactions. Classic avian examples include dunnocks, which nest in polygamous groups with several unrelated co-breeders [4], and pied kingfishers, which have both related and unrelated helpers at the nest [5,6]. However, recent molecular studies, particularly in Australia, Madagascar and the Neotropics, have uncovered a much broader diversity of cooperative breeding systems that involve complex alliances of relatives and non-relatives [7–9]. These cases raise intriguing evolutionary questions that are just beginning to be addressed. How do unrelated individuals assemble into stable social groups? Are these groups maintained by sexual conflict, mutual benefits, or a combination of both? Do societies of non-relatives share common evolutionary origins with kin-based societies, or have they arisen independently?
Despite increasing interest in—and controversy over—the evolutionary mechanisms that maintain non-kin cooperative breeding, its occurrence has not been systematically reviewed. Here, I provide the first survey of kin structure for cooperatively breeding birds based on the empirical literature. Data on genetic relationships among group members, drawn from either molecular genotyping or colour-ringing studies, were available for 213 species (approximately half of the 406 species that have been confirmed to be regular cooperative breeders). Cooperative breeding by unrelated individuals is surprisingly common: even under the most conservative estimate, 44% of species nest in social groups that regularly include unrelated adult helpers or co-breeders. Next, I identify three patterns of dispersal, recruitment and mortality that give rise to social groups with low genetic relatedness. Third, I review the selective pressures that favour the evolution of non-kin cooperation, arguing that in some circumstances, the direct fitness benefits of group membership—including increased survival, access to extra-pair copulations and future breeding opportunities—can equal or exceed the potential indirect fitness benefits derived from staying with kin. Finally, I discuss recent hypotheses for the evolutionary origins of non-kin cooperative breeding. The evidence discussed in this review suggests that non-kin cooperative breeding has arisen several times along separate evolutionary trajectories. In many cases, social groups with low genetic relatedness have evolved from lineages with a phylogenetic history of kin-based cooperation.
2. How common is cooperative breeding by unrelated individuals?
To estimate the occurrence of non-kin cooperative breeding, I used Cockburn's [1] review of avian parental care systems for all 9456 extant bird species. He classified 852 species as cooperative breeders, of which 390 species were suspected or inferred to be cooperative based on phylogenetic relationships. For all 852 species on this initial list, I searched the primary literature for information on the mating system and genetic relatedness of members of breeding groups. Data were available to describe the composition of breeding groups for 213 species, approximately 52% of the total (n = 406) for which cooperative breeding was confirmed to be a regular occurrence (see electronic supplementary material, table S1).
Cooperative species were broadly distributed into two groups with different social systems: those in which breeding was monopolized by a dominant pair and aided by non-breeding helpers (pair nesting), and those in which more than two breeding adults formed stable social groups and shared reproduction in a single clutch, with or without non-breeding helpers (cooperative polygamy). These two categories represent extremes of a continuum rather than an absolute dichotomy, because the extent of reproductive sharing varies greatly in both.
Nuclear family groups, consisting of a breeding pair and their related non-breeding helpers (typically retained offspring), account for the majority of cooperatively breeding species (55%; n = 118; figure 1). These species were defined as those in which unrelated helpers or co-breeders occur in fewer than 20% of groups in the population; or for which genetic data on group composition were not available, and group members were assumed to be relatives based on behavioural observations. Surprisingly, however, many pair-nesting species are regularly aided by a combination of related and unrelated non-breeding helpers (19%; n = 40). These species were defined as those in which at least 20% of helpers are unrelated to any of the breeders in the social group, such that providing alloparental care to unrelated young is a regular occurrence in the population. The remaining species (26%; n = 55) are cooperatively polygamous, breeding in stable social groups with at least three adults that share reproduction and parental care of the mixed clutch. These social groups are sometimes composed entirely of unrelated adults (n = 31), but many species nest in polygamous groups containing a mix of related and unrelated breeding adults, plus related and/or unrelated helpers (n = 24). When pair-nesting and polygamous species are combined, 55% (n = 118) nest primarily with kin, 15% (n = 31) nest primarily with non-kin, and 30% (n = 64) nest in groups that regularly contain both kin and non-kin.
Figure 1.
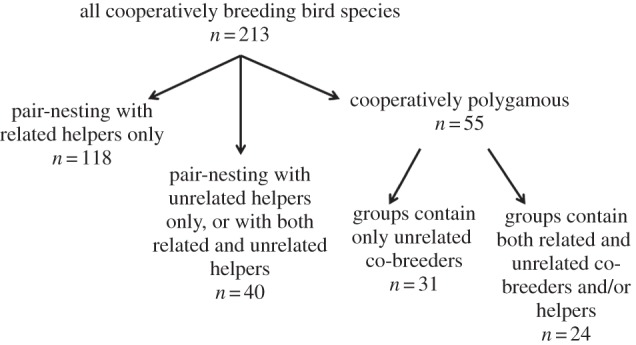
Classification of group composition and social mating system for all 213 species of cooperatively breeding birds for which data are available (see electronic supplementary material, table S1).
Several important patterns emerge from this dataset (see electronic supplementary material, table S1). First, non-kin cooperative breeding has originated many times independently in the avian phylogeny: of 62 taxonomic families in which cooperative breeding has been well described, 46 contain at least one species that breeds in non-kin groups. Social groups with mixed kin structures are particularly prevalent in families in which cooperative breeding is thought to have arisen many times, including the Psittacidae, Rallidae, Accipitridae and Timaliidae [1]. Non-kin cooperation also occurs significantly more frequently in species that are obligate cooperative breeders—species in which at least 95% of breeding units are groups rather than pairs—than in facultative cooperative breeders. Seventy-seven per cent of obligately cooperative species nest in groups that regularly contain non-kin (n = 24 of 31 species), whereas only 38% of facultatively cooperative species do (n = 68 of 181 species; two-way contingency test, χ2 = 17.1, p < 0.0001).
Consistent with previous analyses [10], I found that related non-breeding helpers are more likely to be male than female (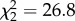 , p < 0.0001). Of 68 species that breed in kin-based groups for which helper sex is known, help is male-biased in 31 species and female-biased in only three, with both sexes helping in 35 species. However, helping by unrelated immigrants in groups with mixed kin structures is equally male-biased (
, p < 0.0001). Of 68 species that breed in kin-based groups for which helper sex is known, help is male-biased in 31 species and female-biased in only three, with both sexes helping in 35 species. However, helping by unrelated immigrants in groups with mixed kin structures is equally male-biased (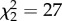 , p < 0.0001). This suggests that sex biases in helping behaviour are not solely a consequence of male philopatry or higher relatedness between group-living males, but may also reflect differences between males and females in their opportunities for direct reproduction. Males in polygamous groups also share reproduction more frequently than females do. Cooperative polyandry is relatively common, occurring in 55 of 63 polygamous species, whereas multiple females share reproduction in a single nest in only 23 of these (
, p < 0.0001). This suggests that sex biases in helping behaviour are not solely a consequence of male philopatry or higher relatedness between group-living males, but may also reflect differences between males and females in their opportunities for direct reproduction. Males in polygamous groups also share reproduction more frequently than females do. Cooperative polyandry is relatively common, occurring in 55 of 63 polygamous species, whereas multiple females share reproduction in a single nest in only 23 of these (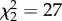 , p < 0.0001). This pattern is consistent with the hypothesis that reproductive sharing by females in the same nest may be limited by clutch size, whereas males may compete for paternity within a clutch without increasing the number of offspring [11].
, p < 0.0001). This pattern is consistent with the hypothesis that reproductive sharing by females in the same nest may be limited by clutch size, whereas males may compete for paternity within a clutch without increasing the number of offspring [11].
This analysis is, necessarily, an oversimplification: within these categories, social groups vary tremendously in their size, stability, reproductive skew and division of labour, and in the strength and nature of social bonds among group members. Nevertheless, it reveals that cooperative breeding by unrelated individuals is widespread in birds. In the following sections, I discuss how these social groups arise and why cooperative interactions may sometimes be favoured even in the absence of kinship.
3. How do non-kin social groups form?
Group formation is well described for species that breed as extended families: offspring typically remain on their natal territory and provide care to younger kin rather than dispersing to breed independently [3]. Alternatively, offspring may disperse only a short distance to breed independently, then return to a relative's territory to help if their nesting attempt is not successful. This pattern of limited dispersal results in local concentrations of relatives known as ‘kin neighbourhoods’ that can also facilitate kin-based cooperation [12]. However, much less is known about the dispersal and recruitment patterns that lead to groups with low genetic relatedness, and data on group formation are available for only a small minority of species. Here, I outline three paths to group formation that result in variable kin structures.
(a). Delayed dispersal with high mortality and/or promiscuous mating
Delayed dispersal does not necessarily result in close kinship among group members. High rates of adult mortality, copulations outside the social group, or conspecific brood parasitism can erode genetic relatedness to such low levels that the indirect fitness benefits of helping are negligible. These processes have been best described in the fairy-wrens (Malurus spp.), in which apparent ‘family’ groups often contain step-parents and extra-pair young. As a consequence, non-breeding helpers often provide care to unrelated nestlings [13]. Although this pattern was considered surprising when it was first described, recent genetic analyses have revealed similarly high rates of extra-group promiscuity and low relatedness in several other species (e.g. Australian magpies Gymnorhina tibicen [14], Seychelles warblers Acrocephalus sechellensis [15] and pied butcherbirds Cracticus nigrogularis [16]). Older reports of societies with high adult mortality and turnover suggest a similar lack of kin structure among group members in speckled mousebirds Colius striatus [17] and black tits Parus niger [18].
(b). Dispersal to join an unrelated breeding pair
Instead of delaying dispersal or providing help at the nest of a relative, lone birds—usually males—may join an unrelated pair or group on its breeding territory. The immigrant typically appears to be seeking extra-pair copulations with the resident female, often providing food to the female or to the brood in order to gain access to the nest. If the immigrant male is unsuccessful in his attempt to reproduce in the current brood, then he becomes a non-breeding helper and may remain with the group until the subsequent breeding season, when he may inherit a mate or breeding position. This route to reproduction is best described in the pied kingfisher, in which unrelated males join breeding groups and act as helpers until breeding [5,6]; but it also occurs in a variety of less well-studied species, including merlins Falco columbarius [19,20], subdesert mesites Monias benschi [7], hoopoes Upupa epops [21], buff-breasted paradise kingfishers Tanysiptera sylvia [22], stripe-backed wrens Campylorhynchus nuchalis [23], riflemen Acanthisitta chloris [24], rufous vangas Schetba rufa [25] and Puerto Rican todies Todus mexicanus [26]. Similarly, in plural-nesting species, male helpers may attend several nests simultaneously within a single breeding territory, and may be related to only a small minority of the young that they provision [27,28]. In most of these cases, the unrelated male delivers food to the nest as frequently as the breeding male and related helpers do, even when he has not contributed offspring to the clutch.
Alternatively, in other species, the immigrant male copulates with the resident female, competing with the resident male for paternity. The resulting social group is a polyandrous trio (or larger group) in which both males reproduce and provide parental care to the mixed brood, although the immigrant male is frequently behaviourally subordinate to the resident male and may sire fewer offspring [4,29,30].
(c). Dispersal to form a coalition
A slightly different route to cooperative polygamy occurs when two or more unrelated individuals—typically of the same sex—form a cooperative coalition, establish or take over a breeding territory together, and then share reproduction and parental care in a single clutch. Well-studied examples include brown skuas Catharacta lonnbergi [31,32], green wood-hoopoes Phoeniculus purpureus [33,34], Henderson reed-warblers Acrocephalus vaughani [35] and Galapagos hawks Buteo galapagoensis [36,37]. Although these polygamous groups are superficially similar in composition to those that form when an immigrant male joins a breeding pair, their behaviours differ in important details. Conflict between group members is low or absent, and reproduction is divided more equally between same-sex group members. A variant of this system occurs in anis Crotophaga spp. and Taiwan yuhinas Yuhina brunneiceps, in which several mated pairs form coalitions, share reproduction equally in a communal nest and cooperate to defend a group territory [38,39].
These three dispersal patterns are not mutually exclusive; social groups may be formed through a combination of delayed dispersal, coalition formation and immigration, leading to complex aggregations of individuals that vary in age, relatedness, social status and reproductive strategy. However, it is clear that delayed dispersal is not the only option available to young birds that cannot establish an independent breeding territory. Joining an unrelated breeding pair, helping at multiple nests simultaneously or allying with an unrelated individual to form a coalition may be preferable to remaining with kin, even if reproduction is delayed.
4. Why cooperate with non-kin?
Non-breeding helpers have been shown to gain a variety of direct fitness benefits from group membership, primarily by increasing their future reproductive success via increased survival, territory inheritance, access to future mates, acquisition of skills relevant to parental care or the acquisition of helpers in their own future breeding attempts (reviewed in [10]). Although it is clear that unrelated helpers can gain opportunities for future reproduction in cooperative groups, it is often difficult to demonstrate that these opportunities are sufficient to favour non-kin helping over other reproductive strategies, such as helping kin or dispersing to breed independently. The most straightforward examples include obligately cooperative species, in which independent breeding is virtually impossible and helpers profit from group membership in predictable ways. In white-winged trumpeters (Psophia leucoptera), for example, unrelated females join cooperative groups as non-breeding subordinates, then inherit the dominant female's breeding position when she dies; this represents the only route to successful breeding, because young birds are evicted from their natal territories and lone pairs are not able to defend an adequate territory to raise young [40]. However, other studies of facultatively cooperative species have explicitly calculated the direct and indirect components of inclusive fitness and have found that helping non-relatives can be evolutionarily stable even when independent breeding is possible [8]. This is most likely to be the case when individuals in groups have significantly higher survival rates than those that disperse to breed alone [41,42].
Even when the direct fitness benefits of helping are substantial, this raises the question of why helpers should not simply remain on their natal territory and gain both direct and indirect fitness benefits by raising kin. In species with promiscuous mating and high turnover, as discussed earlier, remaining with the natal group does not guarantee high relatedness. In other species, individuals join non-relatives only when they are unable to remain with family, and non-kin helping may be a suboptimal strategy. In gray jays (Perisoreus canadensis), the oldest fledgling in a clutch remains on his natal territory and evicts the younger siblings, who each settle with unrelated pairs [43]. The key lesson from these cases is that helpers may prefer to cooperate with kin when possible—highlighting the role that kin selection is likely to have played in the evolution of these societies—but if relatives are not available, cooperation with unrelated individuals may still be more profitable than floating or attempting to nest alone.
In other species, joining an unrelated pair or group may be favoured over staying with the natal group because it provides a more rapid route to direct reproduction. Many studies have found that unrelated male helpers are likely to take over the territory and mate with the female breeder when her male partner has either died or has been ‘divorced’ and replaced by the helper [6,23,44]. The likelihood of the female accepting the helper as a mate can depend on the amount of food that he provided to the previous clutch [45], which may explain the apparently paradoxical observation that unrelated helpers often deliver food to the nest at equal or higher rates than do related helpers [25,46–48]. Young birds can, of course, remain on their parents’ territory and wait for a breeding vacancy to open there, but their path to reproduction may be delayed by the need to avoid incest with other members of the family group or by older relatives that are ahead in the dominance queue [49,50].
In contrast to helper-at-the-nest societies, cooperatively polygamous species have often been excluded from discussions of avian cooperative breeding on the grounds that they are better explained by conventional mating system theory than by theories of cooperation. For this reason, it is important to distinguish between societies in which polygamy arises through competition for reproduction—in which apparently ‘cooperative’ interactions may actually be detrimental to the fitness of some group members—and those in which group living is favoured by common benefits that accrue to all participants. Between these two extremes lie a large number of species with complex systems of mating and parental care, in which both competitive and mutualistic interactions appear to play important roles in maintaining cooperation.
Dunnocks (Prunella modularis) have served as a model for understanding how cooperative breeding can arise through sexual conflict. A female dunnock paired to one male (the ‘alpha’) maximizes her reproductive fitness by mating with a second, subordinate male (the ‘beta’), because the extra parental assistance increases the number of young that can fledge successfully. From the alpha male's perspective, however, the extra young produced in a polyandrous group do not compensate for the paternity that he has lost to the beta male. As a result, males aggressively compete for paternity, and polyandry benefits only the female. Conversely, males maximize their reproductive success by mating polygynously, which is the least desirable system for a female [4]. Similar conflicts of interest may have favoured the evolution of cooperative polygamy in a number of other species, including Smith's longspurs [51] and stitchbirds [52].
In other systems, however, groups can defend territories or feed young more effectively than lone pairs can, and the benefits of cooperative care are great enough to outweigh the costs of sharing reproduction. Clutton-Brock [2,53] considered these to be instances of intraspecific mutualisms, in which the combined actions of group members generate direct, shared benefits that are not easily undermined by cheating. In anis (Crotophaga spp.), trumpeters (Psophia spp.) and Galapagos hawks (B. galapagoensis), group size—and, crucially, the reproductive fitness of individual group members—is positively correlated with territory size or quality [9,36,37,40]. In all three instances, social groups compete with one another for resources, and large groups are able to displace small groups.
Kokko et al. [54] showed mathematically that when the advantages of being in a large group are sufficiently high, individuals could benefit from raising unrelated young simply in order to increase the group size, a hypothesis known as group augmentation. This is probably the best explanation for the bizarre phenomenon of ‘kidnapping’ in white-winged choughs, in which group members recruit and raise unrelated fledglings to serve as helpers in future breeding attempts [55]. But in most bird species that nest in non-kin groups, fledglings disperse from the social group rather than remaining and increasing the size of the group—in fact, it is usually fledgling dispersal that is responsible for low relatedness among group members. It has therefore been difficult to disentangle the specific benefits predicted by group augmentation from the other types of direct and indirect fitness benefits that maintain cooperation in groups with mixed kin structures. A simpler explanation for most non-kin societies is that group members contribute to a common good from which they derive current or future benefits, and in most cases, the opportunity for direct breeding is probably more important than contributions to the group size per se.
Finally, social nesting can increase the survivorship of group members regardless of relatedness, an effect that can favour nest sharing by non-kin [42,56]. In acorn woodpeckers (Melanerpes formicivorus), related males often breed together and share paternity in a clutch. Within a given breeding season, males in duos produce fewer young per capita than do males breeding alone; however, their survivorship, and hence their lifetime reproductive fitness, is significantly higher. Koenig & Mumme [41] calculated that the benefit of increased survivorship in groups is so high that co-breeding should be favoured even when the two males are unrelated, a result that may help explain the high frequency of unrelated male duos in a different population of the same species [57].
5. What are the evolutionary origins of non-kin cooperation?
The traditional view of avian cooperative breeding is that it has evolved along two main routes: via delayed dispersal of offspring, which leads to the formation of family groups in which kin selection plays a major role in promoting cooperation; or, less commonly, via competition for reproduction by unrelated individuals, which leads to the formation of cooperatively polygamous groups in which all adults potentially reproduce [3]. These two processes certainly represent distinct evolutionary trajectories, and there is little doubt that most cooperative avian societies initially arose along one of these two routes. As reviewed earlier, however, there are many instances of more complex social groups that fit neither model. ‘Family’ groups often include unrelated immigrants that may or may not reproduce, and groups with multiple co-breeders may be maintained by mutualistic benefits rather than by competition for reproduction. Perhaps most importantly, genetic relatedness can actually be quite low in groups that form through delayed dispersal. Do these diverse societies represent alternative evolutionary pathways to cooperative breeding, or are they simply variations on a theme?
Cockburn [58] suggested that in some lineages, kin selection may have played a crucial role in the initial evolution of family-based groups, with the direct fitness benefits of cooperation becoming subsequently more important once cooperative breeding was established. In this scenario, monogamous mating and delayed dispersal leads to high genetic relatedness in family groups, providing the founding condition for helpers to stay with their natal groups. Low genetic relatedness, resulting from immigration, parasitism and/or promiscuous mating, is essentially a derived condition. Cornwallis et al. [59] found broad phylogenetic support for the first part of this hypothesis, arguing that evolutionary transitions to cooperative breeding in birds are significantly associated with monogamous mating by females. So far, however, the theoretical emphasis has primarily been on the importance of kin selection in the initial formation of cooperative groups; less attention has been paid to the possibility that direct fitness benefits alone might subsequently be sufficient to maintain cooperation.
Although it is difficult to test this hypothesis directly, recent phylogenetic analyses have revealed several examples of complex social groups with low genetic relatedness that have evolved from lineages with a phylogenetic history of cooperation in smaller, kin-based groups [60] (electronic supplementary material, table S2). For example, the crows and jays (Corvidae) are a large clade in which family cohesion—delayed dispersal of offspring and the formation of stable family groups—appears to be an ancestral behaviour. Family living has apparently predisposed the lineage to the evolution of helping behaviour, and cooperative breeding has evolved many times independently [61]. Some species, such as the Florida scrub-jay (Aphelocoma coerulescens), live in simple family groups in which adults mate monogamously and non-breeding helpers raise full siblings [62]. Others, such as the brown jay (Psilorhinus morio), live in larger groups composed of extended families and unrelated immigrants. Extra-group mating is common, approximately 40% of helpers are unrelated to any of the young that they care for, and the amount of help provided is not dependent on the helper's degree of relatedness to the brood. Female helpers appear to benefit primarily by inheriting a breeding position in the social group, although this may entail several years of waiting. Male helpers may either inherit a breeding position or attempt to mate with the breeding female [63]. Although groups initially form through delayed dispersal and an underlying signature of kinship persists in social groups, it seems likely that the importance of indirect fitness benefits has been greatly diminished by the opportunities for direct breeding that group membership provides. Similar dynamics appear to prevail in several other cooperative corvids [27,30,64].
An entirely separate route has been proposed to explain the evolution of joint nesting by unrelated females. Although cooperative males often share parentage within a single clutch, females rarely do; joint nesting is uncommon in most cooperatively breeding birds, and usually results in severe competition between females and low rates of hatching success [38,65]. In a few species, however, females routinely lay eggs in the same nest, and neither kinship nor polygamous mating is responsible for the formation of social groups [38,39,66]. Vehrencamp & Quinn [56] suggested that these systems might have arisen through the intermediate step of intraspecific brood parasitism, in which a female lays her eggs in another's nest but provides no subsequent parental care. Cooperative brood care could, theoretically, evolve from parasitism if it were mutually beneficial for ‘parasites’ to remain at the nest and care for the young, and for ‘hosts’ to accept the foreign eggs and parents. From this perspective, brood parasitism and joint nesting are simply extremes along a continuum of parental care; joint nesting should be favoured when the costs of providing care are relatively low, and the benefits of cooperative nest defence or provisioning are relatively high.
Direct evidence for this hypothesis is, again, lacking; however, theoretical work suggests that such a transition is possible [67] and phylogenetic patterns of joint-nesting in some families support evolutionary links with parasitism (see electronic supplementary material, table S2). Conspecific brood parasitism is also common in most species in which female joint nesting is the primary breeding system, suggesting that the choice of strategy is flexible [68–70]. The division of labour among members of a joint-nesting group can reflect the same continuum of parental care, ranging from egalitarian to highly unequal [71,72]. However, the rarity of female joint-nesting—especially when compared with the ubiquity of conspecific brood parasitism in birds—suggests that cooperation is a stable endpoint only in very restricted circumstances.
6. Summary
Kin selection has undoubtedly played a central role in the evolution of cooperative breeding in birds: most cooperative groups consist of nuclear families, non-breeding helpers are typically related to the breeding pair, and preferential helping of kin has been documented in many species. However, this review demonstrates that a surprisingly large number of cooperatively breeding species live in social groups that regularly include non-kin. Promiscuous mating, conspecific brood parasitism and the incorporation of unrelated immigrants can all erode genetic relatedness in ‘family’ groups such that the indirect fitness benefits of helping become negligible. Cooperatively polygamous groups are also more common than previously thought, and their formation can be favoured by mutual benefits rather than (or in addition to) conflict over reproduction.
These patterns suggest that the direct fitness benefits of cooperative breeding—specifically, increased survival, territory inheritance and access to current or future mating opportunities—are frequently sufficient to maintain social nesting even when genetic relatedness is low. This is particularly likely to be true when independent nesting is difficult and when individuals are long-lived. Delayed dispersal of offspring is by far the most common route to cooperative breeding, and it is likely that cooperation in kin groups provided the founding condition for many societies in which high genetic relatedness was subsequently lost. However, most instances of cooperative polygamy probably arose via alternative evolutionary pathways, including sexual conflict, conspecific brood parasitism and the formation of territorial coalitions by unrelated adults.
Acknowledgements
I thank J. Armiger, A. Goldizen, R. H. Macedo, S. Oppenheimer, A. Radford, S.-F. Shen, M. Wells, and C. Yamashita for sharing unpublished data. I am grateful to E. G. Leigh Jr., to two anonymous reviewers for their helpful comments on earlier versions of the manuscript, and to I. D. Couzin, S. V. Edwards, E. A. Herre and M. C. Wikelski for their advice and encouragement.
Funding statement
This work was supported by the Harvard Society of Fellows and by the resources of the Ernst Mayr Library of the Museum of Comparative Zoology, Harvard University.
References
- 1.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383 (doi:10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clutton-Brock TH. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72 (doi:10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 3.Hatchwell BJ. 2009. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3217–3227 (doi:10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies NB. 1992. Dunnock behavior and social evolution. New York, NY: Oxford University Press [Google Scholar]
- 5.Reyer H-U. 1984. Investment and relatedness: a cost/benefit analysis of breeding and helping in the pied kingfisher (Ceryle rudis). Anim. Behav. 32, 1163–1178 (doi:10.1016/S0003-3472(84)80233-X) [Google Scholar]
- 6.Reyer H-U. 1986. Breeder–helper interactions in the pied kingfisher reflect the costs and benefits of cooperative breeding. Behaviour 96, 277–303 (doi:10.1163/156853986X00522) [Google Scholar]
- 7.Seddon N, et al. 2005. Mating system, philopatry and patterns of kinship in the cooperatively breeding subdesert mesite Monias benschi. Mol. Ecol. 14, 3573–3583 (doi:10.1111/j.1365-294X.2005.02675.x) [DOI] [PubMed] [Google Scholar]
- 8.von Lippke IS. 2008. Ecology and evolution of cooperation in the Española mockingbird, Nesomimus macdonaldi, Galapagos. PhD thesis, University of California Los Angeles, Los Angeles, CA [Google Scholar]
- 9.Riehl C. 2011. Living with strangers: direct benefits favour non-kin cooperation in a communally nesting bird. Proc. R. Soc. B 278, 1728–1735 (doi:10.1098/rspb.2010.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177 (doi:10.1146/annurev.ecolsys.29.1.141) [Google Scholar]
- 11.Chao L. 1997. Evolution of polyandry in a communal breeding system. Behav. Ecol. 8, 667–674 (doi:10.1093/beheco/8.6.668) [Google Scholar]
- 12.Hatchwell BJ. 2010. Cryptic kin selection: kin structure in vertebrate populations and opportunities for kin-directed cooperation. Ethology 116, 203–216 (doi:10.1111/j.1439-0310.2009.01732.x) [Google Scholar]
- 13.Dunn PO, Cockburn A, Mulder RA. 1995. Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. Lond. B 259, 339–343 (doi:10.1098/rspb.1995.0050) [Google Scholar]
- 14.Hughes JM, Mather PB, Toon A, Ma J, Rowley I, Russell E. 2003. High levels of extra-group paternity in a population of Australian magpies Gymnorhina tibicen: evidence from microsatellite analysis. Mol. Ecol. 12, 3441–3450 (doi:10.1046/j.1365-94X.2003.01997.x) [DOI] [PubMed] [Google Scholar]
- 15.Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T. 2001. Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol. Ecol. 10, 2263–2273 (doi:10.1046/j.0962-1083.2001.01355.x) [DOI] [PubMed] [Google Scholar]
- 16.Robinson A. 1994. Helpers-at-the-nest in pied butcherbirds Cracticus nigrogularis. PhD thesis, Griffith University, Brisbane, Australia [Google Scholar]
- 17.Decoux J-P. 1982. Les particularites demographiques et socioecologiques du Coliou strie dans le nord-est du Gabon. Revue d'Ecologie (La Terre et la Vie) 36, 37–78 [Google Scholar]
- 18.Tarboton WR. 1981. Cooperative breeding and group territoriality in the black tit. Ostrich 52, 216–225 (doi:10.1080/00306525.1981.9633609) [Google Scholar]
- 19.James PC, Oliphant LW. 1986. Extra birds and helpers at the nests of Richardson's merlin. Condor 88, 533–534 (doi:10.2307/1368289) [Google Scholar]
- 20.Sodhi NS. 1991. Pair copulations, extra-pair copulations, and intraspecific nest intrusions in Merlin. Condor 93, 433–437 (doi:10.2307/1368960) [Google Scholar]
- 21.Martín-Vivaldi M, Martinez JG, Palomino JJ, Soler M. 2002. Extrapair paterntity in the hoopoe Upupa epops: an exploration of the influence of interactions between breeding pairs, non-pair males and strophe length. Ibis 144, 236–247 (doi:10.1046/j.1474-919X.2002.00044.x) [Google Scholar]
- 22.Legge S, Heinsohn R. 2001. Kingfishers in paradise: the breeding biology of Tanysiptera sylvia at the Iron Range National Park, Cape York. Aust. J. Zool. 49, 85–98 (doi:10.1071/ZO00090) [Google Scholar]
- 23.Piper WH, Parker PG, Rabenold KN. 1995. Facultative dispersal by juvenile males in the cooperative stripe-backed wren. Behav. Ecol. 6, 337–342 (doi:10.1093/beheco/6.3.337) [Google Scholar]
- 24.Sherley GH. 1990. Co-operative breeding in riflemen (Acanthisitta chloris): benefits to parents, offspring and helpers. Behaviour 112, 1–22 (doi:10.1163/156853990X00653) [Google Scholar]
- 25.Eguchi K, Asai S, Yamagishi S. 2009. Individual differences in the helping behaviors of cooperatively breeding rufous vangas. Ornithol. Sci. 8, 5–13 (doi:10.2326/048.008.0102) [Google Scholar]
- 26.Kepler AK. 1977. Comparative study of the todies (Todidae): with emphasis on the Puerto Rican tody, Todus mexicanus. Cambridge, MA: Nuttall Ornithological Club [Google Scholar]
- 27.Li SH, Brown JL. 2000. High frequency of extrapair fertilization in a plural breeding bird, the Mexican jay, revealed by DNA microsatellites. Anim. Behav. 60, 867–877 (doi:10.1006/anbe.2000.1554) [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein DI. 2006. The evolution of the social and mating systems of the plural cooperatively breeding superb starling, Lamprotornis superbus. PhD thesis, Cornell University, Ithaca, NY [Google Scholar]
- 29.Ekstrom JMM, Burke T, Randrianaina L, Birkhead TR. 2007. Unusual sex roles in a highly promiscuous parrot: the greater vasa parrot Caracopsis vasa. Ibis 149, 313–320 (doi:10.1111/j.1474-919X.2006.00632.x) [Google Scholar]
- 30.Baglione V. 2002. Direct fitness benefits of group living in a complex cooperative society of carrion crows, Corvus corone corone. Anim. Behav. 64, 887–893 (doi:10.1006/anbe.2002.2007) [Google Scholar]
- 31.Phillips RA. 2002. Trios of brown skuas at Bird Island, South Georgia: incidence and composition. Condor 104, 694–697 (doi:10.1650/0010-5422(2002)104[0694:TOBSAB]2.0.CO;2) [Google Scholar]
- 32.Young EC. 1998. Dispersal from natal territories and the origin of cooperatively polyandrous breeding groups in the brown skua. Condor 100, 335–342 (doi:10.2307/1370274) [Google Scholar]
- 33.Ligon JD, Ligon SH. 1982. The cooperative breeding behavior of the green woodhoopoe. Sci. Am. 247, 106–114 (doi:10.1038/scientificamerican0782-126) [Google Scholar]
- 34.Ligon JD, Ligon SH. 1983. Reciprocity in the green woodhoopoe (Phoeniculus purpureus). Anim. Behav. 31, 480–489 (doi:10.1016/S0003-3472(83)80069-4) [Google Scholar]
- 35.Brooke MDL, Hartley IR. 1995. Nesting Henderson reed-warblers (Acrocephalus vaughani taiti) studied by DNA fingerprinting: unrelated coalitions in a stable habitat? Auk 112, 77–86 (doi:10.2307/4088768) [Google Scholar]
- 36.DeLay LS, Faaborg J, Naranjo J, Paz SM, de Vries T, Parker PG. 1996. Paternal care in the cooperatively polyandrous Galapagos Hawk. Condor 98, 300–311 (doi:10.2307/1369148) [Google Scholar]
- 37.Rivera JL, Vargas FH, Parker PG. 2011. Natal dispersal and sociality of young Galapagos hawks on Santiago Island. Open Ornithol. J. 4, 12–16 (doi:10.2174/1874453201104010012) [Google Scholar]
- 38.Vehrencamp SL. 1977. Relative fecundity and parental effort in communally nesting anis, Crotophaga sulcirostris. Science 197, 403–405 (doi:10.1126/science.197.4301.403) [DOI] [PubMed] [Google Scholar]
- 39.Yuan H-W, Liu M, Shen S-F. 2004. Joint nesting in Taiwan yuhinas: a rare passerine case. Condor 106, 862–872 (doi:10.1650/7520) [Google Scholar]
- 40.Sherman PT. 1995. Social organization of cooperatively polyandrous white-winged trumpeters (Psophia leucoptera). Auk 112, 269–309 (doi:10.2307/4088795) [Google Scholar]
- 41.Koenig WD, Mumme RL. 1987. Population ecology of the cooperatively breeding acorn woodpecker. Princeton, NJ: Princeton University Press [Google Scholar]
- 42.Walters JR, Doerr PD, Carter JHI. 1992. Delayed dispersal and reproduction as a life-history tactic in cooperative breeders: fitness calculations from red-cockaded woodpeckers. Am. Nat. 139, 623–643 (doi:10.1086/285347) [Google Scholar]
- 43.Waite TA, Strickland D. 1997. Cooperative breeding in gray jays: philopatric offspring provision juvenile siblings. Condor 99, 523–525 (doi:10.2307/1369960) [Google Scholar]
- 44.Sloane SA. 1996. Incidence and origins of supernumeraries at bushtit (Psaltriparus minimus) nests. Auk 113, 757–770 (doi:10.2307/4088855) [Google Scholar]
- 45.Jones DA. 1998. Parentage, mate removal experiments, and sex allocation in the cooperatively breeding bell miner, Manorina melanophyrs. PhD thesis, University of Melbourne, Melbourne, Australia [Google Scholar]
- 46.Sherley GH. 1994. Co-operative parental care: contribution of the male rifleman (Acanthisitta chloris) to the breeding effort. Notornis 41, 71–81 [Google Scholar]
- 47.Magrath RD, Whittingham LA. 1997. Subordinate males are more likely to help if unrelated to the breeding female in cooperatively breeding white-browed scrubwrens. Behav. Ecol. Sociobiol. 41, 185–192 (doi:10.1007/s002650050378) [Google Scholar]
- 48.McDonald PG, Kazem AJN, Wright J. 2009. Cooperative provisioning dynamics: fathers and unrelated helpers show similar responses to manipulations of begging. Anim. Behav. 77, 369–376 (doi:10.1016/j.anbehav.2008.10.009) [Google Scholar]
- 49.Piper WH, Slater GL. 1993. Polyandry and incest avoidance in the cooperative stripe-backed wren of Venezuela. Behaviour 124, 227–247 (doi:10.1163/156853993X00597) [Google Scholar]
- 50.Ribeiro AM, Lloyd P, Feldheim KA, Bowie RC. 2012. Microgeographic socio-genetic structure of an African cooperative breeding passerine revealed: integrating behavioural and genetic data. Mol. Ecol. 21, 662–672 (doi:10.1111/j.1365-294X.2011.05236.x) [DOI] [PubMed] [Google Scholar]
- 51.Briskie JV, Montgomerie R, Põldmaa T, Boag PT. 1998. Paternity and parental care in the polygynandrous Smith's longspur. Behav. Ecol. Sociobiol. 43, 181–190 (doi:10.1007/s002650050479) [Google Scholar]
- 52.Ewen JG, Armstrong DP, Lambert DM. 1999. Floater males gain reproductive success through extrapair fertilizations in the stitchbird. Anim. Behav. 58, 321–328 (doi:10.1006/anbe.1999.1150) [DOI] [PubMed] [Google Scholar]
- 53.Clutton-Brock TH. 2010. Cooperation between non-kin in animal societies. Nature 462, 51–57 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 54.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinsohn R. 1991. Kidnapping and reciprocity in cooperatively breeding white-winged choughs. Anim. Behav. 41, 1097–1100 (doi:10.1016/S0003-3472(05)80652-9) [Google Scholar]
- 56.Vehrencamp SL, Quinn JS. 2004. Avian joint laying systems. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 177–196 Cambridge, UK: Cambridge University Press [Google Scholar]
- 57.Stacey PB. 1979. Kinship, promiscuity, and communal breeding in the acorn woodpecker. Behav. Ecol. Sociobiol. 6, 53–66 (doi:10.1007/BF00293245) [Google Scholar]
- 58.Cockburn A. 2013. Cooperative breeding in birds: toward a richer conceptual framework. In Cooperation and its evolution (eds Sterelney K, Joyce R, Calcott B, Fraser B.), pp. 223–245 Cambridge, MA: MIT Press [Google Scholar]
- 59.Cornwallis CK, West SA, Davis KE, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 60.Berg EC, Aldredge RA, Townsend Peterson A, McCormack JE. 2012. New phylogenetic information suggests both an increase and at least one loss of cooperative breeding during the evolutionary history of Aphelocoma jays. Evol. Ecol. 26, 43–54 (doi:10.1007/s10682-011-9492-8) [Google Scholar]
- 61.Ekman J, Ericson PG. 2006. Out of Gondwanaland; the evolutionary history of cooperative breeding and social behaviour among crows, magpies, jays and allies. Proc. R. Soc. B 273, 1117–1125 (doi:10.1098/rspb.2005.3431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Townsend AK, Bowman R, Fitzpatrick JW, Dent M, Lovette IJ. 2011. Genetic monogamy across variable demographic landscapes in cooperatively breeding Florida scrub-jays. Behav. Ecol. 22, 464–470 (doi:10.1093/beheco/arq227) [Google Scholar]
- 63.Berg EC, Williams DA. 2007. Studying individual interactions and direct fitness benefits in wild birds: history and practice. Behav. Proc. 76, 163–166 (doi:10.1016/j.beproc.2006.12.020) [DOI] [PubMed] [Google Scholar]
- 64.Berg EC. 2005. Parentage and reproductive success in the white-throated magpie-jay, Calocitta formosa, a cooperative breeder with female helpers. Anim. Behav. 70, 375–385 (doi:10.1016/j.anbehav.2004.11.008) [Google Scholar]
- 65.Zahavi A. 1990. Arabian babblers: the quest for social status in a cooperative breeder. In Cooperative breeding in birds (eds Stacey PB, Koenig WD.), pp. 103–130 Cambridge, UK: Cambridge University Press [Google Scholar]
- 66.Quinn JS, Haselmayer J, Dey C, Jamieson IG. 2012. Tolerance of female co-breeders in joint-laying pukeko: the role of egg recognition and peace incentives. Anim. Behav. 83, 1035–1041 (doi:10.1016/j.anbehav.2012.01.027) [Google Scholar]
- 67.Zink AG. 2000. The evolution of intraspecific brood parasitism in birds and insects. Am. Nat. 155, 395–405 (doi:10.1086/303325) [DOI] [PubMed] [Google Scholar]
- 68.Gibbons DW. 1986. Brood parasitism and cooperative nesting in the moorhen, Gallinula chloropus. Behav. Ecol. Sociobiol. 19, 221–232 [Google Scholar]
- 69.Whitehead PJ, Tschirner K. 1991. Patterns of egg laying and variation in egg size in the magpie goose Anseranas semipalmata: evidence for intra-specific nest parasitism. Emu 91, 26–31 (doi:10.1071/MU9910026) [Google Scholar]
- 70.Riehl C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Curr. Biol. 20, 1830–1833 (doi:10.1016/j.cub.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 71.Sanchez-Lafuente AM. 1993. Breeding systems related to incubation investment in the purple swamphen Porphyrio porphyrio. Ardea 81, 121–124 [Google Scholar]
- 72.Riehl C. 2012. Mating system and reproductive skew in a communally breeding cuckoo: hard-working males do not sire more young. Anim. Behav. 84, 707–714 (doi:10.1016/j.anbehav.2012.06.028) [Google Scholar]


