Abstract
Zoophilous flowers often transmit olfactory signals to attract pollinators. In plants with unisexual flowers, such signals are usually similar between the sexes because attraction of the same animal to both male and female flowers is essential for conspecific pollen transfer. Here, we present a remarkable example of sexual dimorphism in floral signal observed in reproductively highly specialized clades of the tribe Phyllantheae (Phyllanthaceae). These plants are pollinated by species-specific, seed-parasitic Epicephala moths (Gracillariidae) that actively collect pollen from male flowers and pollinate the female flowers in which they oviposit; by doing so, they ensure seeds for their offspring. We found that Epicephala-pollinated Phyllanthaceae plants consistently exhibit major qualitative differences in scent between male and female flowers, often involving compounds derived from different biosynthetic pathways. In a choice test, mated female Epicephala moths preferred the scent of male flowers over that of female flowers, suggesting that male floral scent elicits pollen-collecting behaviour. Epicephala pollination evolved multiple times in Phyllantheae, at least thrice accompanied by transition from sexual monomorphism to dimorphism in floral scent. This is the first example in which sexually dimorphic floral scent has evolved to signal an alternative reward provided by each sex, provoking the pollinator's legitimate altruistic behaviour.
Keywords: Epicephala, floral scent, obligate pollination mutualism, Phyllanthaceae, sexual dimorphism
1. Introduction
So-called sexual dimorphism—phenotypic differences in ornamentation, morphology and behaviour between males and females—is widespread in animals and has generated much interest since the days of Darwin [1]. By contrast, sexual dimorphism in plants has attracted much less attention, and only recently have studies begun to explore the significance of sexual dimorphism in a range of plant traits [2,3]. In angiosperms, unisexual flowers have evolved repeatedly from hermaphroditic flowers, with roughly 30% of angiosperm species producing at least some unisexual flowers [4]. These unisexual flowers sometimes exhibit secondary sex characteristics in size or morphology, and exploring the ecological cause of such dimorphism will help to better understand floral evolution in angiosperms [5].
Male and female flowers by definition differ in their primary functional characteristics (i.e. production of stamens in males and pistils in females). In addition to such differences, most wind- and water-pollinated plants show extensive secondary sex characteristics in their floral and inflorescence characters, which facilitate pollen release in males and pollen reception in females [6]. However, in animal-pollinated species, sexual divergence in floral signals is weak, and traits that specifically serve to attract pollinators (perianth shape, colour or floral scent) rarely differ between the sexes. This is because plants must attract the same animal to both male and female flowers to secure conspecific pollen transfer [7], and thus are selected to produce similar floral signals in the flowers of both sexes. Selection for male and female flowers to resemble each other is particularly strong when one sex (often the female) produces little or no reward and effectively mimics flowers of the other sex [5,8].
Underlying the fact that male and female flowers of animal-pollinated plants resemble each other is that pollinators seek similar rewards (e.g. floral nectar) from flowers of both sexes. Conversely, if pollinator animals tightly associated with a plant species seek different rewards (e.g. pollen and ovule) on male and female flowers, floral characters may diverge between the sexes to signal alternative rewards. Although rare, plants that offer different rewards to species-specific pollinators on male and female flowers are known to possess sexually dimorphic flowers [9].
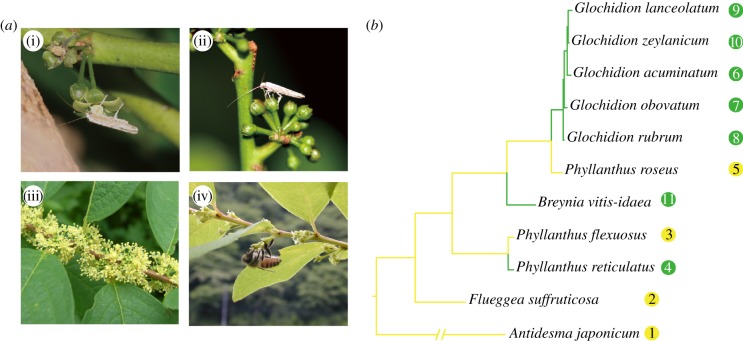
The tribe Phyllantheae (Phyllanthaceae) contains around 1200 species of monoecious or dioecious herbs/shrubs/trees and nearly half of the species are pollinated only by female moths of the genus Epicephala (Gracillariidae) [10,11]. At night, Epicephala females visit male flowers and actively collect pollen grains using their modified proboscises (figure 1a). They then carry pollen grains to female flowers and deliberately deposit them on the stigma (figure 1a), after which they lay eggs into the flowers that have just been pollinated [10]. The larvae are seed consumers and develop by eating a subset of the maturing seeds within a single fruit [11]. Thus, active pollination by Epicephala females is an adaptation that has evolved to secure larval food (seeds) for their offspring. Specificity of the plant–moth association is high, with each Phyllantheae host usually pollinated exclusively by a single Epicephala species. The plants emit a unique blend of floral volatiles at night that matches the olfactory preference of the nocturnally active Epicephala species with which they are associated [13,14]. Thus, floral scent signals are essential for host location in these moths. A phylogenetic study suggests that specialization to Epicephala pollination occurred independently at least five times in Phyllantheae from more generalized pollination systems [12].
Figure 1.
Evolution of floral scent sexual dimorphism in Phyllantheae. (a) A female Epicephala moth (i) collecting pollen grains on a male flower and (ii) pollinating a female flower of G. lanceolatum. (iii) Male flowers and (iv) female flowers of Flueggea suffruticosa, the latter of which is visited by a honeybee. (b) Phylogenetic relationships of the 11 Phyllanthaceae species sampled in this study. The phylogeny is based on the maximum-likelihood tree from a previous study [12]. Numbers in circles represent species numbers in figure 2. Green indicates Epicephala-pollinated species and yellow indicates non-Epicephala-pollinated species.
In generalized pollination systems, pollination is usually a by-product that arises as the animal visitor moves between flowers to gain constant floral rewards [15]. By contrast, pollination by Epicephala is active, which involves collection of pollen from male flowers, and pollen deposition and oviposition on female flowers. Because the ability of Epicephala to distinguish male and female flowers is crucial for successful reproduction of both the moths and the plants, selection may favour divergence in floral scent between the male and female flowers of Epicephala-pollinated Phyllantheae plants. We tested this prediction by analysing sexual differences in floral scent of Epicephala-pollinated species and comparing them in a phylogenetic context with those of other members of Phyllantheae having general pollination systems. We also conducted a behavioural test to determine whether Epicephala moths can distinguish sexual differences in floral scent of the host species.
2. Material and methods
(a). Collection and analysis of floral scent
Floral scent samples were collected from 11 Phyllantheae species (117 individuals in total) using the headspace adsorption technique [13]. Of the 11 species, seven are pollinated nocturnally by Epicephala, while the remaining four are pollinated diurnally by various bees and flies [12]. The details of the study sites and dates, and sample sizes, are given in the electronic supplementary material, tables S1 and S2, respectively. Fifty female flowers and 30 male flowers per tree were removed from the plants and separately put into 5 ml glass vials. Floral volatiles were pumped from the glass vials at 200 ml min−1 for 3 h and adsorbed on Tenax-TA (60 mg; mesh 80–100; GL Sciences, Tokyo, Japan). Collection was done at ambient temperature (25–28°C) either in the field or indoors.
We used gas chromatography–mass spectrometry (GC–MS) to analyse headspace samples using a GCMS-QP2010 system (Shimadzu, Tokyo, Japan) consisting of a model GC-2010 gas chromatograph coupled with a QP2010 electron-impact (EI, 70 eV) mass spectrometer (Shimadzu). Before the analysis, we eluted volatile compounds from the adsorbent with 2 ml of diethyl ether and added 1 µl each of n-hexadecane (1 mg ml−1) and n-eicosane (1 mg ml−1) as internal standards. The eluate was carefully concentrated by N2 flow to 25 µl and topped up with 25 µl of hexane. An aliquot (1 µl) of each sample was injected in splitless mode for 1 min with an injector temperature of 250°C. For GC, we used an Rtx-5SilMS capillary column (30 m × 0.25 mm; film thickness, 250 µm; Restek, Bellefonte, PA) and helium as the carrier gas. The oven temperature was programmed to 40°C for 5 min, followed by an increase of 4°C min−1 to 200°C, and 10°C min−1 to 280°C, where it was held for 5 min. For a preliminary identification of the compounds, we compared the fragments with those contained in the NIST 05 and NIST 05s libraries. We also calculated retention indices for all compounds by using n-alkane (C9–C20) standards and compared them with those reported in the NIST Chemistry WebBook (http://webbook.nist.gov/chemistry) [16] and The Pherobase (http://www.pherobase.com) [17]. The identification of a subset of the compounds was further verified by using the retention indices and MS fragments of authentic compounds whenever possible. The proportion of each volatile compound was calculated as the percentage of its peak area to the total peak area on gas chromatograms.
To discriminate the two enantiomers of linalool, (R)-(−)-linalool and (S)-(+)-linalool, we performed an additional analysis by GC (GC-2010) equipped with a chiral column (InertCap Chiramix capillary column; 30 m × 0.25 mm; film thickness 250 µm; GL Sciences). Helium was used as the carrier gas. The injector was operated in the splitless mode for 1 min. The oven temperature was programmed to 30°C for 5 min, followed by an increase of 1°C min−1 to 180°C, where it was held for 30 min. Before analysing the floral samples, we analysed authentic racemic linalool, (R)-(−)-linalool and (S)-(+)-linalool, with the n-hexadecane standard. Identification of enantiomers was then conducted by comparing the retention time (standardized with n-hexadecane) of floral linalool with that of authentic compounds.
(b). Data analysis
We first calculated dissimilarity indices among individual samples using the Bray–Curtis dissimilarity index [18] based on the relative amount of each compound obtained from the GC analysis. We then used non-metric multi-dimensional scaling (NMDS) to visualize the overlap between male and female floral odour within each species. To further evaluate the extent of sexual differences in floral scent within species, we established a dimorphism index (D), which is a positive value that approaches 0 as the floral scents become more sexually dimorphic, and approaches 1 as the male and female floral scents become more similar. D was obtained by dividing the average of Bray–Curtis dissimilarity indices among all intrasex pairwise comparisons by the average of Bray–Curtis dissimilarity indices among all intersex pairwise comparisons. Thus, when male and female floral scents are similar, D is close to 1, but may slightly exceed 1 depending on how samples are distributed in multivariate space.
We tested for correlated evolution of pollinator type (Epicephala or non-Epicephala) and floral scent sexual dimorphism using independent contrasts [19] as implemented in the PDAP module of Mesquite [20]. Pollinator type was coded as discrete characters, and D was used to represent the degree of floral scent sexual dimorphism for each species. Phylogenetic relationships and branch length information were based on the maximum-likelihood tree produced in a previous study (figure 1b) [12], which investigated the relationships among 46 Phyllantheae species, including all the species sampled in this study except Antidesma japonicum. Because A. japonicum belongs to another tribe apart from Phyllantheae and is distantly related to all the other species sampled in this study, we used Antidesma alexiteria, which was included in the above phylogenetic analysis, as a substitute of A. japonicum to approximate its phylogenetic position.
(c). Behavioural test
To test whether female pollinators have the ability to distinguish male and female flowers by olfactory cues, we conducted a two-choice Y-tube test using female Epicephala bipollenella reared from wild fruits of Glochidion zeylanicum, which were randomly collected from seven trees in June 2013 in Amami-Oshima Island. Fruits were kept in plastic containers under laboratory conditions (temperature 25–28°C, humidity 60–80%) until larvae exited the fruits, pupated and emerged as adults. Because we reasoned that female moths must first mate to become motivated to visit flowers and lay eggs, all behavioural tests were done using mated females. To obtain mated female moths, we kept pairs of male and female moths individually in 50 ml centrifuge tubes with tissue paper immersed in 1% sugar water and kept them in an environment-controlled room under 15.5 L : 8.5 D cycle for approximately 48 h prior to the experiment. Each female moth was used for Y-tube assay only once, after which they were dissected and checked for copulated status based on the presence of a spermatophore in the bursa copulatrix.
Previous studies have shown that Epicephala moths are attracted to the floral scent of their host, both when scents of male and female flowers have been combined as stimulus [13] and tested separately [14], but it is not known whether these moths prefer the scent of one sex over the other. Because a pollinating female Epicephala moth first visits a male flower to collect pollen and then visits female flowers to pollinate and lay eggs [10], we expected that she would be more attracted to male floral scent than to female floral scent at the first encounter if she is capable of distinguishing floral sex based on olfactory cues. We therefore presented the scents of male and female flowers to the above laboratory-mated females in a Y-tube assay to test this prediction. Procedures for the Y-tube test generally followed those in our previous study [14]. We used 10 μl extract of either male or female G. zeylanicum floral headspace sample as test stimulus on each arm of the Y-tube. The solvent was allowed to evaporate for 3 min before the test started. The odour stimuli were applied to small scraps of filter paper (1×1 cm) inserted into a plastic tube, which was connected to the arms of the Y-tube. During the experiment, filter papers were replaced every 20 min, and new stimulus added. The arms of the Y-tube were alternated every five tests to avoid position effects. Data on olfactory response were analysed with a binomial test.
3. Results
We detected a total of 85 volatile compounds from flowers of 11 Phyllantheae species. The floral scent profiles of male and female flowers of each species and their dimorphism indices are shown in the electronic supplementary material, table S2. The difference between floral scents of Epicephala- and non-Epicephala-pollinated plants were difficult to characterize; only one compound, 6-methyl-5-hepten-2-one, was produced by all the Epicephala-pollinated species and not detected in any of the species with non-Epicephala pollination. In general, Epicephala-pollinated species produced more volatile compounds (range, 17–35) than non-Epicephala-pollinated species (range, 6–18). Volatile samples of Epicephala-pollinated plants were closely spaced with each other on the NMDS scatterplot (figure 2).
Figure 2.
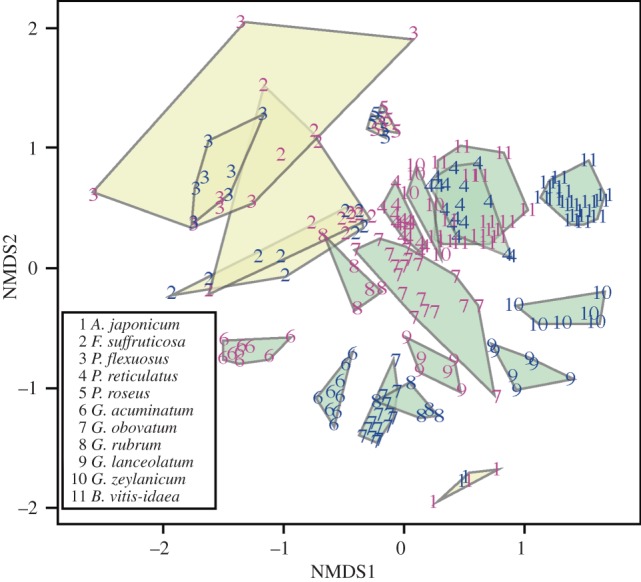
NMDS scatterplot of volatile samples analysed in this study. Species are represented by numbers as shown, and male and female volatile samples are indicated by blue and pink coloration, respectively. Samples of the same sex within each species are boxed and coloured green for Epicephala-pollinated species and yellow for non-Epicephala-pollinated species. Note a clear sexual dimorphism in the floral scents of Epicephala-pollinated plants, whereas a vast overlap can be seen between male and female floral scents in non-Epicephala-pollinated species.
The floral scent of Epicephala-pollinated species showed major qualitative differences between the sexes; on average, 36.5% of the volatiles found in each of the Epicephala-pollinated species were unique to one sex, while gender-specific volatiles were on average 8.9% of all volatiles found in each non-Epicephala-pollinated species (see electronic supplementary material, figure S1). NMDS plots showed clear dimorphism between male and female floral scents in Epicephala-pollinated plants, whereas the floral scents of the two sexes vastly overlapped in plants not pollinated by Epicephala (figure 2). D ranged from 0.14 to 0.64 in Epicephala-pollinated plants and from 0.77 to 1.05 in non-Epicephala-pollinated species (see electronic supplementary material, table S2). Correlation between pollinator type and the degree of dimorphism was significant after controlling for phylogenetic non-independence (regression analysis, r = –0.648, d.f. = 9, p = 0.031; electronic supplementary material, figure S2).
The Y-tube test indicated that mated E. bipollenella females show a preference for the floral scent of male over female G. zeylanicum flowers. The test was conducted using 49 mated females, of which 11 were inactive. Of the remaining 38 moths, 79% chose the male floral scent (binomial test, p < 0.001).
4. Discussion
Our results indicate that the male and female flowers of Epicephala-pollinated plants emit markedly different floral odours. Sexual dimorphism in floral scent was found in multiple Phyllantheae lineages that have independently evolved Epicephala pollination, providing strong support that the observed dimorphism is associated with Epicephala pollination. For logistical reasons, only three of the five documented cases of transition to Epicephala pollination were analysed here, but our results suggest that the same pattern would also be found in the remaining cases. The difference between male and female floral scents involves major qualitative differences in volatile blends. Within each species, roughly a third of the compounds were produced by only one sex, and some of these sex-specific compounds constituted the dominant component of the bouquet. In some cases, the dominant compounds were derived from different biosynthetic pathways; in Glochidion lanceolatum and G. zeylanicum, the major components of female floral scent were terpenoids synthesized by the 2-C-methyl-d-erythritol 4-phosphate pathway, while those of male flowers were benzenoids synthesized by the shikimic acid pathway (see electronic supplementary material, table S2). In other cases, male and female flowers used different enantiomers of the same compound as the major components. For example, in Glochidion obovatum and Glochidion rubrum, only one of the two linalool enantiomers was detected in each sex: R-(–)-linalool in the male and S-(+)-linalool in the female.
Floral scents of male and female flowers are usually similar, if not identical, in animal-pollinated plants, reflecting their need to attract the same animal to flowers of both sexes [8]. Contrary to this prevailing pattern, our results demonstrate a remarkable difference in floral scent between sexes of animal-pollinated plants. We raise two possible evolutionary processes that may be responsible for the observed sexual dimorphism: (i) sexual dimorphism in floral scent is an adaptive divergence that has evolved to promote floral discrimination by Epicephala, or (ii) it is the result of a random process under relaxed selective pressure to produce dissimilar floral signals in male and female flowers. Several lines of evidence indicate that the dimorphism is indeed adaptive. First, if the dimorphism is non-adaptive and simply the product of a random process, the extent of variation among samples of the same sex within a species should be greater in Epicephala-pollinated plants than in plants pollinated by other insects. However, intrasex variation was smaller in Epicephala-pollinated species (figure 2), indicating that a comparable level of purifying selection is acting regardless of pollinator type. Second, because the specificity of Epicephala moths to their host plants is mediated by host-specific floral volatiles [13], any non-adaptive variation in floral odour is likely to disrupt species-specific encounters of the plants and the moths. Third, the plants emitting sex-specific odours are expected to receive better pollination service because the pollinator moths having perceived the signals would locate flowers of the correct sex and transport pollen more efficiently. In support of this idea, our behavioural data indicated that mated E. bipollenella females prefer male floral scent over that of the female at first encounter. Taken together, adaptive divergence remains the most likely explanation for the remarkable floral scent sexual dimorphism found in Epicephala-pollinated Phyllanthaceae plants.
Acknowledgements
We thank Y. Kosaka for field assistance in Laos; M. Tokoro and T. Takanashi for assistance with the behavioural test; and Amami Wildlife Center for logistic support during fieldwork. T.O. and M.K. designed research; T.O., A.K., R.G. and G.P.S. performed research; T.O. and A.K. analysed data; and T.O., A.K. and M.K. wrote the paper.
Funding statement
This work was financially supported by a Japan Society for the Promotion of Science grant to M.K. and a Japan Society for the Promotion of Science Research Fellowships for Young Scientists grant to T.O. and R.G.
References
- 1.Darwin C. 1871. Sexual selection and the descent of man. London, UK: John Murray [Google Scholar]
- 2.Barrett SCH, Hough J. 2013. Sexual dimorphism in flowering plants. J. Exp. Bot. 64, 67–82 (doi:10.1093/jxb/ers308) [DOI] [PubMed] [Google Scholar]
- 3.Ashman TL. 2009. Sniffing out patterns of sexual dimorphism in floral scent. Funct. Ecol. 23, 852–862 (doi:10.1111/j.1365-2435.2009.01590.x) [Google Scholar]
- 4.Lloyd DG, Webb CJ. 1977. Secondary sex characters in plants. Bot. Rev. 43, 177–216 (doi:10.1007/BF02860717) [Google Scholar]
- 5.Willson MF, Ågren J. 1989. Differential floral rewards and pollination by deceit in unisexual flowers. Oikos 55, 23–29 (doi:10.2307/3565868) [Google Scholar]
- 6.Waser NM, Ollerton J. 2006. Plant–pollinator interactions: from specialization to generalization. Chicago, IL: University of Chicago Press [Google Scholar]
- 7.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Syst. 35, 375–403 (doi:10.1146/annurev.ecolsys.34.011802.132347) [Google Scholar]
- 8.Soler CCL, Proffit M, Bessière JM, Hossaert-McKey M, Schatz B. 2012. Evidence for intersexual chemical mimicry in a dioecious plant. Ecol. Lett. 15, 978–985 (doi:10.1111/j.1461-0248.2012.01818.x) [DOI] [PubMed] [Google Scholar]
- 9.Hemborg ÅM, Bond WJ. 2005. Different rewards in female and male flowers can explain the evolution of sexual dimorphism in plant. Biol. J. Lenn. Soc. 85, 97–109 (doi:10.1111/j.1095-8312.2005.00477.x) [Google Scholar]
- 10.Kato M, Takimura A, Kawakita A. 2003. An obligate pollination mutualism and reciprocal diversification in the tree genus Glochidion (Euphorbiaceae). Proc. Natl Acad. Sci. USA 100, 5264–5267 (doi:10.1073/pnas.0837153100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakita A. 2010. Evolution of obligate pollination mutualism in the tribe Phyllantheae (Phyllanthaceae). Plant Spec. Biol. 25, 3–19 (doi:10.1111/j.1442-1984.2009.00266.x) [Google Scholar]
- 12.Kawakita A, Kato M. 2009. Repeated independent evolution of obligate pollination mutualism in the Phyllantheae–Epicephala association. Proc. R. Soc. B 276, 417–426 (doi:10.1098/rspb.2008.1226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto T, Kawakita A, Kato M. 2007. Interspecific variation of floral scent composition in Glochidion and its association with host-specific pollinating seed parasite (Epicephala). J. Chem. Ecol. 33, 1065–1081 (doi:10.1007/s10886-007-9287-0) [DOI] [PubMed] [Google Scholar]
- 14.Svensson GP, Okamoto T, Kawakita A, Goto R, Kato M. 2010. Chemical ecology of obligate pollination mutualisms: testing the ‘private channel’ hypothesis in the Breynia–Epicephala. New. Phytol. 186, 995–1004 (doi:10.1111/j.1469-8137.2010.03227.x) [DOI] [PubMed] [Google Scholar]
- 15.Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Timber Press [Google Scholar]
- 16.Linstrom PJ, Mallard WG. (eds). 2012. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Gaithersburg, MD: National Institute of Standards and Technology, 20899. http://webbook.nist.gov (accessed 7 September 2012) [Google Scholar]
- 17.El-Sayed AM. 2008. The pherobase: database of insect pheromones and semiochemicals. See http://www.pherobase.com
- 18.Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (doi:10.2307/1942268) [Google Scholar]
- 19.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- 20.Maddison WP, Maddison DR. 2008. Mesquite: a modular system for evolutionary analysis (v. 2.5). See http://mesquiteproject.org.