Abstract
While sound is a signal modality widely used by many animals, it is very susceptible to attenuation, hampering effective long-distance communication. A strategy to minimize sound attenuation that has been historically used by humans is to use acoustic horns; to date, no other animal is known to use a similar structure to increase sound intensity. Here, we describe how the use of a roosting structure that resembles an acoustic horn (the tapered tubes that form when new leaves of plants such as Heliconia or Calathea species start to unfurl) increases sound amplification of the incoming and outgoing social calls used by Spix's disc-winged bat (Thyroptera tricolor) to locate roosts and group members. Our results indicate that incoming calls are significantly amplified as a result of sound waves being increasingly compressed as they move into the narrow end of the leaf. Outgoing calls were faintly amplified, probably as a result of increased sound directionality. Both types of call, however, experienced significant sound distortion, which might explain the patterns of signal recognition previously observed in behavioural experiments. Our study provides the first evidence of the potential role that a roost can play in facilitating acoustic communication in bats.
Keywords: acoustic horn, disc-winged bat, roost, social call, waveguide
1. Introduction
Sound, compared with other signal modalities such as vision and smell, is an especially common mode of communication for many animals [1]. However, as sound moves through a medium, it is susceptible to attenuation, or the loss of sound intensity as the distance from the sender increases [2]. Clearly, attenuation can decrease effective communication, because receivers cannot detect or properly interpret very weak signals [3]. Therefore, animals that use acoustic signals for communication should adopt strategies to minimize sound attenuation between the signaller and the receiver. Some of these strategies include vocalizing at times of day when environmental conditions favour distant sound transmission [4,5], emitting low-frequency sounds that suffer reduced loss from spherical spreading [6,7], adjusting vocal signals to match the natural (or resonant) frequency of the spreading medium (thereby enhancing the amplitude of the signal [8,9]) or vocalizing from hollow structures, which often amplify sounds [10–12]. By conveying information over longer distances, animals increase their chances of finding mates, offspring or other group members [13–16], which can impact reproductive success and access to limited resources, such as food or cover [17,18]. Thus, being able to communicate over long distances has the potential to improve the fitness of animals.
Acoustic horns are tapered tubular structures that act as waveguides and have been widely used by humans to increase amplification and directionality of sounds [19]. There are two types of horn: speaking and hearing; the former amplify outgoing sounds, from the narrow end to the wide end of the horn, whereas the latter amplify sounds in the opposite direction. Speaking horns increase sound volume because they match the acoustic impedance of outgoing sounds to the surrounding air [20], and hearing horns amplify sounds when waves are reflected into a progressively narrower area, thereby increasing the sound pressure that reaches the ear [21,22]. In addition, both types of horn aid in acoustic communication because they increase sound directionality [20]. Two major drawbacks of horns are that they cause significant sound distortion [23] and that not all frequencies are equally amplified [22].
Here, we describe how the use of a roosting structure that resembles an acoustic horn has the potential to increase sound amplification of social calls used by Spix's disc-winged bat (Thyroptera tricolor), a bat that is morphologically adapted to using the tapered tubes that form when new leaves of plants such as Heliconia or Calathea species start to unfurl [24,25]. While the role of roosts as refugia from predators and harsh environmental conditions has clearly been established in bats [26], we provide the first evidence of the role that a roost can play in facilitating acoustic communication. Owing to its similarities to an acoustic horn, we believe that the tubular leaves used by T. tricolor could represent a unique example of exploitation of a natural resource for communicative purposes.
Thyroptera tricolor heavily relies on social calls to locate roosts and group members. This species uses two contact calls that allow individuals from a social group to remain cohesive despite changing roosts on a daily basis [27,28]. ‘Inquiry’ calls are simple downward frequency-modulated (FM) sweeps (figure 1a) and are constantly emitted by flying bats to maintain contact with flying and roosting group members. ‘Response’ calls are more complex, composed of multiple U-shaped syllables that form a composite signal with a short upward FM sweep followed by a longer constant frequency (CF) component (figure 1b) and are typically emitted in rapid bouts. These response calls are produced only by bats within roosts after hearing an inquiry call; once response calls are emitted, flying bats rapidly enter the occupied roost [27]. Here, we examine whether inquiry and response calls are amplified by the tubular leaves used for roosting by T. tricolor. We hypothesize that the similarity in shape between leaves and acoustic horns will lead to sounds being amplified and distorted in a manner consistent with hearing and speaking horns [20–23]. We predict that incoming and outgoing sounds will be generally amplified. We further predict that signal fidelity will decrease, with some frequencies being amplified while others are filtered.
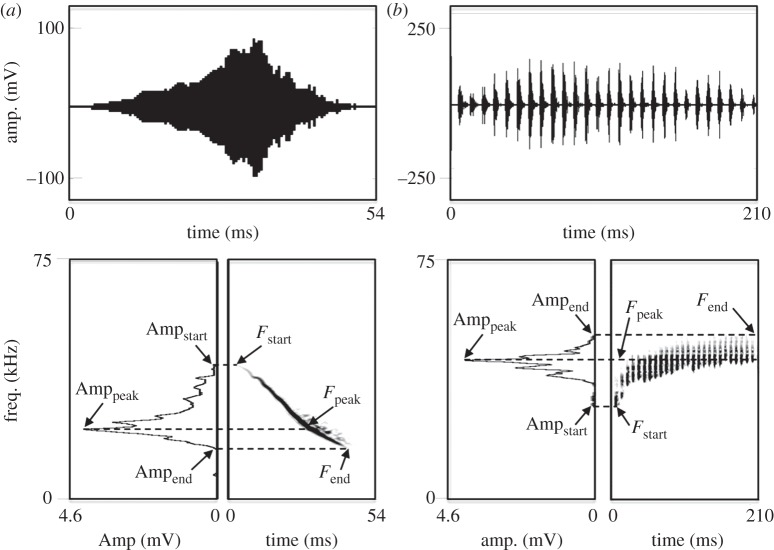
Figure 1.
Depiction of sound measurements taken on (a) inquiry calls and (b) response calls of Spix's disc-winged bats. For each call, the top graph depicts the signal waveform, the bottom left graph depicts the power spectrum and the lower right graph a spectrogram of the respective acoustic signal.
2. Material and methods
The effect of roosts on the social calls of T. tricolor was measured based on two acoustic experiments conducted in April 2013; one experiment measured how the presence of a leaf affects detection of inquiry calls as they pass from the outside environment into the tube (called the ‘incoming inquiry’ experiment; figure 2a), and the other measured how the leaf affects detection of response calls as they pass from inside the tube to the outside environment (called the ‘outgoing response’ experiment; figure 2b). To measure the effect of tubular leaves on inquiry and response call parameters, we gathered recordings of the two signal types from previous field studies [29,30] to broadcast in playback experiments. All signals were recorded from bats in the field using an Avisoft UltraSoundGate (116H or 416H, Avisoft Bioacoustics) onto a laptop computer running Avisoft Recorder. Calls were high-pass filtered to remove unwanted low-frequency signals in Avisoft SAS Lab Pro and we retained calls only for experiments with high signal-to-noise ratios. A total of 79 inquiry calls were used for broadcast in the ‘incoming inquiry’ experiment, whereas 65 response calls were used for broadcast in the ‘outgoing response’ experiment. All signals within a call type were collected from different individuals.
Figure 2.
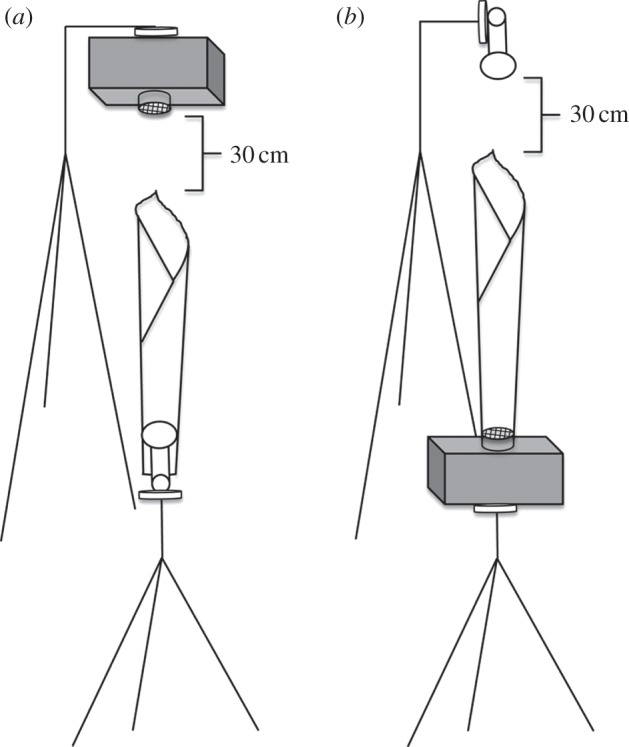
Placement of microphone, speaker and tubular leaf for the (a) incoming inquiry and (b) outgoing response experiments.
For all experiments, we collected fresh tubular leaves every day at nearby lowland tropical forests of the Golfito Wildlife Refuge, southwestern Costa Rica. The region is characterized by an average daily temperature between 24°C and 28°C, and a mean relative humidity of 90% [31]. We attempted to collect a range of leaf sizes according to sizes naturally selected by T. tricolor for roosting. Leaves were used for only one trial of an experiment, and then discarded. For each trial, a leaf was positioned in reference to a microphone (Avisoft CM16) and speaker (Avisoft Ultrasonic Speaker Vifa, no. 60108), according to the experiment being conducted. We calibrated the speaker each day using a sound level meter (model 2236, Brüel & Kjær, Nærum, Denmark) that had previously been calibrated using a 1 kHz tone generator (model 4230, Brüel & Kjær). Calibration proceeded by mounting the sound level meter so that the tip was positioned 50 cm from the speaker, and adjusting broadcast amplitude until the peak frequency of playback calls was detected as 90 dB SPL by the sound level meter [32]. Experiments were conducted in pairs, such that after each experimental session (i.e. leaf present), a control session (i.e. leaf absent) was conducted. This temporal coupling of experimental and control sessions allowed us to minimize the impacts of environmental conditions as they changed throughout the day.
For the incoming inquiry experiment, we collected 14 tubular leaves. In each leaf, we cut the basal portion, so that we could insert the microphone through this opening, and then placed it at a position similar to where bats naturally would be inside the tubular leaf. We snugly fitted the microphone within the leaf's walls such that the width of the microphone was equal to the width of the leaf. In addition, duct tape was used to secure the leaf position to further ensure that there was no opening at the base of the leaf. The speaker was placed above the entrance of the leaf at 30 cm from the leaf tip, pointed downward (figure 2a). An acoustic trial would begin by broadcasting the inquiry calls, and then repeating the broadcast twice more. The leaf was then immediately removed, being careful not to change the position of the microphone and speaker. The same series of inquiry calls was then broadcast three times, as before, in the absence of a leaf. These trials were considered the experimental and control, respectively, as noted earlier. The outgoing response experiment was conducted in a similar manner to the incoming inquiry experiment, although in this experiment the bottom opening of the leaf was attached to the speaker, whereas the microphone was placed above the entrance of the leaf at 30 cm from the leaf tip, pointing downward (figure 2b). The speaker was small enough to fit entirely within the basal opening of the leaf tube; therefore, sound was emitted exclusively within the leaves. In addition, duct tape was used to secure the leaf position to further ensure that there was no opening at the base of the leaf. We used 10 tubular leaves for this experiment.
For call analysis, we generated spectrograms (1024 pt FFT, 93.75% overlap; sampling rate = 250 kHz; frequency resolution = 244 Hz; temporal resolution = 0.256 ms) and power spectra for each recorded call. We took measurements at the start, end and point of maximum amplitude of a call. Measurements at call start and end were essentially instantaneous (i.e. the width of one FFT frame—0.256 ms), whereas point of maximum amplitude was assessed from across the entire call. At each of these positions, we measured amplitude (Ampstart, Ampend and Ampmax), and the dominant frequency associated with that amplitude (Fstart, Fend, and Fmax; see figure 1 for a representation of the link between amplitude and dominant frequency). All measurements for both call types were taken using Avisoft SAS Lab Pro (figure 1). Values for all call parameters for the three repetitions per trial were averaged. After call analysis, we observed the difference in call parameters between experimental and control trials. To do this, we compared the averaged amplitude and dominant frequency measurements for a given call broadcast when the leaf was present and when it was absent. A difference of 0 indicated no difference in call parameters between averaged trials with and without leaves. For each experiment, we determined whether there was a significant difference from a mean of 0 based on 1000 bootstrapped 95% CIs in SPSS v. 20 (IBM Corporation).
In addition to the previous experiments, which show how sound is generally amplified and distorted by tubular leaves, we also assessed two additional characteristics of leaves that might resemble an acoustic horn. In the first experiment, dubbed the ‘vertical effect’ experiment, we sought to determine how leaves amplify signals as sound waves are reflected into a progressively narrower area. For this, we repeated the incoming inquiry experiment, only now the microphone was not left in a single position, but moved after each trial to one of four different depths within the leaf (0, 15, 30 and 45 cm from the tip of the leaf). We used seven tubular leaves for this experiment. Here, we compared Ampmax as a surrogate of sound intensity for calls at different positions within the tubular leaf. Differences in amplitude between experimental and control (same four positions but without a leaf) trials among the four microphone depths were tested using a one-way analysis of variance in SPSS.
In the second experiment, dubbed the ‘horizontal effect’ experiment, we examined issues of directionality for response calls being emitted inside the leaf. For this, we measured the spread of sound along a 20 × 20 cm grid, with and without a tubular leaf. We placed the speaker in the middle of the grid and broadcast response calls in triplicate as described above. Using a single microphone, we measured Ampmax at increasing distances of 10 cm from the centre of the grid in the four cardinal directions. For all horizontal positions on the grid, the height of the microphone was fixed at a vertical distance of 30 cm to the tip of the leaf; this was kept the same during control trials when the leaf was removed. This experiment was conducted on eight different tubular leaves. Data on sound amplitude at each point were analysed using contour figures created in Matlab v. 7.14 (The MathWorks, Inc.). Data for all four experiments are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.9sv83.
3. Results
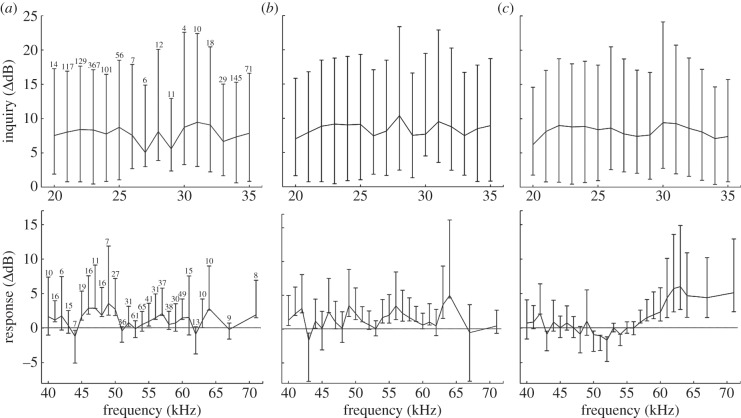
There was a significant increase in inquiry call amplitude for calls of all frequencies as they entered a tubular leaf (figure 3). The trend was similar for Ampstart, Ampend and Ampmax. The mean increase in call amplitude for Ampmax was smallest for frequencies of 20 (6 dB), 28 (7.4 dB) and 34 (7 dB) kHz, but still led to significant signal amplification. The greatest increase in amplitude was observed for frequencies between 22 and 24 kHz (8.7–9 dB), and frequencies between 30 and 32 kHz, with calls in this last frequency up to 9.4 dB louder when the leaf was present. Mean call amplitude was significantly different at all depths within the leaf (F3,1839 = 487.73, p < 0.01), with amplitude increasing steadily as the distance from the leaf tip increased (figure 4). When the microphone was positioned at the leaf entrance, call amplitude increased by less than 1 dB compared with calls emitted at the same position without a leaf. By contrast, calls recorded at a depth of 45 cm from the entrance of the leaf were up to 10 dB louder than calls recorded at the same position in the absence of a leaf.
Figure 3.
Mean difference in call amplitude (in decibels) between experimental (leaf) and control (no leaf) trials for inquiry and response calls. Horizontal axis represents the original frequency of broadcast calls, based on signals recorded during control trials, and only those with n ≥ 4 are included. Vertical lines represent the 95% bootstrap confidence intervals (CIs) for each frequency. Numbers above the CIs represent sample size for each frequency. The horizontal line in graphs for response calls represents a mean value of 0. CIs that cross the 0 line for both types of call represent a frequency for which no differences between experimental and control trials were observed. (a) Ampstart, (b) Ampend and (c) Ampmax.
Figure 4.
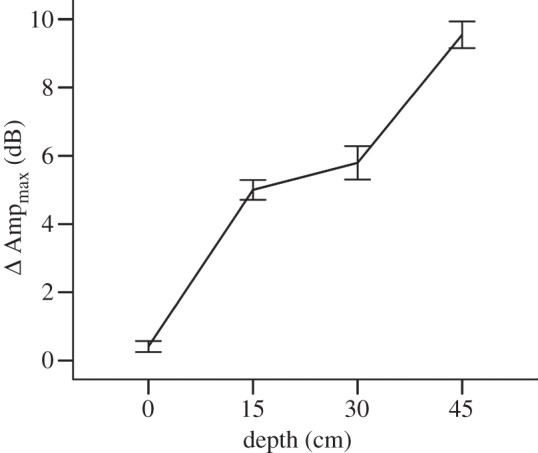
Difference in call amplitude (Ampmax) in decibels for inquiry calls according to the position of the microphone within the leaf. The difference represents the mean amplitude of calls emitted into a leaf minus the mean amplitude of calls without a leaf. Vertical lines represent the 95% CIs.
Response call amplitude also increased with the presence of a leaf for most call frequencies (figure 3). However, most frequencies were amplified only by 1 or 2 dB; the trend was similar for Ampstart, Ampend and Ampmax, although calls with frequencies of 62 and 63 kHz increased up to 6 dB for Ampmax. Response calls emitted from the leaf were highly directional (figure 5), with amplitude quickly decreasing as the microphone was placed horizontally 10 cm from the leaf's entrance. By contrast, amplitude for calls emitted without a tubular leaf decreased steadily at increasing distances from the speaker (see the electronic supplementary material, table S1 for additional details). At the centre of the grid, however, calls emitted from a leaf were generally louder than calls broadcast without a leaf, as noted earlier.
Figure 5.
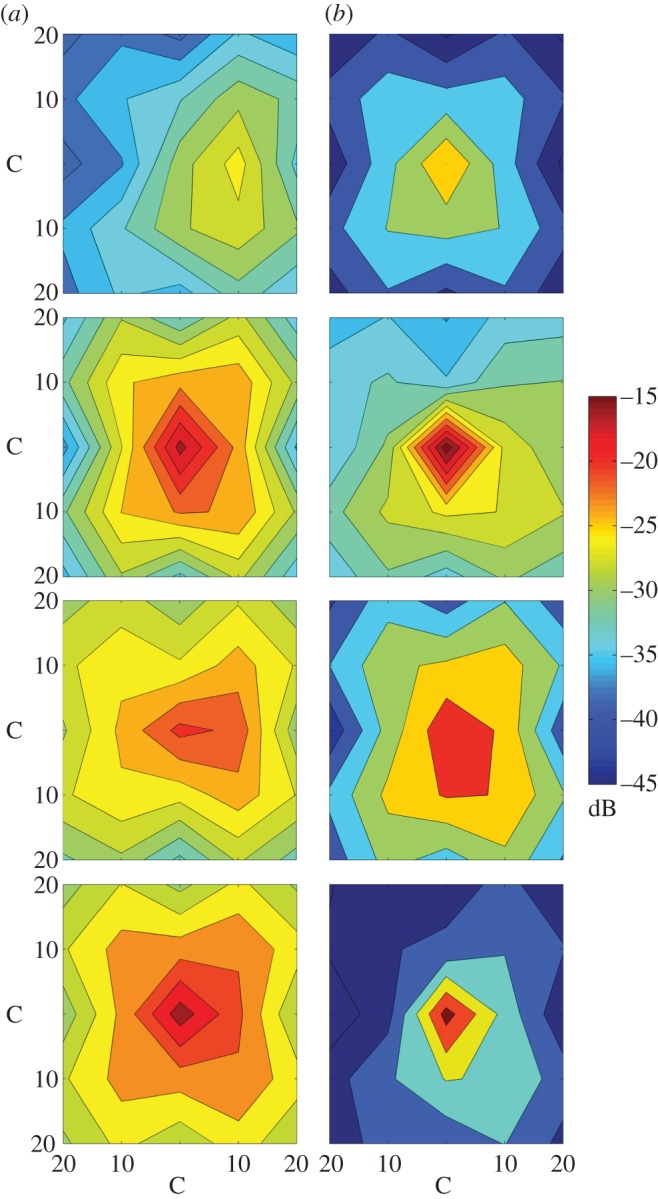
Spread of sound around the speaker without and with a tubular leaf. Each row represents a different leaf. Red depicts higher amplitudes; scale of colour at the right side is in decibels. Axes represent distance from the centre (C) of the grid where the speaker was placed, from 10 to 20 cm. All trials were conducted with the same calls at different times of the day; paired trials (with and without a tubular leaf) were conducted within a short time span of <1 h. (a) No leaf and (b) leaf. (Online version in colour.)
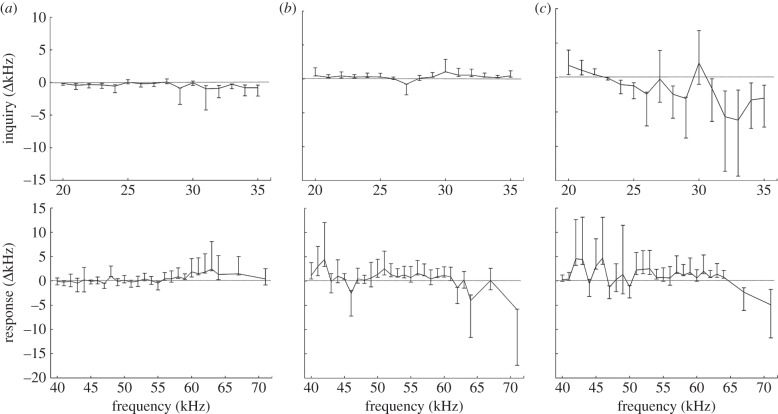
We found that inquiry calls experienced small, but significant, distortions when travelling through a leaf, with some frequencies being amplified while others were degraded. Our standardized measurements of calls that had passed through the leaf revealed a lower Fstart and higher Fend compared with calls emitted in the absence of a leaf, indicating that the most extreme frequencies in the signal were degraded during transmission (figure 6). Fmax experienced a greater variation around most frequencies, with only a few calls around 23, 27 and 30 kHz staying the same as the original signal. Calls lower than 23 kHz entering a leaf measured a higher Fmax compared with the original signal, whereas calls at higher frequencies measured a lower Fmax. This pattern also indicates that peripheral frequencies are most subjected to degradation when a call passes through a leaf tube.
Figure 6.
Mean difference in call frequency (in kHz) between experimental (leaf) and control (no leaf) trials for inquiry and response calls. Horizontal axis represents the original frequency of broadcast calls, based on signals recorded during control trials. Vertical lines represent the 95% bootstrap confidence intervals (CIs) for each frequency. Sample size for each frequency is the same as in figure 3. The horizontal line represents a mean value of 0. CIs that cross the 0 line for both types of call represent a frequency for which no differences between experimental and control trials were observed. (a) Fstart, (b) Fend and (c) Fmax.
Response calls also experienced some modification of spectral structure as signals were emitted through the tubular leaf, yet the trend was more variable than that of inquiry calls (figure 6). Fstart for calls broadcast from the leaf was not significantly different from the original signal for frequencies below 55 kHz; above that, Fstart was recorded at higher frequencies compared with the original, undistorted signal. For frequencies above 65 kHz, we recorded lower call frequencies for the Fend and Fmax of calls that had passed through the leaf tube, although sample sizes were small for this range of calls.
4. Discussion
Sound attenuation represents one of the biggest obstacles hampering effective acoustic communication, and many animals are known to adopt diverse strategies to minimize this problem. While bats rely heavily on sound for social communication [33,34], particularly because most species are nocturnal and highly mobile, there is still no evidence to suggest that these mammals exploit resources to improve sound transmission. While the role of roosts as refugia is clearly demonstrated in bats [26], here we provide for the first time evidence of the acoustic benefits of roost sites in Spix's disc-winged bat, a species that heavily relies on acoustic signals to convey information about location of roost sites to group mates. Through simple acoustic experiments, we show that the tapered tubular structures used as roosts by this bat provide a significant source of sound amplification for at least one type of social call, thereby acting as the acoustic horns historically used by humans to transmit and receive information over long distances [19].
Speaking horns are known to amplify sounds because they match the acoustic impedance between sounds emitted through the horn's narrow end and the air at the wide end of the horn [20]. However, impedance matching is possible only if the sound waves can be confined within the horn's walls, which may not be possible for high-frequency signals of narrow sound beams [35]. Thus, the sound beam of the high-frequency response signals (average Fmax = 57 kHz [27]) emitted by T. tricolor within the tubular leaf is probably too narrow to benefit from acoustic impedance matching, suggesting that these calls are amplified mostly from the sound directionality provided by tubular leaves (figure 5). Sound directionality may not be important for increasing the amplitude of inquiry calls, as roosting bats cannot direct the leaf towards flying bats. Inquiry calls are probably amplified mostly because incoming sound waves are reflected into a progressively narrower area, which increases the sound pressure that reaches the roosting bat [21,22], as demonstrated by our results (figure 4).
High-frequency sounds in lowland tropical regions where T. tricolor is most commonly found rapidly attenuate with distance [36,37]. To understand from how much further this species’s social calls could be perceived with the aid of tubular leaves, we use calculations of atmospheric attenuation based on ISO 9613-1 [36] as a proxy to estimate maximum distance travelled by these signals. For example, Spix's disc-winged bat emits most inquiry signals within the 20–25 kHz range (figure 3). If these calls get an 8 dB boost from entering tubular leaves, this means that these sounds could travel an additional 15–30 m (figure 7). This is certainly an important increase in the area of detection, particularly for a species that has a small home range [38]. By contrast, response calls are amplified less than 10 additional metres owing to the small effects of leaves on call amplitude and high atmospheric attenuation (figure 7). Bats could compensate for this problem by emitting high-intensity calls, which they often appear to do (E. H. Gillam & G. Chaverri 2011, unpublished data). By emitting high-frequency, high-intensity calls, a bat's location remains concealed from most predators whose hearing is not tuned to high-frequency calls [39].
Figure 7.
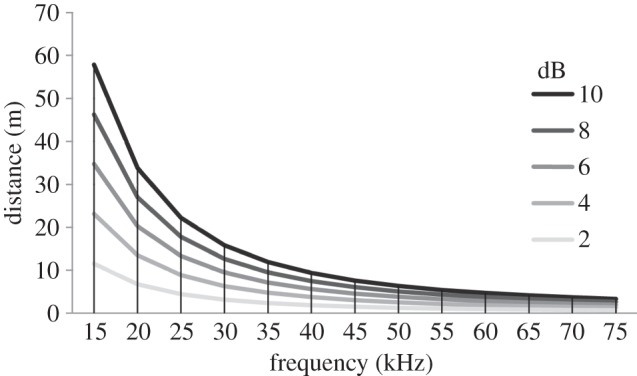
Sound attenuation (in metres) according to sound intensity (in dB) and frequency (in kHz). Calculations based on ISO 9613-1 [36] at 25°C and 90% relative humidity. High-intensity (i.e. 8–10 dB) signals of low frequencies (≤20 kHz), for example, may travel ≥30 m before attenuating, whereas high-intensity and high-frequency signals of ≥40 kHz travel <10 m before attenuating.
In addition to the amplification experienced by the social calls of Spix's disc-winged bat, our experiments also show that tubular leaves decrease sound fidelity. This could potentially represent a source of distortion that significantly affects communication in this species. While the response calls, and to a lesser extent the inquiry calls, used by T. tricolor have signatures that provide sufficient information to allow for individual discrimination [30], previous experiments show that roosting bats are probably unable to differentiate among inquiry calls emitted during flight by group and non-group members, and thus respond indiscriminately [29]. This could be explained by our current findings that incoming inquiry calls experience significant variations in pitch as they enter tubular leaves, which is an important characteristic that provides the individual signatures to these calls [30]. This distortion could be particularly problematic for inquiry calls because these are simple signals that may not encode sufficient information for efficient assessment of individual identity beyond the basic spectral and temporal structure of the call [29]. In fact, while our current findings show that response calls are also affected by the tubular leaves, field experiments demonstrate that flying bats are capable of discriminating between the response calls of group and non-group members, possibly because these complex signals carry a larger amount of information and are thus still recognizable after being degraded by the tubular leaf [29].
The decrease in sound fidelity of incoming and outgoing sounds explained above may be attributed to complex interactions among the original sound wave and multiple reflections within the leaf tube, and to the resonant properties of the tubular leaf. In essence, the tubular leaves used by T. tricolor may act as waveguides, whereby incoming or outgoing sounds are bounced between two parallel boundaries, causing interference among sound waves, and hence frequency pattern distortions [1]. Depending on the resonant properties of the waveguide, such as mass and stiffness of the walls, and width and length of the tube, sounds will be displaced differently according to their frequency [9], causing an additional source of spectral distortion. Thus, because sound waves within waveguides are displaced at different velocities depending on their frequency [40,41], it is possible that broadband calls, like those used by T. tricolor, may suffer the greatest loss of fidelity because some frequency components of the call will appear to be amplified, whereas others will be filtered out.
Because our playback study focused strictly on how known sounds travel through the leaf, we do not know whether bats actively adjust their signals according to the acoustic properties of their current leaf roost in a manner that would reduce signal distortion while maximizing call intensity. In addition, while our study provides initial findings that show how sound is generally amplified and distorted within the tubular leaves used by T. tricolor, it does not yet address how the characteristics of the leaf, such as length, width and plant species, affect sound transmission and how bats may select roost sites based on their acoustic properties. We believe these are potentially rewarding avenues for future research.
Spix's disc-winged bat is one of the few species of bats that specializes in the use of a single roost type, and may even be incapable of using other structures for roosting [42]. The high availability of tubular leaves in the understorey of lowland tropical forests makes them an ideal source of shelter. Yet, the fact that leaves suitable for roosting unfurl in approximately 1 day means that individuals must locate a new structure every day while remaining in a cohesive group. Thyroptera tricolor is quite capable of accomplishing these tasks, and is actually rare among bats for its unusually high levels of group stability [38,43]. Previous studies suggest that group cohesion despite daily roost changes is possible owing to the use of small roosting territories [38,42], by selectively flying near group members, and by being able to discriminate among the response calls of group and non-group members [29]. In addition, our current results suggest that communication of roost location may be further enhanced by the acoustic properties of tubular leaves, as these structures may allow communication over longer distances. This study adds further insights into the important and varied roles that roost sites play in the life of bats.
Acknowledgements
We thank Mauricio Cárdenas Mora and Roberto Sánchez Peraza for their assistance in collecting the calls used in the experiments. We also appreciate the help of Javier Guevara in obtaining research permits.
Funding statement
Funding for this study was partially provided by the Committee for Research and Exploration of the National Geographic Society (grant no. 8973-11 issued to G.C.) and start-up funds issued to E.H.G. from North Dakota State University.
References
- 1.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Forrest TG. 1994. From sender to receiver: propagation and environmental effects on acoustic signals. Am. Zool. 34, 644–654 [Google Scholar]
- 3.Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3, 69–94 (doi:10.1007/BF00300047) [Google Scholar]
- 4.Henwood K, Fabrik A. 1979. A quantitative analysis of the dawn chorus: temporal selection for communicatory optimization. Am. Nat. 114, 260–274 (doi:10.1086/283473) [Google Scholar]
- 5.Larom D, Garstang M, Payne K, Raspet R, Lindeque M. 1997. The influence of surface atmospheric conditions on the range and area reached by animal vocalizations. J. Exp. Biol. 200, 421–431 [DOI] [PubMed] [Google Scholar]
- 6.Marten K, Quine D, Marler P. 1977. Sound transmission and its significance for animal vocalization. II. Tropical forest habitats. Behav. Ecol. Sociobiol. 2, 291–302 (doi:10.1007/BF00299741) [Google Scholar]
- 7.Marten K, Marler P. 1977. Sound transmission and its significance for animal vocalization. I. Temperate habitats. Behav. Ecol. Sociobiol. 2, 271–290 (doi:10.1007/BF00299740) [Google Scholar]
- 8.Lardner B, bin Lakim M. 2002. Tree-hole frogs exploit resonance effects. Nature 420, 475 (doi:10.1038/420475a) [DOI] [PubMed] [Google Scholar]
- 9.Speaks CE. 1999. Introduction to sound: acoustics for the hearing and speech sciences, 3rd edn San Diego, CA: Singular Publishing Group [Google Scholar]
- 10.Bennet-Clark HC. 1987. The tuned singing burrow of mole crickets. J. Exp. Biol. 128, 383–409 [Google Scholar]
- 11.Bailey WJ, Roberts JD. 1981. The bioacoustics of the burrowing frog Heleioporus (Leptodactylidae). J. Nat. Hist. 15, 693–702 (doi:10.1080/00222938100770491) [Google Scholar]
- 12.Penna M. 2004. Amplification and spectral shifts of vocalizations inside burrows of the frog Eupsophus calcaratus (Leptodactylidae). J. Acoust. Soc. Am. 116, 1254–1260 (doi:10.1121/1.1768257) [DOI] [PubMed] [Google Scholar]
- 13.McComb K, Reby D, Baker L, Moss C, Sayialel S. 2003. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 65, 317–329 (doi:10.1006/anbe.2003.2047) [Google Scholar]
- 14.Vehrencamp SL, Ritter AF, Keever M, Bradbury JW. 2003. Responses to playback of local vs. distant contact calls in the orange-fronted conure, Aratinga canicularis. Ethology 109, 37–54 (doi:10.1046/j.1439-0310.2003.00850.x) [Google Scholar]
- 15.Fischer J. 2004. Emergence of individual recognition in young macaques. Anim. Behav. 67, 655–661 (doi:10.1016/j.anbehav.2003.08.006) [Google Scholar]
- 16.Roesli M, Reyer HU. 2000. Male vocalization and female choice in the hybridogenetic Rana lessonae/Rana esculenta complex. Anim. Behav. 60, 745–755 (doi:10.1006/anbe.2000.1519) [DOI] [PubMed] [Google Scholar]
- 17.Evans CS, Evans L. 1999. Chicken food calls are functionally referential. Anim. Behav. 58, 307–319 (doi:10.1006/anbe.1999.1143) [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson GS, Boughman JW. 1998. Social calls coordinate foraging in greater spear-nosed bats. Anim. Behav. 55, 337–350 (doi:10.1006/anbe.1997.0557) [DOI] [PubMed] [Google Scholar]
- 19.Mills M. 2009. When mobile communication technologies were new. Endeavour 33, 140–146 (doi:10.1016/j.endeavour.2009.09.006) [DOI] [PubMed] [Google Scholar]
- 20.Heller EJ. 2013. Why you hear what you hear: an experiential approach to sound, music, and psychoacoustics. Princeton, NJ: Princeton University Press [Google Scholar]
- 21.Geisler CD. 1998. From sound to synapse: physiology of the mammalian ear. Oxford, UK: Oxford University Press [Google Scholar]
- 22.Bossomaier TRJ. 2012. Introduction to the senses: from biology to computer science. Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Black LJ. 1940. A physical analysis of distortion produced by the nonlinearity of the medium. J. Acoust. Soc. Am. 12, 266–267 (doi:10.1121/1.1916101) [Google Scholar]
- 24.Wilson DE, Findley JS. 1977. Thyroptera tricolor Mamm. Species 71, 1–3 (doi:10.2307/3503885) [Google Scholar]
- 25.Findley JS, Wilson DE. 1974. Observations on the neotropical disk-winged bat, Thyroptera tricolor Spix. J. Mammal 55, 562–571 (doi:10.2307/1379546) [PubMed] [Google Scholar]
- 26.Kunz TH. 1982. Roosting ecology of bats. In Ecology of bats (ed. Kunz TH.), pp. 1–50 New York, NY: Plenum Press [Google Scholar]
- 27.Chaverri G, Gillam EH, Vonhof MJ. 2010. Social calls used by a leaf-roosting bat to signal location. Biol. Lett. 6, 441–444 (doi:10.1098/rsbl.2009.0964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaverri G, Gillam EH. 2010. Cooperative signaling behavior of roost location in a leaf-roosting bat. Commun. Integr. Biol. 3, 1–4 (doi:10.4161/cib.3.6.13277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaverri G, Gillam EH, Kunz TH. 2013. A call-and-response system facilitates group cohesion among disc-winged bats. Behav. Ecol. 24, 481–487 (doi:10.1093/beheco/ars188) [Google Scholar]
- 30.Gillam EH, Chaverri G. 2012. Strong individual signatures and weaker group signatures in the contact calls of Spix's disk-winged bat, Thyroptera tricolor. Anim. Behav. 83, 269–276 (doi:10.1016/j.anbehav.2011.11.002) [Google Scholar]
- 31.Kappelle M, Castro M, Acevedo H, Gonzalez L, Monge H. 2002. Ecosistemas del Area de Conservacion Osa (ACOSA). Santo Domingo de Heredia, Costa Rica: Instituto Nacional de Biodiversidad [Google Scholar]
- 32.Kime NM, Turner WR, Ryan MJ. 2000. The transmission of advertisement calls in Central American frogs. Behav. Ecol. 11, 71–83 (doi:10.1093/beheco/11.1.71) [Google Scholar]
- 33.Pfalzer G, Kusch J. 2003. Structure and variability of bat social calls: implications for specificity and individual recognition. J. Zool. (Lond.) 261, 21–33 (doi:10.1017/s0952836903003935) [Google Scholar]
- 34.Fenton MB. 2003. Eavesdropping on the echolocation and social calls of bats. Mammal. Rev. 33, 193–204 (doi:10.1046/j.1365-2907.2003.00019.x) [Google Scholar]
- 35.Sen SN. 1990. Acoustics: waves and oscillations. New Delhi, India: New Age International Publishers [Google Scholar]
- 36.International Organization for Standardization 1993. ISO 9613-1:1993: Acoustics—attenuation of sound during propagation outdoors—part 1: calculation of the absorption of sound by the atmosphere. Geneva, Switzerland: International Organization for Standardization [Google Scholar]
- 37.Griffin DR. 1971. The importance of atmospheric attenuation for the echolocation of bats (Chiroptera). Anim. Behav. 19, 55–61 (doi:10.1016/S0003-3472(71)80134-3) [DOI] [PubMed] [Google Scholar]
- 38.Vonhof MJ, Whitehead H, Fenton MB. 2004. Analysis of Spix's disc-winged bat association patterns and roosting home ranges reveal a novel social structure among bats. Anim. Behav. 68, 507–521 (doi:10.1016/j.anbehav.2003.08.025) [Google Scholar]
- 39.Arch VS, Narins PM. 2008. ‘Silent’ signals: selective forces acting on ultrasonic communication systems in terrestrial vertebrates. Anim. Behav. 76, 1423–1428 (doi:10.1016/j.anbehav.2008.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen FB, Kuperman WA. 1982. Optimum frequency of propagation in shallow water environments. J. Acoust. Soc. Am. 73, 813–819 (doi:10.1121/1.389049) [Google Scholar]
- 41.Mercado E, III, Frazer LN. 1999. Environmental constraints on sound transmission by humpback whales. J. Acoust. Soc. Am. 106, 3004–3016 (doi:10.1121/1.428120) [DOI] [PubMed] [Google Scholar]
- 42.Chaverri G, Kunz TH. 2011. Response of a bat specialist to the loss of a critical resource. PLoS ONE 6, e28821 (doi:10.1371/journal.pone.0028821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaverri G. 2010. Comparative social network analysis in a leaf-roosting bat. Behav. Ecol. Sociobiol. 64, 1619–1630 (doi:10.1007/s00265-010-0975-3) [Google Scholar]