Abstract
Human cytomegalovirus (CMV) infection may be acquired in very low birth weight and extremely low birth weight (ELBW) infants from breast milk. The clinical relevance of such infections is uncertain. There is no consensus on whether screening breast milk for CMV, freezing/pasteurizing milk before feeding, or performing virological monitoring on at-risk infants is warranted. We describe an ELBW infant who acquired CMV postnatally from breast milk and developed CMV sepsis syndrome and clinical evidence of necrotizing enterocolitis (NEC) at ∼5 weeks of age. The availability of serial dried blood spots from day of life (DOL) 4 to 21, coincidentally obtained for a metabolic study, provided the novel opportunity to retrospectively test for and quantify the magnitude of CMV DNAemia. DNAemia was present for several weeks before the onset of severe CMV disease, first being noted on DOL 18 and increasing in magnitude daily to 4.8 log10 genomes/mL on DOL 21, approximately 8 days before the onset of abdominal distension and 15 days before the onset of CMV sepsis syndrome and NEC. After surgical resection, supportive care, and ganciclovir therapy, the infant recovered. This case underscores the importance of including CMV infection in the differential diagnosis of sepsis and NEC in premature infants. This case also suggests the value of prospective virological monitoring in at-risk low birth weight and ELBW infants. Future studies should examine the potential utility of preemptive monitoring for, and possibly treatment of, CMV DNAemia in premature infants, which may herald the onset of serious disease.
Keywords: cytomegalovirus, premature infant, necrotizing enterocolitis (NEC), DNAemia (viral load), breast milk
Introduction
Very low birth weight (VLBW) and extremely low birth weight (ELBW) infants are at risk for symptomatic postnatal cytomegalovirus (CMV) infections, most commonly acquired from breast milk. Most CMV-seropositive women reactivate virus postpartum in the mammary gland upon lactation, and shed CMV in breast milk.1 Breast milk-acquired CMV infections are typically asymptomatic in term infants, but can cause serious disease in VLBW and ELBW infants. Such infections may present with sepsis-like syndrome, thrombocytopenia, neutropenia, pneumonitis, and cholestatic hepatitis.1,2 Enteritis may also be a manifestation of CMV and can mimic necrotizing enterocolitis (NEC), occasionally requiring surgical intervention.2–15
There is no consensus to guide surveillance, prevention, and therapy of CMV infections acquired via breast milk in the premature infant. Some experts stress the generally innocuous course of these infections, and argue that special precautions to prevent transmission are unnecessary. A recently published policy statement by the American Academy of Pediatrics (AAP) Section on Breastfeeding stressed the benefits of breast milk in reducing the risk of complications of prematurity, and stated that “the value of routinely feeding human milk from seropositive mothers to preterm infants outweighs the risks of clinical disease” attributable to CMV.16 The AAP also noted that although freezing breast milk before feeding can reduce the viability of CMV in milk, it does not eliminate the risk of transmission. Pasteurization, although capable of completely inactivating CMV, unfortunately inactivates some of the salutary components of milk.17–20 Therefore, the AAP recommends fresh maternal breast milk for routine feeding in premature infants.
We report here a case of sepsis syndrome and NEC in a premature infant infected with CMV acquired via maternal breast milk. The enrollment of the infant in a clinical study requiring daily heel-stick blood samples in the weeks before the onset of CMV disease presented us with the unique opportunity to retrospectively identify CMV DNAemia, by polymerase chain reaction (PCR) of DNA extracted from dried blood spots (DBS), before symptom onset. We show that high-grade CMV DNAemia was present several weeks before the onset of severe CMV end-organ disease. PCR of control blood spots was negative for CMV DNA, confirming the specificity of our PCR assay. We speculate that surveillance PCR for CMV DNA could serve as an important prognostic and predictive marker for preterm infants at high risk of developing end-organ disease, and suggest that this is testable hypotheses that could be evaluated in future studies of preemptive monitoring and possibly antiviral intervention.
Patient Presentation
A preterm male infant born at 24 and 5/7 weeks’ gestation and weighing 800 g was admitted to a tertiary care NICU with respiratory distress syndrome. Physical examination excluded microcephaly, hepatosplenomegaly, and rash. Empirical antibiotics were discontinued at 48 hours of age after documentation of negative blood and urine cultures. Congenital CMV infection was excluded by negative urine CMV PCR obtained at day of life (DOL) 12.21 Feeding with breast milk initiated on DOL 14. The infant was fed frozen maternal breast milk, stored at −20°C, via gavage. On DOL 26 his abdomen became distended. Abdominal radiograph revealed dilated bowel loops. Antibiotics were administered because of concern for NEC. Bacterial cultures of blood and urine were negative. Seven days later (on DOL 33) he had tachycardia and temperature instability. His abdomen remained distended. White blood cell count was 12 × 109/L. Platelet count was 24 × 109/L. Exploratory laparotomy revealed perforations in the jejunum, and histology demonstrated variable ischemic necrosis and CMV inclusions. Immunohistochemical stain of bowel using a monoclonal antibody to CMV (DDG9/CCH2) was positive (Fig 1). The diagnosis of systemic CMV infection was confirmed by PCR of urine and blood, which demonstrated 4.4 log10 copies/mL and 6.2 log10 copies/mL, respectively. Ganciclovir (GCV) was commenced intravenously on DOL 35, at 6 mg/kg/dose every 12 hours for 3 weeks. Response to therapy was confirmed by clinical improvement and resolution of DNAemia (Fig 2).
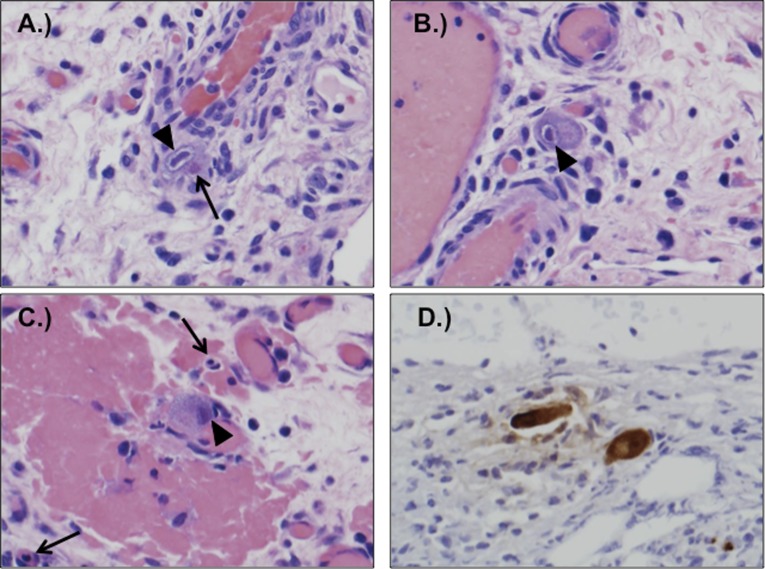
FIGURE 1.
A–C, Sections of surgically resected bowel showed enlarged endothelial cells with viral cytopathic changes, including eosinophilic cytoplasmic inclusions and basophilic nuclear inclusions. Arrows point to cytoplasmic (open arrows) and nuclear (arrowheads) inclusions. D, Immunoperoxidase staining with anti-CMV antibody was positive in affected cells.
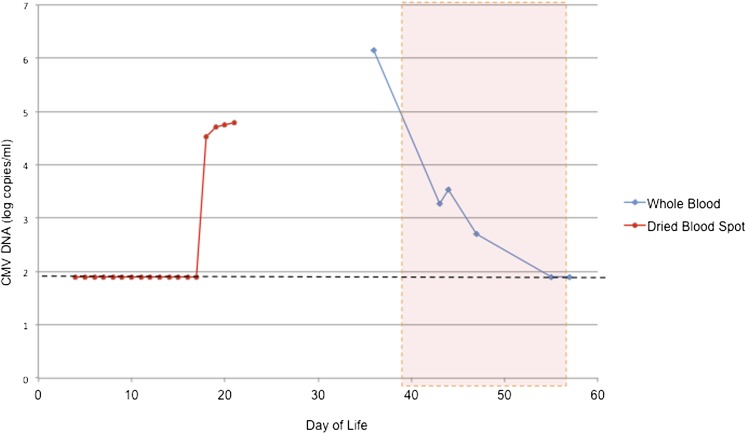
FIGURE 2.
CMV viral load by real-time PCR from dried blood spot (analyzed retrospectively) and whole blood. The dotted line shows the detection limit of the assay. Asymptomatic CMV DNAemia was noted as early as DOL 18, preceding the development of clinical evidence of CMV end-organ disease by 8 days. The dotted red box shows duration of ganciclovir treatment.
Of interest, daily DBS had been collected from this infant on DOL 4 to 21 for an ongoing clinical study of immunoreactive trypsinogen and its potential association with NEC and intestinal perforation in ELBW infants (http://clinicaltrials.gov/ct2/show/NCT01530828). After Institutional Review Board approval and informed consent, these DBS were examined for CMV DNA by PCR. Five 3-mm punches were collected from each DBS, and DNA extraction and PCR amplification were performed as previously described.22 This analysis demonstrated that CMV DNAemia commenced on DOL 18, 8 days before onset of symptoms and 4 days after initiation of breast milk feeds (Fig 2). As a control for the PCR, 10 DBS obtained in a comparison of CMV infections in infants who pass or fail newborn hearing screening were tested in parallel with this infant’s samples. These DBS (from CMV-uninfected controls with normal hearing) were all negative for CMV DNA.22 PCR analyses of 4 samples of frozen breast milk, independently obtained at different dates, all demonstrated high levels of CMV DNA, with a range in viral load from 5.7 to 6.6 log10 copies/mL (data not shown). The breast milk and blood CMV isolates were identical (gB genotype group 1 strains; data not shown).23
Discussion
Postnatal CMV infections are transmitted via breast milk in 6% to 59% of breastfed premature infants, and up to 34% of infected infants become symptomatic.24–26 Early appearance of viral DNA in milk, the presence of viral DNA in milk whey, and the overall magnitude of the breast milk viral load are risk factors for transmission.24 In this case report, we demonstrate by retrospective analyses of DBS samples that CMV DNAemia may be present well in advance of the onset of symptoms in the VLBW infant. DNAemia occurred quickly after initiation of feeds, with low-grade levels of viral DNA noted in blood within 4 days of initiation of breast milk (DOL 18; Fig 2). CMV PCR of a urine sample obtained on DOL 12 almost certainly excluded the possibility of congenital infection, given the known sensitivity and specificity of urine PCR for the diagnosis of congenital CMV.21,27 Despite an initial absence of symptoms, this infant’s systemic viral load continued to rise to 4.8 log10 genomes/mL on DOL 21 (the last day that DBS samples were available through the immunoreactive trypsinogen study protocol). Although no samples were available to test for CMV DNA between DOL 21 and DOL 33, the viral load on DOL 33 when the patient demonstrated a CMV sepsis syndrome and required surgical intervention for NEC was ∼6 log10 genomes/mL. Whether earlier recognition and possibly treatment of asymptomatic (but high-grade) DNAemia could have prevented the development of severe end-organ disease in this patient is speculative, but this question may warrant further research in future prospective studies.
It was of interest that this patient had an NEC-like syndrome caused by CMV infection. We identified 14 previously reported cases of perinatal CMV infections associated with NEC or NEC-like syndrome (Table 1).3 This infant’s intraoperative specimens demonstrated histopathological findings of ulceration, inflammation, granulation tissue, necrosis, and stricture.8 The pathogenesis of NEC-like syndrome triggered by CMV infection is unclear. CMV enteritis often occurs in immunocompromised adults, including solid organ transplant (SOT) or hematopoietic stem cell transplant (HSCT) patients, but it is uncommon in the neonate and only rarely has been associated with severe enough pathology to require surgical resection. In a recent report of CMV-associated NEC, it was proposed that CMV infection may increase vulnerability to secondary bacterial invasion and may also drive proinflammatory immune responses,15 further exacerbating the pathology of NEC. Some of the other potential mechanisms for CMV-associated NEC proposed in this report15 included increased intestinal mucosal permeability,28 enhanced proinflammatory cytokine production,29–31 and alterations in the normal interactions between host cells and commensal microorganisms.32,33 In addition to inducing proinflammatory cytokines, CMV also encodes gene products that modulate the function of host leukocytes, including T cells, neutrophils, and natural killer cells.34,35 CMV-induced modulation of the host immune response could in turn lead to disruptions in mucosal immune homeostasis, potentiating the development of NEC.
TABLE 1.
Summary of Reported Cases of CMV Associated With NEC
| Ref | Gestational Age (wk) | Weight (g) | Clinical Presentation | Breast Milk Given | Operative Findings | Histopathology | Other Clinical Features | GCV |
|---|---|---|---|---|---|---|---|---|
| 3 | 29 | 1490 | Vomiting, distended abdomen, lethargy | Yes | Stricture in ascending and transverse colon | Ulcerating and granulating inflammation of colonic mucosa | None | No |
| 4 | Term | 3500 | Fever, diarrhea, distended abdomen, hepatosplenomegaly | No | Ileal perforation | Ileal perforation, transmural inflammation and granulation tissue formation | None | No |
| 5 | 34 | 2695 | Distended abdomen | NA | Colonic strictures | Severe colitis with mucosal ulceration and transmural acute and chronic inflammation | None | No |
| 6 | 36 | NA | Distended abdomen | NA | Ileal ulceration | Ileal ulcer with inflammatory changes | Hearing impairment | No |
| 7 | 33 | 2200 | Vomiting, distended abdomen | NA | Stricture of descending colon | Ischemic colonic stricture | Hearing impairment | No |
| 8 | 37 | 2490 | Distended abdomen, biliary vomiting mimicking Hirschsprung disease | Yes | Stricture at colon-sigmoid junction | Inflammatory colonic stricture with vasculitis, endothelialitis, mucosal ulceration covered by fibrinopurulent exudate | Chorioretinitis | Yes |
| 9 | 30 | NA | Distended abdomen, vomiting | NA | Inflamed ileocecal area, perforated tip of appendix | Ileocecal necrosis with acute and chronic inflammation | None | No |
| 10 | 27 | 490 | Distended abdomen, pale and mottled skin, temperature instability, tachycardia | Yes | Stricture of ascending and transverse colon | Inflamed, ulcerated cecum and colon with area of full thickness necrosis | None | No |
| 11 | 28 | 790 | Distended abdomen, hepatosplenomegaly | NA | Stricture of colon | NA | NA | No |
| 30 | 990 | Distended abdomen, hepatosplenomegaly, sepsis | NA | Colonic ulceration | NA | None | No | |
| 12 | 235/7 | 580 | Vomiting, diarrhea, distended abdomen | Yes | Ileal ulceration | Ileal ulceration and mixed inflammation | None | No |
| 13 | 37 | 2490 | Biliary vomiting, distended abdomen | Yes | Colonic stricture with multiple ulcers | Colonic stricture with multiple ulcers | Chorioretinitis | Yes |
| 14 | 274/7 | 1130 | Blood stool | Yes | Distal ileum | NA | None | No |
| 15 | 251/7 | 653 | Pneumatosis intestinalis | Yes | Fulminant | NA | None | No |
| 16 | 245/7 | 800 | Distended abdomen | Yes | Jejunum | CMV inclusions; positive viral antigen staining | None | Yes |
CMV, cytomegalovirus; GCV, ganciclovir; NEC, necrotizing enterocolitis
As reviewed previously, the availability of a collection of DBS obtained sequentially between DOL 4 and 21 allowed for retrospective examination of DNAemia that evolved during the course of illness, well before the onset of any signs or symptoms of disease. We propose a possible analogy between premature infants and immunocompromised SOT or HSCT patients with respect to CMV DNAemia analysis. Monitoring for DNAemia in HSCT and SOT recipients is of critical importance in identifying patients who are at high risk for CMV disease, and initiation of preemptive antiviral therapy dramatically reduces CMV morbidity and mortality in these settings.36–38 Similarly, it is tempting to speculate that this same preemptive therapy approach could be applied if future studies confirm that DNAemia heralds a substantial risk for subsequent end-organ disease in premature infants. Interestingly, in our patient the doubling time of CMV DNAemia was 1.2 days, similar to that reported in transplant patients.39,40 Weekly screening for CMV DNAemia as a part of routine laboratory studies in premature infants might identify those at high risk for development of symptomatic CMV disease and guide preemptive antiviral therapy. Studies in HSCT patients indicate that a viral load of 1000 copies/mL (3 log10) is an appropriate cut-off for the initiation of preemptive therapy.41–43 Whether an approach aimed at preemptively treating asymptomatic high-grade DNAemia in the ICU setting could prevent the development of CMV end-organ disease or yield additional benefits is unproven, but may merit future research.44
We chose to treat the infant described in this report with GCV, based on recommendations by the infectious diseases consultant involved in this case. In infants with congenital CMV infection involving the central nervous system, 6 weeks of GCV therapy has been associated with improved audiologic and neurodevelopmental outcomes.45,46 There are no clinical trials to date suggesting a benefit of GCV therapy for postnatally acquired CMV infections, outside of the setting of severe immune compromise. However, given the severity of this infant’s CMV sepsis syndrome and end-organ disease, the clinicians caring for this child believed that GCV therapy was warranted. A 3-week course of therapy was recommended, rather than 6 weeks, because this was sufficient both to resolve DNAemia and to produce other clinical signs of improvement. It is unclear whether GCV therapy has any impact on the neurodevelopmental prognosis of premature infants who acquire CMV infection postnatally from breast milk. A recent study suggested less favorable cognitive and motor function outcomes in preterm infants with postnatally acquired CMV infection, as compared with those without CMV infection,47 but other studies have not demonstrated an increased risk.48 Future clinical studies are needed to determine whether the appearance of DNAemia in asymptomatic preterm infants is predictive of the development of end-organ disease, and to assess whether there are any short- or long-term benefits to be realized from the use of preemptive antiviral interventions, such as GCV and/or CMV immune globulin, in the absence of clinical evidence of CMV disease.
Glossary
- AAP
American Academy of Pediatrics
- CMV
cytomegalovirus
- DBS
dried blood spots
- DOL
day of life
- ELBW
extremely low birth weight
- GCV
ganciclovir
- HSCT
hematopoietic stem cell transplant
- NEC
necrotizing enterocolitis
- PCR
polymerase chain reaction
- SOT
solid organ transplant
- VLBW
very low birth weight
Footnotes
Dr Tengsupakul performed the CMV PCR studies (in the laboratory of Dr Schleiss), wrote the first draft of the manuscript, and participated in data analysis and figure preparation; Dr Birge was the co-Principal Investigator on the immunoreactive trypsinogen parent study with Dr Bendel, coordinated the procurement of blood spots, obtained informed consent, and helped draft the report; Dr Bendel was the co-Principal Investigator on the immunoreactive trypsinogen parent study with Dr Birge, coordinated the procurement of blood spots, and helped draft the report; Dr Reed performed the histopathologic analyses, helped draft the paper, prepared the figures, and was involved in data analysis; Ms Bloom helped in procurement of blood spots, helped write the paper, and provided perspective on blood spot screening and the case report study; Ms Hernandez designed the real-time CMV PCR assay, taught the technique and supervised Dr Tengsupakul, and reviewed and helped write the paper; and Dr Schleiss conceived of the retrospective blood spot testing, conceived of preemptive monitoring of DNAemia, and wrote the final revised draft of the paper.
This manuscript has not been published previously and is not under consideration elsewhere, and all authors are responsible for reported research, have participated in the concept and design, analysis and interpretation of data, and drafting or revising of the manuscript, and have approved the manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health grants T32 HD068229 and R01 HD044864. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev Med Virol. 2006;16(2):73–82 [DOI] [PubMed] [Google Scholar]
- 2.Kurath S, Halwachs-Baumann G, Müller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010;16(8):1172–1178 [DOI] [PubMed] [Google Scholar]
- 3.Gessler P, Bischoff GA, Wiegand D, Essers B, Bossart W. Cytomegalovirus-associated necrotizing enterocolitis in a preterm twin after breastfeeding. J Perinatol. 2004;24(2):124–126 [DOI] [PubMed] [Google Scholar]
- 4.Huang YC, Lin TY, Huang CS, Hseun C. Ileal perforation caused by congenital or perinatal cytomegalovirus infection. J Pediatr. 1996;129(6):931–934 [DOI] [PubMed] [Google Scholar]
- 5.Stiskal J, Jacquette M, Kaplan G, Ritterman R, Stavis R, Palder S. Congenital cytomegalovirus infection with gastrointestinal involvement. J Pediatr. 1997;131(1 Pt 1):168. [DOI] [PubMed] [Google Scholar]
- 6.Hakim A, Mimouni F, Payandeh F, Morawski J. Immunochemical staining in congenital cytomegalovirus-induced ileal ulceration. J Pediatr. 1997;131(1 Pt 1):168–170 [DOI] [PubMed] [Google Scholar]
- 7.Reyes C, Pereira S, Warden MJ, Sills J. Cytomegalovirus enteritis in a premature infant. J Pediatr Surg. 1997;32(11):1545–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekema G, Pedersini P, Milianti S, Ubertazzi M, Minoli D, Manciana A. Colonic stricture mimicking Hirschsprung’s disease: a localized cytomegalovirus infection. J Pediatr Surg. 2006;41(4):850–852 [DOI] [PubMed] [Google Scholar]
- 9.Terry NE, Fowler CL. Cytomegalovirus enterocolitis complicated by perforated appendicitis in a premature infant. J Pediatr Surg. 2006;41(8):1476–1478 [DOI] [PubMed] [Google Scholar]
- 10.Shetty A, Barnes R, Lazda E, Doherty C, Maxwell N. Cytomegalovirus: a cause of colonic stricture in a premature infant. J Infect. 2007;54(1):e37–e39 [DOI] [PubMed] [Google Scholar]
- 11.Bonnard A, Le Huidoux P, Carricaburu E, et al. Cytomegalovirus infection as a possible underlying factor in neonatal surgical conditions. J Pediatr Surg. 2006;41(11):1826–1829 [DOI] [PubMed] [Google Scholar]
- 12.Srinivasjois RM, Kava MP, Thomas A, Rao SC. Cytomegalovirus-associated ileal stricture in a preterm neonate. J Paediatr Child Health. 2008;44(1-2):80–82 [DOI] [PubMed] [Google Scholar]
- 13.Tzialla C, Decembrino L, Di Comite A, Bollani L, Colombo R, Stronati M. Colonic stricture and retinitis due to cytomegalovirus infection in an immunocompetent infant. Pediatr Int. 2010;52(4):659–660 [DOI] [PubMed] [Google Scholar]
- 14.Lee SL, Johnsen H, Applebaum H. Cytomegalovirus enterocolitis presenting as abdominal compartment syndrome in a premature neonate. World J Pediatr. 2012;8(1):80–82 [DOI] [PubMed] [Google Scholar]
- 15.Tran L, Ferris M, Norori J, et al. Necrotizing enterocolitis and cytomegalovirus infection in a premature infant. Pediatrics. 2013;131(1). Available at: www.pediatrics.org/cgi/content/full/131/1/e318 [DOI] [PubMed] [Google Scholar]
- 16.Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e827 [DOI] [PubMed] [Google Scholar]
- 17.Forsgren M. Cytomegalovirus in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004;56(4):526–528 [DOI] [PubMed] [Google Scholar]
- 18.Hamprecht K, Maschmann J, Müller D, et al. Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004;56(4):529–535 [DOI] [PubMed] [Google Scholar]
- 19.Goelz R, Hihn E, Hamprecht K, et al. Effects of different CMV-heat-inactivation-methods on growth factors in human breast milk. Pediatr Res. 2009;65(4):458–461 [DOI] [PubMed] [Google Scholar]
- 20.Maschmann J, Hamprecht K, Weissbrich B, Dietz K, Jahn G, Speer CP. Freeze-thawing of breast milk does not prevent cytomegalovirus transmission to a preterm infant. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F288–F290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries JJ, van der Eijk AA, Wolthers KC, et al. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2012;53(2):167–170 [DOI] [PubMed]
- 22.Choi KY, Schimmenti LA, Jurek AM, et al. Detection of cytomegalovirus DNA in dried blood spots of Minnesota infants who do not pass newborn hearing screening. Pediatr Infect Dis J. 2009;28(12):1095–1098 [DOI] [PubMed] [Google Scholar]
- 23.Chou SW, Dennison KM. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991;163(6):1229–1234 [DOI] [PubMed] [Google Scholar]
- 24.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518 [DOI] [PubMed] [Google Scholar]
- 25.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001;33(12):1998–2003 [DOI] [PubMed]
- 26.Buxmann H, Miljak A, Fischer D, Rabenau HF, Doerr HW, Schloesser RL. Incidence and clinical outcome of cytomegalovirus transmission via breast milk in preterm infants </=31 weeks. Acta Paediatr. 2009;98(2):270–276 [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger Y, Halle D, Eidelman AI, et al. Urine polymerase chain reaction as a screening tool for the detection of congenital cytomegalovirus infection. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F371–F374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Maar EF, Kleibeuker JH, Boersma-van Ek W, The TH, van Son WJ. Increased intestinal permeability during cytomegalovirus infection in renal transplant recipients. Transpl Int. 1996;9(6):576–580 [DOI] [PubMed] [Google Scholar]
- 29.Pulliam L, Moore D, West DC. Human cytomegalovirus induces IL-6 and TNF alpha from macrophages and microglial cells: possible role in neurotoxicity. J Neurovirol. 1995;1(2):219–227 [DOI] [PubMed] [Google Scholar]
- 30.Rahbar A, Boström L, Lagerstedt U, Magnusson I, Söderberg-Naucler C, Sundqvist VA. Evidence of active cytomegalovirus infection and increased production of IL-6 in tissue specimens obtained from patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9(3):154–161 [DOI] [PubMed] [Google Scholar]
- 31.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clin Perinatol. 2013;40(1):109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wardwell LH, Huttenhower C, Garrett WS. Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr Infect Dis Rep. 2011;13(1):28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CJ, Nelson A, Scribbins D, et al. Bacterial and fungal viability in the preterm gut: NEC and sepsis. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F298–F303. [DOI] [PubMed] [Google Scholar]
- 34.Schleiss MR. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect Disord Drug Targets. 2011;11(5):449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J. 2009;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325(23):1601–1607 [DOI] [PubMed] [Google Scholar]
- 37.Hodson EM, Craig JC, Strippoli GF, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008; (2):CD003774. [DOI] [PubMed] [Google Scholar]
- 38.Strippoli GF, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation. 2006;81(2):139–145 [DOI] [PubMed] [Google Scholar]
- 39.Mattes FM, Hainsworth EG, Hassan-Walker AF, et al. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191(1):89–92 [DOI] [PubMed] [Google Scholar]
- 40.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355(9220):2032–2036 [DOI] [PubMed] [Google Scholar]
- 41.Halfon P, Berger P, Khiri H, et al. Algorithm based on CMV kinetics DNA viral load for preemptive therapy initiation after hematopoietic cell transplantation. J Med Virol. 2011;83(3):490–495 [DOI] [PubMed] [Google Scholar]
- 42.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limaye AP, Boeckh M. CMV in critically ill patients: pathogen or bystander? Rev Med Virol. 2010;20(6):372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimberlin DW, Lin CY, Sánchez PJ, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25 [DOI] [PubMed] [Google Scholar]
- 46.Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46(Suppl 4):S22–S26. [DOI] [PMC free article] [PubMed]
- 47.Bevot A, Hamprecht K, Krägeloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167–e172 [DOI] [PubMed] [Google Scholar]
- 48.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23(4):322–327 [DOI] [PubMed] [Google Scholar]