Abstract
The proven effectiveness of biologics and other immunomodulatory products in inflammatory rheumatic diseases has resulted in their widespread use as well as reports of potential short- and long-term complications such as infection and malignancy. These complications are especially worrisome in children who often have serial exposures to multiple immunomodulatory products. Post-marketing surveillance of immunomodulatory products in juvenile idiopathic arthritis (JIA) and pediatric systemic lupus erythematosus is currently based on product-specific registries and passive surveillance, which may not accurately reflect the safety risks for children owing to low numbers, poor long-term retention, and inadequate comparators. In collaboration with the US Food and Drug Administration (FDA), patient and family advocacy groups, biopharmaceutical industry representatives and other stakeholders, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) and the Duke Clinical Research Institute (DCRI) have developed a novel pharmacosurveillance model (CARRA Consolidated Safety Registry [CoRe]) based on a multicenter longitudinal pediatric rheumatic diseases registry with over 8000 participants. The existing CARRA infrastructure provides access to much larger numbers of subjects than is feasible in single-product registries. Enrollment regardless of medication exposure allows more accurate detection and evaluation of safety signals. Flexibility built into the model allows the addition of specific data elements and safety outcomes, and designation of appropriate disease comparator groups relevant to each product, fulfilling post-marketing requirements and commitments. The proposed model can be applied to other pediatric and adult diseases, potentially transforming the paradigm of pharmacosurveillance in response to the growing public mandate for rigorous post-marketing safety monitoring.
Keywords: medication safety, immunomodulatory therapy, biologic products, juvenile rheumatic disease
Current State of Safety Research In Pediatric Rheumatology
In the late 1990s, the first immunomodulatory products for rheumatic diseases were introduced, revolutionizing care for adults and children who have inflammatory arthritis.1–3 The proven effectiveness of tumor necrosis factor (TNF) inhibitors fueled the development of other products targeting cytokines and cellular receptors involved in the pathophysiology of various inflammatory and autoimmune diseases. Although the benefits of targeted immunomodulatory products are clear, the short- and long-term risks in children and adolescents are uncertain. These risks are particularly important for children, given their possible impact on the developing immune system and the potential for life-long exposure. In addition, the pharmacokinetics of drugs often differ in children,4 so safety information collected in adults may not be generalizable to children.
In November 2009, the US Food and Drug Administration (FDA) placed a Boxed Warning for TNF inhibitors, informing prescribers of malignancies in children and young adults. The warning was based on analysis of voluntary post-marketing reports of malignancies in pediatric patients receiving TNF inhibitors for various conditions.5 However, voluntary reporting systems are limited owing to event underreporting, inadequate clinical detail, no knowledge about the total number of patients exposed (denominator) necessary to calculate an incidence rate, and the lack of a relevant comparator group of unexposed children. The FDA Boxed Warning understandably caused alarm among parents of children taking TNF inhibitors and may have prompted decisions not to use TNF inhibitors, trading an effective treatment with a potential yet uncertain risk for cancer for less effective treatments carrying no perceived risk. Subsequent studies have demonstrated an increased baseline rate of malignancy among all children who have juvenile idiopathic arthritis (JIA) compared with the general population even without biologic exposure.6–8,9 However, other studies have not revealed increased malignancy risk in JIA, highlighting the need for more robust longer-term studies with active comparators rather than historical controls.10 Other events of interest in children treated with immunomodulatory products include opportunistic infections, lupus or lupus-like illnesses, demyelinating diseases, pulmonary hypertension, inflammatory bowel disease, and uveitis.11–14
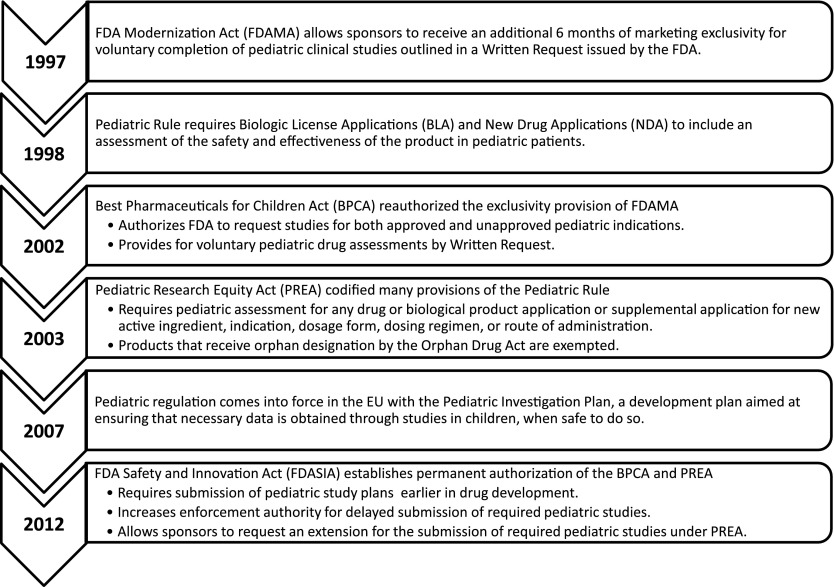
Historically children were excluded from clinical trials and most products lacked adequate pediatric safety and efficacy data. However, appropriate labeling of clinical safety and efficacy data for children has improved in the United States over the last decade through a series of federal legislative actions, culminating in the permanent authorization of the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act (PREA) under the FDA Safety and Innovation Act of 2012.15–19 European pediatric legislation has also developed in parallel with that in the United States. These legislative developments are summarized in Fig 1.
FIGURE 1.
Pediatric pharmaceutical regulation timeline. The major federal legislative actions targeting improving appropriate labeling of clinical safety and efficacy data for children are shown.
Under this legislation, a pediatric assessment is required for all drug or biological product applications or supplemental applications for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration.20 Therefore, drugs and biological products seeking approval for the treatment of adult rheumatoid arthritis as a new indication would be subject to PREA. Importantly, pediatric studies can only be required under PREA for the specific reasons listed above. However, sponsors may receive additional marketing exclusivity for pediatric conditions that may not occur in adults through the issuance of a Written Request under the Best Pharmaceuticals for Children Act. Unique subpopulations such as systemic JIA with systemic involvement and few joints may also be put forward for FDA approval. The recent Biologics License Application approval of tocilizumab in systemic JIA (www.clinicaltrials.gov identifier NCT00642460) is one such example.
The pivotal phase 3 trials of biologic agents in polyarticular-course JIA have involved relatively small patient populations because of the low prevalence of JIA. In addition, the use of novel trial designs in these studies, such as the randomized withdrawal design, limit placebo exposure,2,21–23 such that serious and/or uncommon adverse events cannot reliably be detected in these registration trials. Most children are exposed to multiple agents over time, such that pinpointing adverse event association with a single product is problematic. Lastly, as previously described, having rheumatic diseases may predispose children to increased risks for adverse events irrespective of therapy,6,24 although the magnitude of this baseline risk is poorly quantified.
The long-term safety of immunomodulatory products in JIA is currently monitored in 3 ways: open-label, long-term extension studies of randomized controlled trials; passive adverse event surveillance systems; and sponsor-led product-specific observational registries that have traditionally fulfilled industry post-marketing commitments and requirements. Each has significant limitations as outlined in Table 1. Although the FDA’s recently implemented Sentinel Program will significantly improve current passive surveillance methods through actively surveying diverse data holders, the consolidated safety registry approach will provide richer clinical data and insure longer follow-up of patients beyond what is available in insurance claims to allow better adjustment for potential confounders, including disease severity.
TABLE 1.
Limits of Existing Methods of Post-Marketing Surveillance
| Long-Term Extensions of Clinical Trials | Passive Adverse Event Surveillancea | Single-Product Phase IV Registries |
|---|---|---|
| Small sample size | Limited or incomplete clinical information | Challenging recruitment leads to inability to reach enrollment targets |
| Restrictive eligibility requirements | Underreporting and other reporting biases | Retention issues lead to small numbers of exposed patients by the end of study |
| Excludes patients with comorbidities | No method to determine total number of exposed patients | Multiple competing JIA patient registries unsustainable |
| Randomized withdrawal clinical trial design | Comparator group information is often unobtainable | |
| Limit placebo exposure (no comparator group) | ||
| Selection bias: eliminates nonresponder patients and patients with adverse events |
The FDA Sentinel System has been implemented to augment the passive surveillance systems by actively querying multiple data systems, such as public and private payers and pharmacy databases.
These limitations are illustrated in the etanercept post-marketing registry in polyarticular course JIA,25 a 3-year study comparing methotrexate alone, etanercept alone, and the combination of etanercept and methotrexate. The etanercept registry enrolled 594 participants (of whom 397 patients received at least 1 dose of etanercept), but only 245 (41%) completed 3 years of observation, of whom only 179 had been exposed to etanercept, showing that single-product registries often do not provide the robust long-term data sought by investigators and patients. The 2 most recently approved immunomodulatory products for polyarticular-course JIA (www.clinicaltrials.gov identifier NCT00783510 [adalimumab], identifier NCT01357668 [abatacept]), are fulfilling post-marketing requirements and commitments for single-product registries with 10 years of follow-up and greater numbers of participants exposed to the product compared with the etanercept registry; however, the high discontinuation rates seen over just 3 years in the etanercept registry25 as well as the limited numbers of available participants for competing registries are likely to lead to vanishingly small numbers of participants over the life of each product-specific study.
The lack of comprehensive pediatric safety data on immunomodulatory products is a hardship for both families and health care providers when weighing the risks and benefits of powerful products. With large numbers of immunomodulatory products under study in current or planned clinical trials, clinical investigators will be unable to meet enrollment targets for multiple, single-product registries. Biopharmaceutical companies compete for the same pool of patients and investigators globally with significant overlap of the collected information for each product-specific registry. Interpretation of collected data is complicated by serial use of different immunomodulatory products by participants over the duration of each registry, as well as the use of concomitant medications. Clearly a model that better meets the public mandate for efficient and relevant safety surveillance of these agents is urgently needed.
A Model for Safety Surveillance: Consolidated Safety Registry
Over the past 3 years, the Childhood Arthritis and Rheumatology Research Alliance (CARRA)26 and the Duke Clinical Research Institute (DCRI)27 in collaboration with the FDA, industry representatives, other governmental organizations, as well as patient and family advocacy groups, have developed a novel pharmacosurveillance model that will be based on an established multicenter observational pediatric rheumatic disease registry (The CARRA Registry). Contributors and advisors to this effort included representatives from CARRA, DCRI, Pediatric Rheumatology Collaborative Study Group, National Institutes of Health (NIH), FDA, Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, Arthritis Foundation (AF), Lupus Foundation of America, Friends of CARRA, Pediatric Rheumatology International Trials Organization, Pediatric Rheumatology European Society, European Medicines Agency, Pharmaceutical Research and Manufacturers of America, Biotechnology Industry Organization, and representatives of individual pharmaceutical companies. The effort to establish a consolidated safety registry was initially launched at a public workshop sponsored by the FDA in May 2009,28 and further expanded in a stakeholder meeting held June 2011, leading to the development of the CARRA-Consolidated Safety Registry (CARRA-CoRe).
A consolidated disease-based safety registry can address many limitations of product-specific registries as shown in Table 2. For patients, families, clinicians, and biopharmaceutical companies, a consolidated safety registry offers the opportunity to more efficiently evaluate the risk/benefit ratio of treatments with less redundant information, and reflects treatment usage within the context of usual clinical care. Importantly, the consolidated safety registry allows for a more scientifically robust approach to collecting safety data on products of interest while fulfilling biopharmaceutical companies’ post-marketing requirements at the same or lower cost. The following sections describe critical elements of the model and the accompanying development plan and protocol for CARRA-CoRe.
TABLE 2.
Benefits of CARRA-CoRe Compared With Traditional Product-Specific Registries
| Characteristics of Consolidated Disease-Based Safety Registries | Benefits |
|---|---|
| Broad inclusion criteria allowing enrollment of all patients with specific diseases into a single registry | Larger sample sizes |
| Inclusion of comparator groups | |
| More closely represents the target population | |
| Improved ability to detect serious and/or rare adverse events | |
| Systematic, active surveillance of adverse events and outcomes | |
| No competition for patients as occurs with multiple single-product registries | |
| Long-term retention of patients in registry as medication usage, age, and geographic location change | |
| Insight into how medications are actually used in the target population by practitioners | |
| Consolidated safety registry infrastructure, standardized data entry and management, policies, procedures | Decreased variability in data entry, improving quality while limiting monitoring and data cleaning burden, and improving regulatory compliance |
| Leverage existing rapid start agreements with sites, site performance training and regulatory processes, improving efficiency of study start-up and conduct | |
| Allows multiple concurrent safety surveillance projects for different products | |
| Long-term follow-up into adulthood improves assessment of events with long latency times | |
| Collaborative study design includes industry input while assuring scientific independence | |
| Standard of care outpatient visits and diagnostic studies decreases the registry’s cost per patient | |
| Ability to perform multiple concurrent projects of interest to stakeholders: investigators, industry partners, funding agencies, and patients | |
| Comprehensive and cohesive scientific oversight platform for design and data analysis | Scientifically sound and efficient approach to meet post-marketing requirements and commitments for registries |
| Improved assessment of the risk/benefit ratio of treatment options for patients, families, and providers | |
| Ability to compare multiple exposed and unexposed groups to more accurately analyze the strength of the association of adverse events to products | |
| Improved ability to understand contribution of disease course and severity to adverse events and outcomes | |
| Ability to incorporate patient- and family-centered goals and patient-reported outcomes | |
| Engagement of patients and families in design and governance of the consolidated safety registry | Patients, families, and clinicians have input into the evaluation of the risk/benefit ratio of treatment options |
| Improved patient and family trust in immunomodulatory products and the pharmaceutical industry owing to transparent governance and scientific oversight |
Development of the CARRA Consolidated Safety Registry
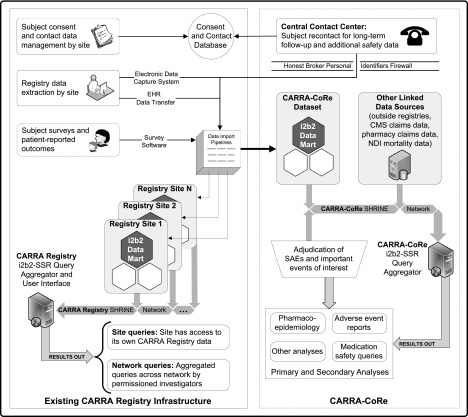
CARRA was founded in 2002 by pediatric rheumatologists with the mission to prevent, treat, and cure rheumatic diseases in children and adolescents through fostering, facilitating, and conducting high-quality clinical and translational research. There are now 107 CARRA sites with 376 active members, representing the vast majority of pediatric rheumatologists in the United States and Canada. CARRA’s administrative structure is funded by the AF, and the majority of its trials and research studies have been funded by the NIH and foundations. CARRA’s scientific committee structure continuously drives a diverse project portfolio that currently includes 27 active projects and multiple completed studies and trials.26 The CARRA Registry was initially funded by an NIH American Recovery and Reinvestment Act of 2009 award with additional support from AF and Friends of CARRA (a nonprofit parent run organization supporting CARRA). The CARRA Registry is based on a 21CFR11-compliant electronic data entry foundation with a modular, flexible, extensible research data storage and framework.29,30 Registry enrollment started in May 2010 and includes longitudinal data on all major pediatric rheumatic diseases from 60 US pediatric rheumatology centers with the goal of following at least 10 000 participants for over 10 years. Table 3 shows enrollment in the CARRA Registry as of February 2013. The establishment of CARRA-CoRe will build on the existing infrastructure of CARRA and the CARRA Registry, which will necessitate expansion of the existing registry data collection to include more extensive safety surveillance and detailed medication use. Figure 2 describes the data architecture of the CARRA Registry and CARRA-CoRe, and Table 4 explains the data flow and the elements in the figure.
TABLE 3.
Overview of Participants in the CARRA Registry (February 2013)
| Number of Participants | % Current or Previous Exposure to Biologicsa | % Current or Previous Exposure to DMARDS or Immune Suppressive Medicationsb | |
|---|---|---|---|
| Total number enrolled | 8211 | 34.5 | 75.9 |
| Specific diagnosesc | |||
| JIA | 5823 | 42.9 | 72.3 |
| SLE | 834 | 11.5 | 95.1 |
| Mixed connective tissue disease | 141 | 23.4 | 92.9 |
| Juvenile dermatomyositis | 552 | 11.2 | 94.4 |
| Vasculitis | 170 | 27.1 | 82.4 |
| Systemic sclerosis | 48 | 2.1 | 87.5 |
| Localized scleroderma | 316 | 2.9 | 86.7 |
| Sjogren syndrome | 16 | 18.8 | 81.3 |
| Autoinflammatory disease | 52 | 25.0 | 23.1 |
| Idiopathic uveitis | 70 | 55.7 | 82.9 |
| Sarcoidosis | 45 | 53.3 | 77.8 |
| Pain syndromes | 144 | 2.1 | 5.6 |
DMARDS, disease-modifying antirheumatic drugs; SLE, systemic lupus erythematosus.
Includes abatacept, adalimumab, anakinra, belimumab, canakinumab, certolizumab, etanercept, golimumab, infliximab, rilonacept, rituximab, and tocilizumab. Excludes intravenous immunoglobulin.
Includes azathioprine, cyclophosphamide, cyclosporine, hydroxychloroquine, leflunomide, methotrexate, mycophenylate mofetil, sulfasalazine, and tacrolimus. Excludes corticosteroids.
Additional diagnoses added as of February 2, 2012 include Sjogren syndrome, autoinflammatory diseases, and idiopathic uveitis.
FIGURE 2.
CARRA Registry and CARRA-CoRe data architecture. The current CARRA Registry infrastructure is shown on the left panel of the figure. The right panel illustrates components that extend the existing CARRA Registry infrastructure for CARRA-CoRe functions.
TABLE 4.
Key Elements of the CARRA Registry and CARRA-CoRe Data Architecture
| Data Flow | Key Features |
|---|---|
| Data entry and imports | The CARRA Registry has for FDA 21CFR11 compliant site data entry using an electronic data capture system and, at certain sites, electronic health record data transfer. |
| Subjects are consented at each site. | |
| Contact information and copies of informed consents are maintained in the Consent and Contact Database that is managed by the Long-Term Follow-up Group at the DCRI. | |
| Access to clinical data and contact data are separated by a Personal Identifiers Firewall (at both organizational and electronic levels) to ensure privacy and protect the usage of contact and personal health information. | |
| Patient-reported outcomes and surveys are collected and entered into the electronic data system or entered directly by subjects. | |
| Data exports | Imported data are securely routed to site-specific i2b2 datamarts corresponding to the originating sites. |
| Multiple virtual i2b2 databases are hosted on a central i2b2 server “farm,” allowing scalability to add sites, other sources of data entry, or specific projects such as CARRA-CoRe. | |
| Permissioned sharing of data is governed through the Shared Health Research Information Network. | |
| The CARRA Registry i2b2-Self Scaling Registry Query Aggregator and User Interface permits each site to query its own CARRA Registry data. | |
| Permissioned investigators can perform network queries to access and analyze aggregated data across 2 or more network sites. | |
| CARRA-CoRe has a virtual i2b2 datamart incorporating data imports from the CARRA Registry with additional CARRA-CoRe-specific fields. | |
| Additional data from review of medical records for serious adverse events or prespecified important medical events are entered into the CARRA-CoRe datamart. | |
| Additional linked data sources are added as appropriate to the CARRA-CoRe requirements. | |
| CARRA-CoRe queries can provide multiple opportunities for primary and secondary analyses relevant to pharmacoepidemiology, adverse events, medication safety, and other areas of interest. |
The CARRA-CoRe protocol includes approaches to general data collection, management, and analysis, with enhancements if requested by industry partners. The overall approach includes initial online electronic data capture with eventual direct electronic health record transfer. Follow-up occurs twice yearly (approximately every 6 months). Active surveillance is performed either at the site or centrally. The DCRI will actively survey patients no longer connected with a site or lost to follow-up. Serious adverse events and prespecified important medical events are collected and centrally categorized using the Medical Dictionary for Regulatory Activities. Incoming data are monitored for irregularities that may prompt “for cause” site monitoring at any time in addition to periodic routine on-site monitoring. For each product of interest, methodologic and clinical factors will be considered in selecting the most appropriate study design (cohort, case control, or case only design), defining study populations and comparators, assessing and categorizing exposure status over time, accounting for potential confounders, and considering effect modifications by patient factors. After carefully designing studies for specific products of interest, the best analytical approaches will be chosen, including multivariable regression, propensity scoring, and methods to account for time-varying confounding such as marginal structural modeling.
CARRA-CoRe Governance and Scientific Oversight
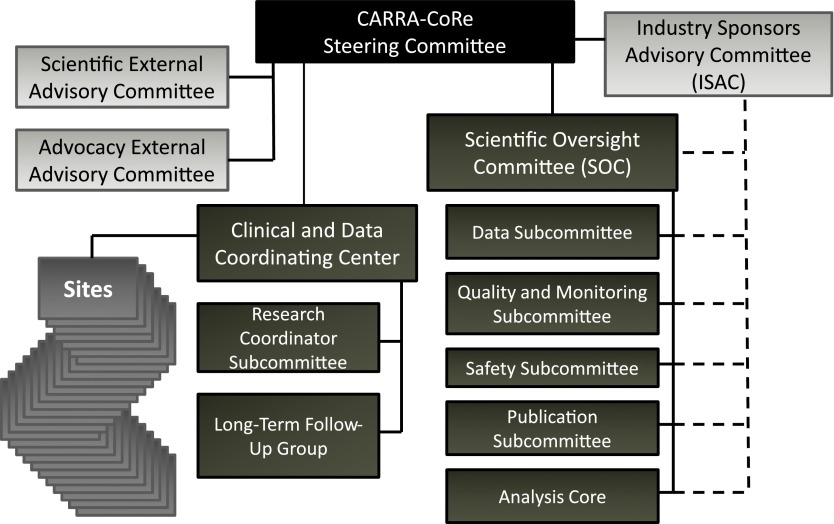
The proposed governance plan (Fig 3) ensures representation by all relevant stakeholders and optimizes scientific collaboration while maintaining scientific independence. Key elements that form the foundation for a robust governance plan are shown in Table 5. The CARRA-CoRe Steering Committee is responsible for setting strategic directions, allocating resources for registry infrastructure and operations, appointing committee members, providing ethics oversight, and approving policies and new studies presented by the Scientific Oversight Committee (SOC). Steering Committee membership includes pediatric and adult rheumatologists with relevant expertise, a research coordinator representative, the SOC Chair, DCRI representatives, patient/family advocates, and representatives from industry sponsors (non-voting). The Steering Committee does not participate directly in project design or development of the analysis plan, and does not make decisions regarding data access, approval of publications, or safety reporting. These responsibilities are delegated to the SOC to insure scientific independence and manage conflicts of interest. The SOC consists of members with expertise in areas including pharmacoepidemiology, epidemiology, biostatistics, pediatric rheumatology, registries, and informatics. Through subcommittees, the SOC provides independent scientific oversight, including assessment of new projects, registry data design, data query requests, data access, data analyses, adjudication of safety events, publications, and identification of safety trends and signals. The Industry Sponsors Advisory Committee (ISAC) is designed to interact directly with the Steering Committee and the SOC. Each major industry sponsor has a non-voting representative on the Steering Committee. The ISAC ensures ongoing dialogue and collaboration between the Steering Committee, the SOC, and industry partners by facilitating the inclusion of industry expertise on SOC subcommittees addressing registry design and data analysis plans. This interaction will leverage national experts and ensure success in meeting post marketing commitments and requirements. There are 2 External Advisory Committees (EAC), the Scientific EAC and the Advocacy EAC. The Scientific EAC, composed of scientific experts, including NIH and FDA representatives, reviews the scientific approach, organization, and progress, making recommendations to the Steering Committee. The Advocacy EAC includes advocacy groups and family/patient representatives who review and advise the Steering Committee regarding general principles and progress. CARRA-CoRe’s Data Coordinating Center (DCRI) provides significant expertise in contracting, research coordinator support, regulatory compliance, site management, registries and large databases, and safety monitoring and reporting. DCRI has executed rapid start agreements with the current CARRA Registry sites. DCRI has an established administrative center for managing subject verification as well as long-term follow-up. It verifies that a valid informed consent exists for each subject, maintains a central repository of contact information that is firewalled from the CARRA-CoRe clinical database to protect patient confidentiality (“honest broker”),31 and manages a centralized contact center for long-term follow-up that will be especially important as participants transfer out from pediatric to adult medical services. Data access will be governed by the rigorous CARRA Data and Sample Sharing policies and overseen by the SOC. Industry partners will have full access to data query outputs and datasets for their respective products along with input into analysis plans in accordance with the policies and procedures of the SOC and the ISAC.
FIGURE 3.
CARRA-CoRe organizational structure. The CARRA-CoRe structure is designed to insure scientific independence through the Scientific Oversight Committee, while the Steering Committee is responsible for strategy, operational oversight, resources, involvement of industry sponsors, and input from Advisory Committees.
TABLE 5.
Key Elements of CARRA-CoRe Governance Plan
| Principle | Challenge Addressed | CARRA-CoRe Solution |
|---|---|---|
| Formal role for patients, families, and patient advocates in governance | Ensuring governance appropriately reflects the social contract to protect the safety of patients | Steering Committee membership includes family/patient advocates and the Advocacy External Advisory Committee reviews progress and future directions, making recommendations as indicated. |
| Formal industry role in governance and scientific design | Protecting industry interests in decision-making and scientific design | The Industry Sponsor Advisory Committee appoints 2 Steering Committee members as well as ad hoc industry experts to advise the SOC (see below) on study design. |
| Independent scientific oversight | Ensuring a process to mitigate potential conflicts of interest | The SOC is solely responsible for scientific design, study conduct, and analysis without formal industry or other private or public membership. Scientific participation from industry is assured through the ISAC providing input to the SOC to manage conflicts of interest among industry participants with competing products. The Scientific EAC reviews the scientific progress and direction, making recommendations where indicated. |
| Efficient policies and procedures for data quality and monitoring | Balancing high-quality data collection and management with efficient and less costly data verification and monitoring procedures | Efficient policies and procedures optimize data integrity through use of electronic monitoring where appropriate, supplemented with routine and for cause on-site monitoring.a |
| Reliable adverse event adjudication and reporting | Ensuring a reliable process for efficient detection and management of adverse events | The SOC is responsible for the evaluation, adjudication, and appropriate notification and reporting of all serious adverse events and events of special interest. Adjudication will include subspecialty experts as necessary. Validated definitions of pediatric SAEs and events of interest are being developed with international partners. |
| Risk attribution in the presence of multiple exposures | Balancing patient care needs requiring treatment adjustments with interpretation of adverse events in the context of multiple exposures over time | Fair and transparent policies and procedures developed by the SOC with input from industry govern analyses of serious adverse events in the context of multiple exposures, risk windows, and other approaches. |
| Long-term follow-up | Implementing processes and procedures to follow participants who relocate or transfer to adult care | The Long-Term Follow-Up Group within the DCRI Clinical and Data Coordinating Center manages a centralized communication repository, including confidential contact information for participants. Efforts to harmonize a validated set of outcome tools and patient-reported outcome measures that can be used across the lifespan are underway. In addition, the protected contact repository will facilitate potential future linkage to large administrative and billing databases including the Centers of Medicare and Medicaid Services, pharmacy databases, insurance databases, cancer registries, death index, and other registries and resources. |
| Patient confidentiality | Assuring the highest level of personal health information protection | Participants’ personal health information is sequestered from clinical data at the sites or by the Long-Term Follow-Up Group (Honest Broker)31 |
| Data access and sharing | Insuring responsible and transparent stewardship of data analysis and dissemination and publication of results | Policies and procedures are under development to govern data access, analysis, utilization, dissemination and publication of results to guarantee meeting safety and regulatory requirements and responsible conduct of research. |
| Management of intellectual property and antitrust issues | Balancing protection of public safety with intellectual property interests and antitrust issues | The Steering Committee is responsible for overseeing potential conflicts regarding ethical and regulatory reporting of safety issues with intellectual property or antitrust issues. |
The FDA agreed to this monitoring approach in concept, and the approach is generally consistent with that described in the FDA’s draft monitoring guidance issued in August 2011 and a letter from the Division of Pulmonary, Allergy, and Rheumatology Products, Office of Drug Evaluation II, Center for Drug Evaluation and Research, FDA, regarding CARRA-CoRe monitoring plan dated December 21, 2010.
Sustainability of CARRA-CoRe
The long-term success of a consolidated safety registry depends on adequate sustained funding to support participating sites and central infrastructure, enabling retention of participants into adulthood. Establishing CARRA-CoRe through a public-private partnership agreement addresses the diverse interests of the stakeholders involved: public value and trust, transparency, collaboration, shared financial responsibility, scientific rigor, and future research and development opportunities. The DCRI will work with CARRA, FDA, NIH, nonprofit voluntary health and advocacy organizations, as well as industry funders. The interactive infrastructure of CARRA, CARRA Registry, CARRA-CoRe, and Duke University (DCRI) enables not only organizational sustainability, but facilitates following patients into adulthood.
The financial model proposes public and nonprofit investments in database infrastructure and private industry funding for registry operations with additional joint investments in research and development. Development of the database architecture and infrastructure was enabled by awards from the NIH and nonprofit organizations. Each industry funder will contribute to the ongoing yearly operational costs of CARRA-CoRe and will receive standard data outputs in return. In addition, nonprofit or governmental agencies may contribute to operational costs and sponsor specific data requests of CARRA-CoRe. Industry funders may enter into contracts to meet post-marketing requirements or other organizational objectives. With participation by multiple sponsors, a benefit to industry funders is the ability to include new therapeutic agents without significant additional infrastructure investment. To insure sustainability over time, critical design elements of the CARRA-CoRe public-private partnership include a single point of negotiations based on the existing rapid start agreements of the CARRA Registry consortium, and policies addressing intellectual property, antitrust issues, and conflict resolution. Industry funders will benefit significantly from participation in a public-private partnership through operational efficiencies gained by using a high-functioning centralized network, elimination of competition for participant enrollment, improved ability to accurately assess adverse events, and improved public trust in product safety information.
Future Considerations
Moving forward requires endorsement of a shared commitment to the sustainability and success of CARRA-CoRe by public and private nonprofit and for-profit stakeholders. Industry investment in CARRA-CoRe would ideally replace current expenditures on product-specific safety registries, which are resource-intensive and are unable to yield scientifically rigorous long-term safety information of immunomodulatory agents in children who have rheumatic diseases. The FDA issued a statement that a consolidated safety registry such as CARRA-CoRe (previously called JIA-CoRe) could fulfill post-marketing requirements and replace the current use of product specific registries:
“FDA understands the scientific benefits of a consolidated disease-based registry, such as JIA [sic] CoRe, as this type of registry may overcome some of the limitations of individual product registries, such as small sample size, inadequate “real-world setting” assessments, inability to evaluate disease contribution to the adverse outcomes, and lack of assessments of serial exposures to a variety of anti-rheumatic medications.
Section 505(o) of the Federal Food, Drug, and Cosmetic Act authorizes FDA to require post-marketing studies or clinical trials at the time of approval or after approval if FDA becomes aware of new safety information about a prescription drug or biological product. If the nature of the risk(s) assessed in the post-marketing requirements and commitments can be addressed by either utilizing the existing data elements from JIA [sic] CoRe or conducting prospective data collection within the JIA [sic] CoRe infrastructure, then such data could provide the information necessary for individual companies to satisfy post-marketing requirements and commitments and obviate the need for an individual product registry. Additionally, such a consolidated registry would be superior to multiple sponsors-maintained registries for the reasons outlined above.”
Excerpt from letter dated December 9, 2011 from Janet Woodcock, MD (Director, Center for Drug Evaluation and Research, FDA)
Essential to the success of CARRA-CoRe is the continued engagement of pediatric rheumatologists and their commitment to a long-term longitudinal registry. One of the benefits of CARRA-CoRe and its links to the CARRA Registry is the research opportunities already available to CARRA investigators who have a vested interest in improving outcomes of children who have rheumatic diseases. The engagement of CARRA investigators is demonstrated by the current achievements of the CARRA Registry, which has successfully enrolled over 8211 participants from 60 sites as of February 2013 (Table 2). Data from the registry has already resulted in multiple national presentations and publications.24,32–34 CARRA-CoRe can also provide realistic estimates of available participants to inform the appropriate design of pre- and post-marketing studies. Importantly, industry funders can use CARRA-CoRe and the CARRA Registry to address additional questions of interest and to identify other areas of potential collaboration with the pediatric rheumatology community. Coordination with similar efforts ongoing in Europe is underway to facilitate harmonization of adverse event data for analysis of post-marketing safety on a global scale. There are existing European national JIA safety registries that continue to collect data, and the Paediatric Rheumatology European Society along with the Pediatric Rheumatology International Trials Organization has launched PHARMACHILD, a JIA disease-specific pharmacovigilance registry for biologic products, supported by the European Union (7th Framework Program) and pharmaceutical industry. Opportunities to use mapping ontologies to combine data from the various registries are also being explored.
Conclusions
A consolidated safety registry provides many advantages over current methods of pharmacosurveillance for immunomodulatory products used to treat pediatric rheumatic diseases. By collaborating with industry partners, advocacy groups, DCRI, and government agencies such as the FDA, NIH, and European Medicines Agency, a consolidated safety registry such as CARRA-CoRe can overcome barriers to markedly improve the ability to capture and understand long-term and rare adverse events. This critical knowledge will translate directly into better medication usage and improved patient care and safety. Ultimately this model can be a powerful and efficient tool to understand rare and serious adverse events as well as events with long latency in other childhood and adult chronic and complex diseases.
Acknowledgments
The authors acknowledge the contributions of Marelle Molbert, who organized the June 2011 meeting entitled “Disease-wide Consolidated Registries: A New Model for Capturing Rare Adverse Events in Juvenile Idiopathic Arthritis (JIA) and Pediatric Rheumatic Diseases,” as well as the following contributors for their critical input and advice in conceptualizing this project: Hermine Brunner, MD, MSc, MBA, Robert Califf, MD, Robert H. Carter, MD, Mary R. Creed, MSN, Esi Morgan DeWitt, MD, MSCE, Denise Dougherty, PhD, Edward Giannini, MSc, DrPH, John Hardin, MD, Robert Harrington, MD, Jennifer Hootman, PhD, ATC, Larissa Lapteva, MD, MHS, Daniel J. Lovell, MD, MPH, Kelly L. Mieszkalski, MA, CCRC, Daniel Rotrosen, MD, Nicola Ruperto, MD, MPH, Susana A. Serrate-Sztein, MD, and Sally Seymour, MD. The authors also acknowledge industry attendees who participated in the June 2011 meeting: Ken Abrams, MD, Novartis Pharmaceuticals; Michele Hooper, MD, MS, Amgen; Gregory F. Keenan, MD, Human Genome Sciences; Stephan Korte, PhD, Novartis Pharma AG; Charles A. Mebus, MD, Pfizer, Inc; John Medich, PhD, AbbVie; Ronald Portman, MD, Bristol-Myers Squibb; Ralph Preiss, MD, Novartis Pharmaceuticals; Debbie K. Tokimoto, MPH, AbbVie; Raymond C. Votzmeyer, MBA, AbbVie; Joseph Wajdula, PhD, Pfizer, Inc; and Debra J. Zack, MD, PhD, Amgen.
Glossary
- AF
Arthritis Foundation
- CARRA
Childhood Arthritis and Rheumatology Research Alliance
- CARRA-CoRe
CARRA Consolidated Safety Registry
- DCRI
Duke Clinical Research Institute
- EAC
external advisory committees
- FDA
Food and Drug Administration
- ISAC
Industry Sponsors Advisory Committee
- JIA
juvenile idiopathic arthritis
- NIH
National Institutes of Health
- PREA
Pediatric Research Equity Acts
- SOC
Scientific Oversight Committee
- TNF
tumor necrosis factor
Footnotes
Dr Lionetti participated in the conceptualization and design of the project, organized and drafted the initial manuscript, and reviewed and edited subsequent drafts; Dr Beukelman participated in the conceptualization and detailed design of the project and assisted in drafting the manuscript; Drs Schanberg, Wallace, and Ilowite participated in the conceptualization and detailed design of the project and the registry database and assisted in drafting and editing the manuscript; Ms Winsor and Dr Natter participated in the conceptualization and detailed design of the registry database and reviewed and revised the manuscript; Ms Fox, Drs Sundy, Brodsky, and Jahreis participated in the conceptualization and detailed design of key aspects of the project and reviewed and revised the manuscript; Drs Curtis, Iyasu, Meeker-O’Connell, Mittleman, Murphy, Peterson, Raymond, Setoguchi, Siegel, Sobel, Solomon, Southwood, Vesely, White, Wulffraat, and Mr Del Gaizo participated in the conceptualization and design of key aspects of the project and reviewed and revised the manuscript; Drs Kimura and Sandborg conceptualized and designed the entire project and assisted in drafting and editing the manuscript; and all authors approved the final manuscript as submitted.
The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by NIH grant AR063890 and NIH ARRA grant AR058934, Arthritis Foundation, Friends of CARRA, and contributions from Abbott Laboratories, Amgen, Inc, Bristol Myers Squibb Company, Genentech, Inc, Human Genome Sciences, Inc, Novartis Pharmaceuticals, and Pfizer, Inc. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Beukelman has received grant support from Pfizer, Inc and has been on the advisory board for Novartis and Genentech, Inc. Dr Schanberg has been on the advisory board for Novartis and has acted as a consultant for UCB and GlaxoSmithKline. Dr Wallace has been on the advisory boards for Novartis and Genentech, Inc and has received research grants from Amgen and Pfizer, Inc. Dr Sundy has received grant support from Abbott, Janssen Pharmaceuticals, Regeneron, Bristol Myers Squibb, and Genentech, Inc. He also has acted as a consultant for Novartis, Regeneron, Bristol Myers Squibb, and Pfizer, Inc. Dr Curtis has acted as a consultant for and has received honoraria and grant support from Roche, UCB, Janssen, CORRONA, Amgen, Bristol Myers Squibb, Crescendo, AbbVie, Genentech, and Pfizer, Inc. Dr Jahreis is an employee of Genentech, Inc and has stock and stock options in Roche. Dr Peterson has acted as a consultant for Janssen Pharmaceuticals, Pfizer, Inc, and Boerhinger Ingelheim. He has received grant support from Janssen Pharmaceuticals and Lilly. Dr Siegel is an employee at Genentech, Inc. Dr Sobel is an employee and shareholder of Pfizer, Inc. Dr Ilowite has acted as the Macrophage Activation Syndrome Adjudication Committee Chair for Novartis and the Data and Safety Monitoring Board Chair for Janssen, and is the Principal Investigator for an NIH sponsored clinical trial for which Regeneron has provided drug and placebo. Dr Solomon has received grant support from Lilly, Amgen, and CORRONA. He has received royalties related to chapters from UpToDate and advisory board membership honoraria from the American Orthopedic Association. He has served as an unpaid member on the executive committee for a trial on NSAIDs led by Pfizer, Inc and as an unpaid member on the Data and Safety Monitoring Board for a trial on canakinumab led by Novartis. Dr Wulffraat has served on the advisory boards for Novartis, Astellas, and Pfizer, Inc. He has received educational grants from Roche, AbbVie, and GlaxoSmithKline. Dr Kimura has served on the advisory boards for Novartis and Genentech, Inc. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hayward K, Wallace CA. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovell DJ, Giannini EH, Reiff A, et al. Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342(11):763–769 [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340(4):253–259 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez W, Selen A, Avant D, et al. Improving pediatric dosing through pediatric initiatives: what we have learned. Pediatrics. 2008;121(3):530–539 [DOI] [PubMed] [Google Scholar]
- 5.Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62(8):2517–2524 [DOI] [PubMed] [Google Scholar]
- 6.Beukelman T, Haynes K, Curtis JR, et al. Safety Assessment of Biological Therapeutics Collaboration . Rates of malignancy associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(4):1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison M, Nordstrom B, Gu Y, Mercaldi C, Aquino P, Mines D. Risk of malignancy in biologics-naive juvenile arthritis patients [abstract]. Ann Rheum Dis. 2010;69(suppl 3):631
- 8.Nordstrom BL, Mines D, Gu Y, Mercaldi C, Aquino P, Harrison MJ. Risk of malignancy in children with juvenile idiopathic arthritis not treated with biologic agents. Arthritis Care Res (Hoboken). 2012;64(9):1357–1364 [DOI] [PubMed] [Google Scholar]
- 9.Simard JF, Neovius M, Hagelberg S, Askling J. Juvenile idiopathic arthritis and risk of cancer: a nationwide cohort study. Arthritis Rheum. 2010;62(12):3776–3782 [DOI] [PubMed] [Google Scholar]
- 10.Bernatsky S, Rosenberg AM, Oen KG, et al. Malignancies in juvenile idiopathic arthritis: a preliminary report. J Rheumatol. 2011;38(4):760–763 [DOI] [PubMed] [Google Scholar]
- 11.Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk of new-onset uveitis in patients with juvenile idiopathic arthritis treated with anti-TNFalpha agents. J Pediatr. 2006;149(6):833–836 [DOI] [PubMed] [Google Scholar]
- 12.van Dijken TD, Vastert SJ, Gerloni VM, et al. Development of inflammatory bowel disease in patients with juvenile idiopathic arthritis treated with etanercept. J Rheumatol. 2011;38(7):1441–1446 [DOI] [PubMed] [Google Scholar]
- 13.Winthrop KL, Chen L, Fraunfelder FW, et al. Initiation of anti-TNF therapy and the risk of optic neuritis; from the Safety Assessment of Biologic ThERapy (SABER) Study. Am J Ophthalmol. 2012;. 155(1):183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y, Weiss JE, Haroldson K, Lee T, Punaro M, Oliveira S, et al; CARRAnet Investigators. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res. 2013;65(5):745–752. [DOI] [PMC free article] [PubMed]
- 15.Safe and Effective Medicines for Children Pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. In: Marilyn J, ed. Institute of Medicine. Washington, DC: Field TFB; 2012 [PubMed] [Google Scholar]
- 16.Fact Sheet: Pediatric provisions in the Food and Drug Administration Safety and Innovation Act (FDASIA). Available at: www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm311038.htm
- 17.Mathis LL, Iyasu S. Safety monitoring of drugs granted exclusivity under the Best Pharmaceuticals for Children Act: what the FDA has learned. Clin Pharmacol Ther. 2007;82(2):133–134 [DOI] [PubMed] [Google Scholar]
- 18.Smith PB, Benjamin DK, Jr, Murphy MD, et al. Safety monitoring of drugs receiving pediatric marketing exclusivity. Pediatrics. 2008;122(3). Available at: www.pediatrics.org/cgi/content/full/122/3/e628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field MJ, Ellinger LK, Boat TF. IOM Review of FDA—approved biologics labeled or studied for pediatric use. Pediatrics. 2013;131(2):328–335 [DOI] [PubMed] [Google Scholar]
- 20.Section 505B of the Federal Food, Drug, and Cosmetic Act [21U.S.C. 355c] 2002: 124. Available at: www.epw.senate.gov/FDA_001.pdf.
- 21.Lovell DJ, Ruperto N, Goodman S, et al. Pediatric Rheumatology Collaborative Study Group. Pediatric Rheumatology International Trials Organisation . Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810–820 [DOI] [PubMed] [Google Scholar]
- 22.Ruperto N, Lovell DJ, Cuttica R, et al. Paediatric Rheumatology International Trials Organisation. Pediatric Rheumatology Collaborative Study Group . A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096–3106 [DOI] [PubMed] [Google Scholar]
- 23.Ruperto N, Lovell DJ, Quartier P, et al. Paediatric Rheumatology INternational Trials Organization. Pediatric Rheumatology Collaborative Study Group . Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–391 [DOI] [PubMed] [Google Scholar]
- 24.Beukelman T, Xie F, Chen L, et al. SABER Collaboration . Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(8):2773–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini EH, Ilowite NT, Lovell DJ, et al. Pediatric Rheumatology Collaborative Study Group . Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(9):2794–2804 [DOI] [PubMed] [Google Scholar]
- 26.Childhood Arthritis and Rheumatology Research Alliance (CARRA). Available at: www.carragroup.org. Accessed June 2013
- 27.Duke Clinical Research Institute. Available at: https://dcri.org/. Accessed June 2013
- 28.Smith MY, Sobel RE, Wallace CA. Monitoring the long-term safety of therapies for children with juvenile idiopathic arthritis: time for a consolidated patient registry. Arthritis Care Res (Hoboken). 2010;62(6):800–804 [DOI] [PubMed] [Google Scholar]
- 29.Natter MD, Quan J, Ortiz DM, et al. An i2b2-based, generalizable, open source, self-scaling chronic disease registry. J Am Med Inform Assoc. 2013;20(1):172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber GM, Murphy SN, McMurry AJ, et al. The Shared Health Research Information Network (SHRINE): a prototype federated query tool for clinical data repositories. J Am Med Inform Assoc. 2009;16(5):624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin W, Singh H, Pople AK, et al. A decade of experience in the development and implementation of tissue banking informatics tools for intra and inter-institutional translational research. J Pathol Inform. 2010;1(Aug 10):ii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss PF, Beukelman T, Schanberg LE, Kimura Y, Colbert RA, CARRA Registry Investigators . Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol. 2012;39(12):2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beukelman T, Ringold S, Davis TE, et al. CARRA Registry Investigators . Disease-modifying antirheumatic drug use in the treatment of juvenile idiopathic arthritis: a cross-sectional analysis of the CARRA Registry. J Rheumatol. 2012;39(9):1867–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringold S, Beukelman T, Nigrovic PA, Kimura Y, CARRA Registry Site Principal Investigators . Race, ethnicity, and disease outcomes in juvenile idiopathic arthritis: a cross-sectional analysis of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J Rheumatol. 2013;40(6):936–942 [DOI] [PubMed] [Google Scholar]