Abstract
BACKGROUND:
The rising population of individuals identified with an autism spectrum disorder (ASD) calls for further investigation of its underlying etiology. A disturbance in the fetal steroid hormone environment may be a mechanism in which environmental and genetic risk factors interact. The mother, fetus, and placenta collectively create the fetal steroid environment. Prepregnancy BMI and pregnancy weight gain have served as markers for fetal steroid hormone exposure in other disease states. This study’s objective is to determine whether prepregnancy BMI and pregnancy weight gain are associated with increased ASD risk across study designs and cohorts while controlling for important confounding variables.
METHODS:
A population-based Utah ASD cohort (n = 128) was ascertained in a 3-county surveillance area and gender- and age-matched to 10 920 control subjects. A second, research-based ASD cohort of Utah children (n = 288) and their unaffected siblings (n = 493) were ascertained through participation in an ASD genetics study. Prenatal variables were obtained from birth certificate records.
RESULTS:
ASD risk was significantly associated with pregnancy weight gain (adjusted odds ratio = 1.10, 95% confidence interval: 1.03 to 1.17; adjusted odds ratio = 1.17, 95% confidence interval: 1.01 to 1.35 for each 5 pounds of weight gained), but not prepregnancy BMI, in population and research-based cohorts, respectively. When analyses were restricted to ASD cases with normal IQ, these associations remained significant.
CONCLUSIONS:
ASD risk associated with a modest yet consistent increase in pregnancy weight gain suggests that pregnancy weight gain may serve as an important marker for autism’s underlying gestational etiology. This justifies an investigation into phenomena that link pregnancy weight gain and ASD independent of prepregnancy BMI.
Keywords: autism, prenatal, weight gain, risk factors, epidemiology
What’s Known on This Subject:
Previous studies have found links between prepregnancy BMI and/or pregnancy weight gain and autism spectrum disorders (ASD) risk. Several contributing factors to BMI and pregnancy weight gain (ie, prematurity, advanced maternal age, parental education, and parity) overlap with established ASD risk factors.
What This Study Adds:
This study identifies an association between ASD risk and prenatal weight gain, but not prepregnancy BMI, and accounts for important confounding variables excluded in previous analyses. It provides the first within-mother comparison of these factors by including unaffected sibling controls.
Autism spectrum disorders (ASD) are neurobehavioral disorders manifested by a range of impaired social interactions, abnormal language development, and stereotypic behavior and interests.1 The rising population of individuals identified with ASD2–4 calls for further investigation of its underlying etiology. Although established risk factors for autism traditionally fall into distinct genetic and environmental categories, these can converge when environmental triggers directly mediate ASD phenotypes in genetically vulnerable individuals. Steroid hormones in the fetal environment are environmental factors that directly affect fetal gene transcription and expression through DNA binding during vulnerable periods of embryonic development.5–8 Termed the “maternofetoplacental unit,” mother, fetus, and placenta collectively create the in utero steroid environment through the complex exchange of steroid precursors.9 A disturbance in this steroid production, hereafter referred to as “in utero steroid dysregulation,” may be a potentially modifiable ASD environmental risk factor.
Proxies for in utero steroid hormone exposure have included prepregnancy BMI and pregnancy weight gain because of their association with adiposity and metabolic syndrome.10–14 Steroid dysregulation characterizes the most common metabolic syndrome among reproductive-aged women, polycystic ovary syndrome, in which 40% to 80% of those affected experience excess weight.15,16 Prepregnancy BMI and pregnancy weight gain have been found to correlate with maternal prenatal hormone levels17–20 and hormone-mediated cancer risk in offspring.11,12,14,21,22 Data from the Utah Pregnancy Risk Assessment Monitoring System from 2000 through 2005 indicate a 19% rise in excessive pregnancy weight gain23; these data also show that during the same time period, the proportion of women who were obese before pregnancy rose 25%.24
Maternal BMI and gestational weight gain have previously been associated with increased risk for developmental disability. This effect has been demonstrated in a Danish study that examined 2 separate birth cohorts separated by 2 decades.25 Heikura et al (2008) identified prepregnancy BMI ≥30 as a risk factor for intellectual disability (ID) in a mid-1980s birth cohort but not in a mid-1960s cohort, suggesting this risk factor had emerged over the interim years. Other previous studies have found an association between prepregnancy obesity and/or weight gain during pregnancy and ID.26–28 More recently, studies have extended this investigation to include autism spectrum disorders. The Nurses Health Study II found a link between an elevated maternal BMI at 18 years of age and increased risk of having a child with ASD.29 However, BMI measures more proximal to the birth of the affected child did not correlate with ASD risk. In a database cohort study of infants born between 1990 and 2002, Dodds et al (2011) reported prepregnancy obesity and excessive weight gain during pregnancy among the risk factors identified for ASD.30 These weight-related risk factors were of more importance for nonfamilial autism, defined as only 1 affected child in a family and negative maternal psychiatric or neurologic history.
These studies support the connection between maternal obesity and ASD, but confounding factors potentially shared by ASD and obesity (such as obstetrical complications, parity, advanced maternal age, and socioeconomic status) were not considered in the obesity/weight gain analyses. Krakowiak et al (2012) compared prepregnancy BMI and other metabolic conditions among children with ASD, developmental disabilities (DD) only, and typical development, using several established ASD risk factors as covariates.27 Obesity alone or obesity together with 1 of 3 additional metabolic conditions (type 2 diabetes, gestational diabetes, hypertension) during the prenatal period significantly increased the risk of ASD or DD compared with the control group. This study controlled for some confounding factors including maternal age but did not control for parity or gestational age. These confounding variables merit consideration because prepregnancy BMI increases progressively with parity and, at its extremes, can be associated with premature birth. Weight gain during pregnancy was also not reported in this study. Interestingly, prenatal obesity and other metabolic conditions conferred a higher risk for DD than for ASD. These early findings need replication with rigorous attention to potentially confounding prenatal variables, familial risk, and co-occurring ID. The current study uses 2 samples of individuals with ASD to investigate the potential role of prepregnancy maternal BMI and weight gain during pregnancy as proxies for in utero steroid dysregulation in the underlying etiology of ASD. The first study group is a population-based ASD sample of children ascertained at 8 years of age using modern strategies and current ASD case definition. The second is an ASD cohort and their unaffected siblings participating in an ongoing ASD genetics study.
Methods
Population-Based 2002 Autism and Developmental Disabilities Monitoring (Utah Site) Surveillance Cohort
Population Characteristics
Surveillance activities targeted the total population of 8-year-old children (26 108) born in 1994, residing in 2002 in Utah’s 3 most populous counties (Salt Lake, Davis, and Utah Counties) where 67% percent of the state’s population resided. Males comprised 52% of the total 8-year-old study population. These children were 81% white non-Hispanic, and 13% were Hispanic. Proportions of other racial categories were significantly less and included 3.4% Asian/Pacific Islander, 1.4% African American, and 0.7% Native American.
Surveillance Methods and Case Definitions
Data were collected as part of the Autism and Developmental Disabilities Monitoring (ADDM) Network in collaboration with Centers for Disease Control and Prevention (CDC) and the Utah Registry of Autism and Developmental Disabilities under the health code reporting rule. Approval was obtained from the Institutional Review Boards of the University of Utah and Utah Department of Health. A full description of the study methodology has been published previously.31,32 A synopsis of the approach follows. Multiple-source screening was conducted at all public schools within the catchment area and all major health sites including, but not limited to, state health clinics, hospitals, clinics, diagnostic centers, and individual providers specializing in services for children with disabilities (33 sites in Utah). In medical settings, records selected for abstractor review were identified through an electronic query of ∼200 International Classification of Diseases, Ninth Revision codes selected by the CDC to include a broad spectrum of child neurodevelopmental disorders and mental health diagnoses. In educational settings, the records of all children receiving special education services during the 2001–2002 and/or 2002–2003 school years were selected for abstractor review. Following CDC protocol, records were abstracted from each site, and case determination was based on the records review and coding methodology developed by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP) and used in the 2002 ADDM Network.31,32 The ADDM ASD case definition was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria for autistic disorder, Asperger disorder, and pervasive developmental disorder, not otherwise specified (including atypical autism). Clinician reviewers for Utah achieved and maintained coding reliability with CDC project staff, other ADDM clinician reviewers, and each other.
Using school and medical records, 196 children met the ADDM case definition of an ASD. Utah birth certificate records from the surveillance area were available for 132 of these children (115 boys, 17 girls). The remaining 64 children were born outside of the study area. There were no statistically significant differences between these 64 children and the 132 children born in the surveillance area with regard to gender, race, presence of ID, and history of regressive onset of autism. All multiple birth pregnancies were removed from the case (n = 4) and control (n = 387) groups because of potential large effects of multiple births on maternal weight gain during pregnancy resulting in 128 ASD cases for analysis. Each of the 128 ASD cases was matched by gender and birth year to 84 controls (the maximum number of available gender-matched controls in the surveillance area) from the birth certificate database. A weighted scheme was applied in which the probability of selection was based on maternal residential zip code so that the selection of controls reflected the geographic distribution of the overall population. Twenty-four percent (n= 31) of birth certificate linked ASD cases were also identified as having ID during the same surveillance study. ID was defined as having an IQ ≤70 on the most recent IQ test or a statement describing the child’s functioning level as being in the ID range during previous psychometric testing if no IQ score were available. The controls were born in the study area and were not identified as having an ASD or ID during the surveillance study. Also, children found to have a medical record containing ≥1 of the 200 International Classification of Diseases, Ninth Revision codes used to identify children for ASD abstraction were removed from the pool of potential controls. These criteria minimize but do not eliminate the possibility of cases existing within the control group. The control group consisted of 9660 boys and 1260 girls resulting in 10 920 total controls. Characteristics of the cases and controls are listed in Table 1.
TABLE 1.
Descriptive characteristics of the ADDM Network and Utah Genetics Study Samples
| Characteristics | ASD Cases | Controls | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | |
| ADDM Network Sample | ||||||
| Maternal age (y) | 128 | 27.33 (5.89) | 16 to 41 | 10920 | 26.54 (5.52) | 14 to 48 |
| Paternal age (y) | 119 | 29.91 (6.36) | 17 to 50 | 10126 | 29.09 (5.99) | 15 to 68 |
| Maternal education (y) | 126 | 13.65 (2.21) | 6 to 17 | 10818 | 13.39 (2.22) | 0 to 19 |
| Paternal education (y) | 117 | 14.00 (2.62) | 1 to 17 | 9977 | 14.13 (2.35) | 0 to 20 |
| Gestational age (wk) | 125 | 38.46 (2.68) | 24 to 45 | 10908 | 39.02 (2.02) | 23 to 51 |
| Parity | 128 | 1.13 (1.38) | 0 to 6 | 10912 | 1.27 (1.48) | 0 to 15 |
| Birth yeara | — | — | — | — | — | — |
| Prepregnancy BMI | 120 | 23.61 (5.19) | 16.06 to 44.38 | 10920 | 23.26 (4.78) | 12.08 to 63.48 |
| Maternal wt gain during pregnancy (lb) | 120 | 33.6 (15.92) | –5 to 140 | 10920 | 30.95 (12.44) | –67 to 144 |
| Utah Genetics Study Sample | ||||||
| Maternal age (y) | 288 | 27.50 (5.41) | 16 to 43 | 493 | 28.88 (5.34) | 17 to 47 |
| Paternal age (y) | 287 | 29.53 (5.97) | 18 to 59 | 487 | 31.05 (6.12) | 19 to 57 |
| Maternal education (y) | 285 | 14.31 (1.72) | 10 to 17 | 490 | 14.43 (1.74) | 11 to 17 |
| Paternal education (y) | 284 | 14.65 (1.93) | 9 to 17 | 478 | 14.78 (2.00) | 10 to 18 |
| Gestational age (wk) | 288 | 38.97 (1.86) | 25 to 43 | 493 | 38.86 (1.95) | 24 to 44 |
| Parity | 288 | 1.30 (1.64) | 0 to 12 | 492 | 1.95 (1.90) | 0 to 15 |
| Birth year | 288 | 1997.60 (3.95) | 1989 to 2005 | 493 | 1999.26 (5.12) | 1989 to 2008 |
| Prepregnancy BMI | 277 | 24.25 (4.89) | 15.95 to 42.77 | 470 | 24.92 (5.51) | 15.45 to 48.86 |
| Maternal wt gain during pregnancy (lb) | 279 | 33.25 (12.49) | –10 to 74 | 478 | 30.48 (12.20) | –24 to 70 |
Birth year is 1994 for entire sample.
Utah Genetics Study Cohort
Participant Characteristics
Individuals with ASD (n = 392) and their siblings (n = 529) were ascertained from ongoing Utah studies of the genetics of autism. Participants were included in the current analysis if they were a singleton birth, linked to a Utah birth certificate in the Utah Population Data Base (UPDB), and born after 1988. This cutoff date reflects the 1989 implementation year of the National Center for Health Statistics’ recommendations, which improved birth certificate accuracy and completeness.33–35 The UPDB is a population-based medical research resource that links to many sets of high-quality, population-based, individual-level records including Utah birth certificate records. Because the analysis focused on within- and between-mother comparison of characteristics of pregnancies resulting in affected versus unaffected children, only children in families with both affected and unaffected children were included. Subsequently, 288 ASD cases and 493 unaffected sibling controls participated in the study. Participant characteristics are presented in Table 1. These children were members of 252 families, the characteristics of which are presented in Table 2. Most families had only 1 affected child and ≥1 unaffected siblings, although some families (n = 23) had >1 affected child.
TABLE 2.
Utah Genetics Study Family Composition
| Total number of children in family | Number of children with autism spectrum disorder in family | Total families | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 2 | 85 | 0 | 0 | 0 | 85 |
| 3 | 82 | 9 | 0 | 0 | 91 |
| 4 | 49 | 4 | 2 | 0 | 55 |
| 5 | 11 | 1 | 1 | 1 | 14 |
| 6 | 1 | 0 | 0 | 3 | 4 |
| 7 | 0 | 0 | 0 | 1 | 1 |
| 8 | 0 | 1 | 0 | 0 | 1 |
| 9 | 1 | 0 | 0 | 0 | 1 |
| Grand total | 229 | 15 | 3 | 5 | 252 |
All families had ≥1 child with an autism spectrum disorder and at least one unaffected sibling.
ASD case status was based on the Autism Diagnostic Inventory, Revised36,37 and the Autism Diagnostic Observation Schedule, Generic.38 Additional sample characteristics including ascertainment and assessment methods have been reported previously.39 IQ scores (based on the Wechsler Intelligence Scale for Children,40 Differential Abilities Scale,41 or Mullen42) were available on 63% (n = 181) of ASD cases. Thirty-six percent (n = 66) of these cases had ID, defined as having an IQ ≤70. Sibling controls were ≥2 years of age. If caregivers reported developmental or behavioral concerns, siblings were screened for ASD using the Social Communication Questionnaire43 and Social Responsiveness Scale.44 Approval for the use of these data were granted by the University of Utah Institutional Review Board and the Resource for Genetic and Epidemiologic Research Review Committee, an oversight body that regulates UPDB access.
Risk Factors
Information regarding the following variables was obtained from birth certificate records through the Utah Department of Health (ADDM cohort) and UPDB (Utah Genetics cohort): maternal height and weight at pregnancy onset, weight gain during pregnancy, maternal age, paternal age, parity, gestational age, birth year, and indication of multiple birth. Durations of maternal and paternal education were obtained as indicators of socioeconomic status. Maternal residential address zip code at time of birth was also acquired from birth certificate records for the ADDM cohort.
Statistical Analysis
The correlation of all pairwise combinations of covariates was assessed for multicollinearity using Pearson product-moment and Spearman rank correlations. Correlations of Spearman’s ρ > 0.5 and/or Pearson’s r ≥ 0.5 or r ≤ –0.5 were considered indicative of a high correlation. Maternal age, paternal age, maternal education, paternal education, and parity met this threshold. Therefore, a principle component analysis (PCA) of these 5 factors was conducted in the large population-based ADDM surveillance cohort resulting in 5 PCA factors. The first 2 PCA factors (Factor 1, parental ages and parity; Factor 2, parental education) collectively accounted for 79% of the variance in the original 5 variables. Weights from these 2 PCA factors were used to create factor scores in the Genetics Study data set. These factors were then used in the multiple logistic regression models to adjust for the confounding effects of these variables on the main predictor variables (maternal prepregnancy BMI and pregnancy weight gain).
For the ADDM surveillance cohort, multiple logistic regression models were formulated to assess the relationship between prepregnancy BMI and ASD risk, controlling for parental ages, parity (PCA Factor 1); parental education (PCA Factor 2); and gestational age. A similar model assessing pregnancy weight gain was also adjusted for prepregnancy BMI. For the Utah Genetics cohort, a conditional logistic regression analysis stratified by mother was conducted to determine risk associated with the selected factors independent of within mother effects. The modeling approach for the Genetics cohort included the same factors as outlined above for the ADDM cohort with the addition of birth year (as a centered variable) and gender as covariates. (The ADDM cohort addressed these confounding variables by using case-control gender matching before analysis and restricting analysis to 1 birth cohort ascertained at age 8.)
These analyses were repeated with ADDM and Genetics case groups (n = 97 and n = 222, respectively) that excluded individuals with ID to determine the impact of ID on the association of the foregoing predictive factors with ASD. All statistical analyses were conducted by using SAS software version 9.3,45 and statistical significance was assessed at α = .05.
Results
Population-Based 2002 ADDM (Utah Site) Surveillance Cohort
The multiple logistic regression models revealed a significant association between maternal weight gain during pregnancy and ASD risk in the ASD sample with and without comorbid ID (for each 5 pounds of weight gained, adjusted odds ratio (aOR) = 1.10, 95% confidence interval (CI): 1.03 to 1.17 and aOR = 1.12, 95% CI: 1.05 to 1.20, respectively). The relationship between maternal BMI at pregnancy onset and ASD risk was not found to be significant in the multiple logistic regression models in either ASD case population (Table 3).
TABLE 3.
ASD Risk Associated With Weight Gaina During Pregnancy and Prepregnancy BMIb in the Population-Based ADDM Network and Research-Based Utah Genetics Study cohorts
| Condition | Cases vs Controls | Cases Without ID vs Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n | Controls, n | aOR | 95% CI | P | Cases, n | Controls, n | AOR | 95% CI | P | |
| ADDM Network cohortc | ||||||||||
| Maternal wt gain during pregnancy | 110 | 9753 | 1.10 | 1.03 to 1.17 | <.01 | 83 | 9753 | 1.12 | 1.05 to 1.21 | <.01 |
| BMI at start of pregnancy | 110 | 9753 | 1.0 | 0.97 to 1.05 | .84 | 83 | 9753 | 1.00 | 0.96 to 1.05 | .76 |
| Utah Genetics Study cohortd | ||||||||||
| Maternal wt gain during pregnancy | 268 | 450 | 1.17 | 1.01 to 1.35 | .03 | 209 | 355 | 1.20 | 1.01 to 1.42 | .05 |
| BMI at start of pregnancy | 271 | 456 | 0.93 | 0.84 to 1.0 | .18 | 211 | 361 | 0.93 | 0.84 to 1.03 | .15 |
Weight gain effect measures risk of autism based on a 5-lb increase.
BMI effect measures risk of autism based on cohort 1 kg/m2 increase.
Binary logistic regression.
Conditional logistic regression; controls are unaffected siblings.
Utah Genetics Study Cohort
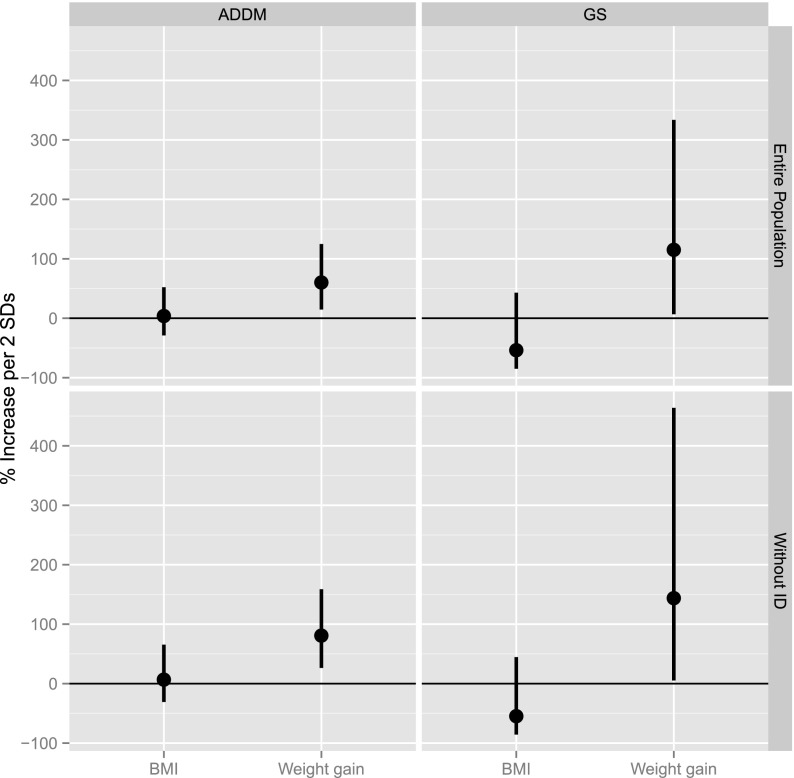
The conditional logistic regression models indicated that weight gain during pregnancy was significantly associated with ASD (aOR = 1.17, 95% CI: 1.01 to 1.35 for each 5 pounds of weight gained). This relationship remained significant when children with comorbid ID were removed from the case group (aOR = 1.20, 95% CI: 1.01 to 1.42 for each 5 pounds of weight gained). BMI at pregnancy onset was not significantly associated with ASD in the full cohort or following the exclusion of children with comorbid ID (Table 3). Figure 1 illustrates the ASD risk associated with a 2 SD increase in prepregnancy BMI and pregnancy weight gain in the ADDM and Genetics cohort samples.
FIGURE 1.
Risk of autism spectrum disorder associated with a 2 SD from the mean increase in prepregnancy BMI (9.57 in the ADDM cohort and 10.6 in the Genetics Study cohort) and pregnancy weight gain (25.00 lb in the ADDM cohort and 24.74 lb in the Genetics Study cohort) in the ADDM and Utah Genetics Study (GS) samples with and without comorbid intellectual disability.
Discussion
The ADDM and Genetics cohorts demonstrated remarkably similar findings particularly in light of their differences in case ascertainment methods, control group selection, and participant demographics. A significant ASD risk associated with maternal pregnancy weight gain, but not prepregnancy BMI, was identified in both cohorts. Similarly, when the analyses were restricted to ASD cases with a normal IQ, the association between ASD and pregnancy weight gain remained significant, suggesting that this finding is independent of the presence of comorbid ID among ASD cases. Although ASD risk associated with pregnancy weight gain appears to extend beyond an underlying association with global developmental delay, the absence of IQ scores for one-third of Genetics cases merits caution when interpreting this finding in this cohort.
The examination of ASD risk associated with prepregnancy maternal BMI and weight gain during pregnancy was conducted in 2 ASD cohort and control groups. Each study group, from a research perspective, had its strengths and weaknesses. The population-based ADDM cohort, through its surveillance-based ascertainment, provided a representative sample of Utah children with ASD and used a nested case-control design matching for age, birth year, and gender, thus maximizing statistical efficiency and generalizability. As a surveillance study, direct assessment of cases and controls was not possible. Case status, sample characterization, and risk-factor assessment were limited to the contents of ascertainment records and birth certificate data. The research-based Genetics Study sample provided a case group that was well characterized as a result of active study participation. ASD case status was defined by in-person, gold standard assessments, and extensive phenotypic and genotyping data were available. The use of an unaffected sibling control group allowed for a unique examination of within-mother effects that essentially allowed each mother to act as her own control. This provided an opportunity to investigate the relationship between ASD risk and the selected risk factors independent of the mother. To our knowledge, this is the first study to report on metabolic risk factors associated with ASD using this study design.
This study’s findings provide preliminary evidence in our 2 Utah samples of a significant connection between ASD and a potential biomarker for the metabolic environment in utero that is independent of maternal BMI at pregnancy onset. However, the absolute difference in pregnancy weight gain between case and control groups (∼3 pounds) is considered clinically insignificant in current obstetrical practice. This finding was not unexpected; we hypothesize that excess pregnancy weight gain serves as a marker of gestational phenomena leading to ASD rather than as a direct risk factor. If ASD is, in part, caused by in utero steroid dysregulation, the maternal contribution of abnormal steroid regulation to this gestational phenomenon may increase maternal adipose tissue and sex-steroid production. As an established peripheral producer of estradiol, the excess adipose tissue may then contribute further to excess maternal sex-steroid production. The persistence of ASD risk associated with pregnancy weight gain in the Genetics sample suggests that even in genetically vulnerable offspring, the proposed underlying ASD etiology may be mediated by potentially modifiable factors.
Conclusions
The results of this study justify further investigation of the connection between in utero steroid dysregulation and ASD. We are pursuing measurement of steroid and related endocrine biomarkers in maternal serum collected during the pregnancy of ASD cases, unaffected siblings, and population controls. If prenatal biomarkers are identified that indicate pregnancies at elevated risk, a window of clinical opportunity could emerge for this mechanism of injury to be prevented or attenuated. The recognition of gene-environment interactions could provide intervention targets that may span the range from maternal education and wellness programs to novel drug development.
Acknowledgments
We thank the Utah Department of Health, Utah State Office of Education, the CDC, and the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the UPDB. We are grateful to the Genetics Study families whose participation contributed to this study.
Glossary
- ADDM
Autism and Developmental Disabilities Monitoring
- aOR
adjusted odds ratio
- ASD
autism spectrum disorder
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ID
intellectual disability
- PCA
principle component analysis
- UPDB
Utah Population Database
Footnotes
Dr. Bilder conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted; Dr Bakian carried out the initial analysis for the Autism and Developmental Disabilities Monitoring cohort, wrote the corresponding Methods section, reviewed and revised the manuscript, and approved the final manuscript as submitted; Mr Viskochil carried out the initial analysis for the Utah Genetics Study cohort, wrote the corresponding Methods section, and approved the final manuscript as submitted; Dr Clark provided critical insight from an obstetrical perspective on the design of the study and through her review and contribution to the background and discussion sections; Dr Botts provided the initial draft of the background section and assisted with the conceptualization of the study through her review of the relevant literature; Dr Smith contributed to the conceptualization of the study from the perspective of conducting population research and reviewed and revised the manuscript; Mr Pimentel coordinated the data collection for the Genetics Study cohort and created the linkage between the Genetics cohort and the Utah Population Database where risk factor data were stored and reviewed and approved the final manuscript as submitted; Dr McMahon provided expertise in the conceptualization of the study, reviewed and revised the manuscript, and approved the final version of the manuscript as submitted; Dr Coon provided critical insight into all aspects of this study’s conceptualization, design, and both Autism and Developmental Disabilities Monitoring and Utah Genetics Study cohort analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr Botts is currently completing her child and adolescent psychiatry fellowship at Vanderbilt University.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Partially funded by the Centers for Disease Control and Prevention under Cooperative Agreement CCU822365 to establish Population-Based Surveillance of Autism Spectrum Disorders, the Utah Registry of Autism and Developmental Disabilities, the National Institute of Mental Health grants R01 MH069359 and R01 MH094400, National Institute on Aging grant R01 AG022095, and Autism Speaks. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Bilder has served as a consultant and advisory board member for BioMarin Pharmaceuticals, has been a coinvestigator and consultant on an Autism Speaks grants, is co–principal investigator of a Centers for Disease Control and Prevention (CDC) Autism and Developmental Disabilities Monitoring (ADDM) grant, and is a consultant on an autism-related National Institute of Mental Health (NIMH) grant. Dr Bakian is a coinvestigator for a CDC ADDM grant and consultant on an autism-related NIMH grant. Dr McMahon has served as an advisory board member for BioMarin Pharmaceuticals, holds a patent with Lineagen, has been a coinvestigator on Autism Speaks grants, is principal investigator of a CDC ADDM grant, and is a coinvestigator on an autism-related NIMH grant. Hilary Coon has been a co– principal investigator and coinvestigator of Autism Speaks grants. Drs Botts, Clark, and Smith, Mr Pimentel, and Mr Viskochil have indicated they have no potential conflicts of interest to disclose.
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed. text revision Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 2.Centers for Disease Control and Prevention, Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators . Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Morb Mortal Wkly Rep. 2009;58(SS10):1–20 [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention, Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Morb Mortal Wkly Rep. 2012;61(SS03):1–19 [PubMed] [Google Scholar]
- 4.Pinborough-Zimmerman J, Bakian AV, Fombonne E, Bilder D, Taylor J, McMahon WM. Changes in the administrative prevalence of autism spectrum disorders: contribution of special education and health from 2002–2008. J Autism Dev Disord. 2012;42(4):521–530 [DOI] [PubMed] [Google Scholar]
- 5.Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100(3):264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7(1):38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti B, Dudbridge F, Kent L, et al. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2(3):157–177 [DOI] [PubMed] [Google Scholar]
- 8.Knickmeyer R, Baron-Cohen S, Fane BA, et al. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm Behav. 2006;50(1):148–153 [DOI] [PubMed] [Google Scholar]
- 9.Kallen CB. Steroid hormone synthesis in pregnancy. Obstet Gynecol Clin North Am. 2004;31(4):795–816, x [DOI] [PubMed] [Google Scholar]
- 10.Petridou E, Katsouyanni K, Hsieh CC, Antsaklis A, Trichopoulos D. Diet, pregnancy estrogens and their possible relevance to cancer risk in the offspring. Oncology. 1992;49(2):127–132 [DOI] [PubMed] [Google Scholar]
- 11.Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L. Pregnancy weight gain and breast cancer risk. BMC Womens Health. 2004;4(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musselman JR, Georgieff MK, Ross JA, Tomlinson GE, Feusner J, Krailo M, Spector LG. Maternal pregnancy events and exposures and risk for hepatoblastoma: a Children’s Oncology Group (COG) study [published online ahead of print January 8, 2013]. Cancer Epidemiol. 10.1016/j.canep.2012.12.005 [DOI] [PMC free article] [PubMed]
- 13.Lumey LH. Prenatal oestrogens and breast cancer. Paediatr Perinat Epidemiol. 1998;12(4):361–365 [DOI] [PubMed] [Google Scholar]
- 14.Sanderson M, Williams MA, Daling JR, et al. Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12(4):397–407 [DOI] [PubMed] [Google Scholar]
- 15.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749 [DOI] [PubMed] [Google Scholar]
- 16.Sam S. Obesity and polycystic ovary syndrome. Obes Manag. 2007;3(2):69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troisi R, Potischman N, Roberts J, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States). Cancer Causes Control. 2003;14(4):347–355 [DOI] [PubMed] [Google Scholar]
- 18.Wuu J, Hellerstein S, Lipworth L, et al. Correlates of pregnancy oestrogen, progesterone and sex hormone-binding globulin in the USA and China. Eur J Cancer Prev. 2002;11(3):283–293 [DOI] [PubMed] [Google Scholar]
- 19.Kaijser M, Jacobsen G, Granath F, Cnattingius S, Ekbom A. Maternal age, anthropometrics and pregnancy oestriol. Paediatr Perinat Epidemiol. 2002;16(2):149–153 [DOI] [PubMed] [Google Scholar]
- 20.Lof M, Hilakivi-Clarke L, Sandin S S, de Assis S, Yu W, Weiderpass E. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health. 2009;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin CC, Baptiste MS, Schymura MJ, Zdeb MS, Nasca PC. Perinatal risk factors for neuroblastoma. Cancer Causes Control. 2009;20(3):289–301 [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163(9):818–828 [DOI] [PubMed] [Google Scholar]
- 23.Utah Department of Health. Pregnancy weight gain in Utah. Available at: www.health.utah.gov/mihp/pdf/Weight_Gain.pdf. Accessed December 27, 2012
- 24.Utah Department of Health. Bloebaum L, Baksh L, McGarry J. Utah PRAMS Data Book 2004–2005. Available at: www.health.utah.gov/mihp/pdf/04_05_Data_Book.pdf. Accessed December 27, 2012
- 25.Heikura U, Taanila A, Hartikainen AL, et al. Variations in prenatal sociodemographic factors associated with intellectual disability: a study of the 20-year interval between two birth cohorts in northern Finland. Am J Epidemiol. 2008;167(2):169–177 [DOI] [PubMed] [Google Scholar]
- 26.Jedrychowski W, Perera F, Jankowski J, et al. Effect of exclusive breastfeeding on the development of children’s cognitive function in the Krakow prospective birth cohort study. Eur J Pediatr. 2012;171(1):151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5). Available at: www.pediatrics.org/cgi/content/full/129/5/e1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neggers YH, Goldenberg RL, Ramey SL, Cliver SP. Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet Gynecol Scand. 2003;82(3):235–240 [DOI] [PubMed] [Google Scholar]
- 29.Lyall K, Pauls DL, Santangelo SL, Spiegelman D, Ascherio A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the Nurses Health Study II [published correction appears in J Autism Dev Disord. 2011;41(5):628]. J Autism Dev Disord. 2011;41(5):618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902 [DOI] [PubMed] [Google Scholar]
- 31.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators. Centers for Disease Control and Prevention . Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56(1, SS1):12–28 [PubMed] [Google Scholar]
- 32.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55 [DOI] [PubMed] [Google Scholar]
- 33.Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. Am J Public Health. 1993;83(8):1163–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobie SA, Baldwin LM, Rosenblatt RA, Fordyce MA, Andrilla CH, Hart LG. How well do birth certificates describe the pregnancies they report? The Washington State experience with low-risk pregnancies. Matern Child Health J. 1998;2(3):145–154 [DOI] [PubMed] [Google Scholar]
- 35.Green DC, Moore JM, Adams MM, Berg CJ, Wilcox LS, McCarthy BJ. Are we underestimating rates of vaginal birth after previous cesarean birth? The validity of delivery methods from birth certificates. Am J Epidemiol. 1998;147(6):581–586 [DOI] [PubMed] [Google Scholar]
- 36.Le Couteur A, Lord C, Rutter M. The Autism Diagnostic Interview—Revised (ADI-R). Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 37.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685 [DOI] [PubMed] [Google Scholar]
- 38.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223 [PubMed] [Google Scholar]
- 39.Allen-Brady K, Robison R, Cannon D, et al. Genome-wide linkage in Utah autism pedigrees. Mol Psychiatry. 2010;15(10):1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: The Psychological Corporation; 1991 [Google Scholar]
- 41.Elliott CD. Differential Ability Scales. San Antonio, TX: The Psychological Corporation; 1990 [Google Scholar]
- 42.Mullen EM. Mullen Scales of Early Learning (AGS edition). Circle Pines, MN: American Guidance Service; 1995 [Google Scholar]
- 43.Rutter M, Bailey A, Berument SK, Lord C, Pickles A. Social Communication Questionnaire (SCQ) (Lifetime Version). Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 44.Constantino JN, Gruber CP. Social Responsiveness Scale (SRS). Los Angeles, CA: Western Psychological Services; 2005 [Google Scholar]
- 45.Statistical Analysis System. SAS. Version 9.3 [computer software]. Cary, NC: SAS Institute, Inc [Google Scholar]