Abstract
OBJECTIVE:
The etiology of childhood cancers is largely unknown. Studies have suggested that birth characteristics may be associated with risk. Our goal was to evaluate the risk of childhood cancers in relation to fetal growth.
METHODS:
We conducted a case-control study nested within Nordic birth registries. The study included cancer cases diagnosed in Denmark, Finland, Norway, and Sweden among children born from 1967 to 2010 and up to 10 matched controls per case, totaling 17 698 cases and 172 422 controls. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were derived from conditional logistic regression.
RESULTS:
Risks of all childhood cancers increased with increasing birth weight (Ptrend ≤ .001). Risks of acute lymphoid leukemia and Wilms tumor were elevated when birth weight was >4000 g and of central nervous system tumors when birth weight was >4500 g. Newborns large for gestational age were at increased risk of Wilms tumor (OR: 2.1 [95% CI: 1.2–3.6]) and connective/soft tissue tumors (OR: 2.1 [95% CI: 1.1–4.4]). In contrast, the risk of acute myeloid leukemia was increased among children born small for gestational age (OR: 1.8 [95% CI: 1.1–3.1]). Children diagnosed with central nervous system tumors at <1 year of age had elevated risk with increasing head circumference (Ptrend < .001). Those with head circumference >39 cm had the highest risk (OR: 4.7 [95% CI: 2.5–8.7]).
CONCLUSIONS:
In this large, Nordic population-based study, increased risks for several childhood tumors were associated with measures of fetal growth, supporting the hypothesis that tumorigenesis manifesting in childhood is initiated in utero.
Keywords: birth weight, childhood cancer, fetal growth, nested case-control study, Nordic countries
What’s Known on This Subject:
The etiology of childhood cancers is largely unknown. However, excessive fetal growth has been associated with some childhood cancers. One of the most consistent findings is that high birth weight is associated with an increased risk of childhood leukemia.
What This Study Adds:
Examining large, population-based birth and cancer registry data from 4 Nordic countries, high birth weight was the most strongly associated with risk of many childhood cancers among several measures of fetal growth that have not previously been extensively assessed.
Cancer is rare before age 15 years. However, in Europe, the incidence rate among children has increased by 1.0% per year since the 1970s, reaching 140 cases per 1 million children in the 1990s.1 In the Nordic countries (2000–2009), the age-standardized incidence rates were 17.1 and 15.2 per 100 000 in boys and girls, respectively (the NORDCAN database).
The etiology of childhood cancers is largely unknown. Based on the early age at diagnosis, it has been speculated that some childhood tumors may have a fetal origin. A complex interplay of genetic, hormonal, nutritional, and environmental factors determines fetal growth, and excessive fetal growth has been associated with some childhood cancers.2
One of the most consistent findings is that high birth weight is associated with an increased risk of childhood leukemia, both acute lymphoid leukemia (ALL)3 and acute myeloid leukemia (AML).4 High birth weight has also been linked to development of other childhood tumors such as cancers of the central nervous system (CNS), lymphomas, and Wilms tumors (nephroblastoma), but results have been inconclusive.5–7 Earlier Nordic population-based studies have also shown that increased head circumference is positively associated with childhood brain cancer, suggesting that brain pathologic conditions originate in fetal life.8,9
Studies of pregnancy risk factors are made difficult by the low relative frequency of different childhood tumors. Pooling data from birth and cancer registries in several countries, however, makes it possible to achieve a sufficient study size.
In the current study, we report on a large, joint Nordic population-based study undertaken to evaluate the risk of childhood cancers in relation to several measures of fetal growth (birth weight, length, ponderal index, and head circumference).
Methods
Data Sources
The population-based Nordic medical birth registries contain information on all births in Denmark, Finland, Norway, and Sweden since 1973, 1987, 1967, and 1973, respectively.10 Information from national hospital patient registries, when available, was used to supplement birth registry data. Danish data were included starting in 1977 because some variables were obtained from the Danish National Hospital Register established in that year.11 Reporting of cancer cases is compulsory in the Nordic countries, and the cancer registries of Denmark, Finland, Norway, and Sweden cover the entire population starting in 1943, 1952, 1953, and 1958.12
Every resident in the Nordic countries is assigned a unique country-specific personal identification number used in all administrative and medical registries. This identification number makes accurate record linkage possible.
Study Design
The data were analyzed within a nested case-control design. The study included cancer cases diagnosed during 1977–2010 in Denmark (n = 3675), 1987–2010 in Finland (n = 2536), 1967–2009 in Norway (n = 4513), and 1973–2009 in Sweden (n = 6974) among children aged <15 years. Twins, higher-order multiples, and children with Down syndrome were excluded. Ten controls per case who were alive at the time of the case’s diagnosis and not diagnosed with cancer (other than non-melanoma skin cancer) were sampled from the birth registries and matched to cases on gender, birth country, and birth year. A total of 17 698 cases and 172 422 controls were included in the study.
The current study focused on all childhood cancers as well as 8 specific types: Wilms tumors, retinoblastoma, CNS tumors, bone tumors, connective/soft tissue tumors, lymphoma, ALL, and AML. The cancer types were classified according to International Classification of Diseases for Oncology, 3rd Edition,13 in Denmark, Finland, and Norway and according to International Classification of Diseases, Revision 7/Systematized Nomenclature of Medicine codes in Sweden (before 1993, a separate coding system for histology was used).14
Prenatal and perinatal characteristics of cases and controls were available from the birth registries. The following growth measures were examined: birth weight (500–1999, 2000–2499, 2500–2999, 3000–3499, 3500–3999, 4000–4499, and 4500–6000 g), birth length (40–49, 50–52, 53, and 54–62 cm), ponderal index (<24.0, 24.0–29.9, and ≥30.0 kg/m3), and head circumference (20–32, 33–34, 35–36, 37–38, and 39–45 cm). Data on head circumference were available starting in 1997, 2004, 1978, and 1973 in Denmark, Finland, Norway, and Sweden, respectively. Measures of weight for gestational age, small for gestational age (SGA), and large for gestational age (LGA) were defined as birth weight <2 and >2 standard deviations (SDs) below and above the mean weight for gestational age, respectively.15 The reference group, appropriate for gestational age, was defined as children who were neither SGA nor LGA, with birth weight within 2 SDs of the mean weight for gestational age. Gestational age was measured as completed weeks of gestation. Data for newborns with extreme measures were excluded from the analyses; that is, gestational age <23 weeks and >44 weeks (0.2%), birth weight <500 g and >6000 g or extremely small or large for gestational age (±4 SDs away from the published weights by gestational age15 with gestational age 23–44 completed weeks) (0.2%), length <40 cm and >62 cm (0.3%), and head circumference <20 cm and >45 cm (0.04%).
Information on maternal BMI before pregnancy or in early pregnancy, categorized as <18.5, 18.5 to 24.9, 25.0 to 29.9 and ≥30.0 kg/m2, was available in Denmark and Finland since 2004, and in Sweden since 1992. Information on maternal smoking early and/or late in pregnancy was available in Denmark, Finland, Norway, and Sweden since 1997, 1987/1990, 1999, and 1982/1999, respectively; this information was categorized as a dichotomized variable. Maternal diabetes (pre-existing diabetes or gestational diabetes) data, available in Finland until 2004 only, was also categorized as a dichotomized variable.
Statistical Analysis
Odds ratios (ORs) and their 95% confidence intervals (95% CIs) were derived from conditional logistic regression models. We denote the increased/decreased ORs as increased/decreased risks. With relatively rare childhood cancers as the outcome, ORs closely approximate relative risks, although the difference between the 2 types of estimates increases at high/low risks.16
The base model included gestational age, birth weight, maternal age, and parity. For the various cancers, we performed analyses stratified on gender and age, and in some instances also on country (matching criteria). Further adjustments were made for maternal BMI, smoking, and diabetes in subanalyses. Tests for trend were performed by using continuous variables. If a U-shaped relation was indicated by examination of the categorical variable estimates, a quadratic term was included in the regression model. The data were analyzed by using PASW Statistics 18 (SPSS Inc, Chicago, IL) and Stata/IC 12.1 (Stata Corp, College Station, TX; www.stata.com).
Ethical and Legal Considerations
Ethics approvals were obtained from review boards in Norway and Sweden, and from the US National Cancer Institute. In Denmark, the study was approved by the Data Protection Agency. In Finland, the National Institute for Health and Welfare gave permission to use its health registry data after approval by the data protection authority.
Results
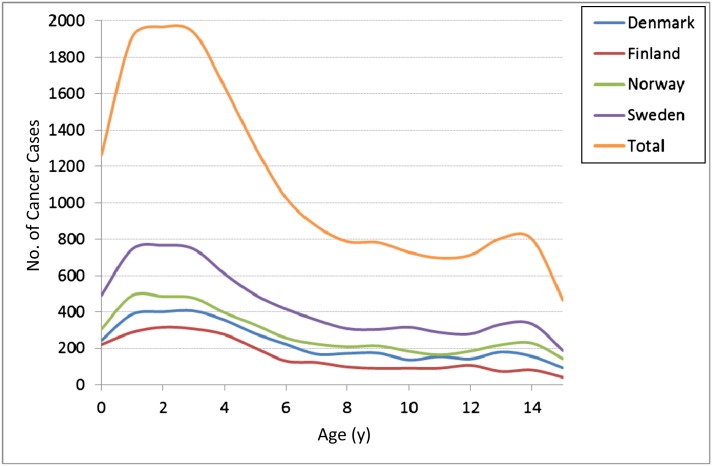
Figures 1 and 2 and Table 1 show the number of cases with major childhood cancers according to age and country. The age distribution was similar in all 4 Nordic countries. Mean age at diagnosis was 6 years, and 53% of cases were diagnosed in children aged <5 years. CNS tumors (29%), ALL (24%), and lymphomas (8%) were most common, with mean ages at diagnosis of 6.2, 5.2, and 8.4 years, respectively.
FIGURE 1.
Number of childhood cancer cases according to age and country.
FIGURE 2.
Number of major childhood cancer cases according to age.
TABLE 1.
Number of Major Childhood Cancer Cases by Country
| Childhood Cancer | Country | ||||
|---|---|---|---|---|---|
| Denmark (1977–2010) | Finland (1987–2010) | Norway (1967–2009) | Sweden (1973–2009) | Total (1967–2010) | |
| Wilms tumor | 166 | 160 | 222 | 413 | 961 |
| Retinoblastoma | 90 | 64 | 139 | 193 | 486 |
| CNS tumor | 1006 | 650 | 1363 | 2144 | 5163 |
| Bone tumor | 149 | 58 | 174 | 239 | 620 |
| Connective/soft tissue tumor | 139 | 111 | 151 | 311 | 712 |
| Lymphoma | 265 | 148 | 269 | 715 | 1397 |
| ALL | 923 | 685 | 1033 | 1680 | 4321 |
| AML | 146 | 88 | 174 | 260 | 668 |
| Other | 791 | 572 | 988 | 1019 | 3370 |
| Total | 3675 | 2536 | 4513 | 6974 | 17 698 |
Table 2 shows maternal and child characteristics of the study population. The cases generally had higher birth weight, length, and head circumference and were more often LGA at birth than the controls.
TABLE 2.
Characteristics of the Study Population
| Characteristic | Controls | Cases | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Maternal characteristics | ||||
| Maternal age, y | ||||
| <25 | 47 050 | 27.3 | 4612 | 26.1 |
| 25–29 | 62 099 | 36.0 | 6403 | 36.2 |
| 30–34 | 43 609 | 25.3 | 4601 | 26.0 |
| ≥35 | 19 663 | 11.4 | 2082 | 11.8 |
| Missing data | 1 | 0 | 0 | 0 |
| Parity | ||||
| No previous children | 73 035 | 42.4 | 7607 | 43.0 |
| 1 previous child | 60 862 | 35.3 | 6176 | 34.9 |
| 2 previous children | 26 066 | 15.1 | 2629 | 14.9 |
| ≥3 previous children | 11 477 | 6.7 | 1183 | 6.7 |
| Missing data | 982 | 0.6 | 103 | 0.6 |
| Smoking at onset of pregnancya | ||||
| No | 63 973 | 37.1 | 6556 | 37.0 |
| Yes | 15 395 | 8.9 | 1607 | 9.1 |
| Missing data | 93 054 | 54.0 | 9535 | 53.9 |
| Smoking at end of pregnancyb | ||||
| No | 39 156 | 22.7 | 4015 | 22.7 |
| Yes | 6622 | 3.8 | 685 | 3.9 |
| Missing data | 126 644 | 73.5 | 12 998 | 73.4 |
| Maternal BMI, kg/m2c | ||||
| <18.5 | 886 | 0.5 | 81 | 0.5 |
| 18.5–24.9 | 17 708 | 10.3 | 1825 | 10.3 |
| 25.0–29.9 | 6080 | 3.5 | 635 | 3.6 |
| ≥30.0 | 2575 | 1.5 | 257 | 1.5 |
| Missing data | 145 173 | 84.2 | 14 900 | 84.2 |
| Maternal diabetesd | ||||
| No | 168 864 | 97.9 | 17 315 | 97.8 |
| Yes | 1247 | 0.7 | 139 | 0.8 |
| Missing data | 2311 | 1.3 | 244 | 1.4 |
| Child characteristics | ||||
| Gender | ||||
| Male | 93 770 | 54.4 | 9617 | 54.3 |
| Female | 78 652 | 45.6 | 8081 | 45.7 |
| Missing data | 0 | 0.0 | 0 | 0.0 |
| Cesarean delivery | ||||
| No | 152 537 | 88.5 | 15 472 | 87.4 |
| Yes | 19 879 | 11.5 | 2226 | 12.6 |
| Missing data | 0 | 0.0 | 0 | 0.0 |
| Assisted reproductive technologye | ||||
| No | 74 768 | 43.4 | 7708 | 43.6 |
| Yes | 886 | 0.5 | 100 | 0.6 |
| Missing data | 96 768 | 56.1 | 9890 | 55.9 |
| Gestational age, wk | ||||
| 23–36 | 8030 | 4.7 | 920 | 5.2 |
| 37–41 | 144 422 | 83.8 | 14 769 | 83.5 |
| 42–44 | 15 362 | 8.9 | 1581 | 8.9 |
| Missing data | 4608 | 2.7 | 428 | 2.4 |
| Birth weight, gf | ||||
| 500–1999 | 1973 | 1.1 | 223 | 1.3 |
| 2000–2499 | 3773 | 2.2 | 392 | 2.2 |
| 2500–2999 | 18 075 | 10.5 | 1754 | 9.9 |
| 3000–3499 | 55 114 | 32.0 | 5230 | 29.6 |
| 3500–3999 | 61 011 | 35.4 | 6302 | 35.6 |
| 4000–4499 | 25 974 | 15.1 | 2946 | 16.6 |
| 4500–6000 | 5879 | 3.4 | 762 | 4.3 |
| Missing data | 623 | 0.4 | 89 | 0.5 |
| Birth length, cm | ||||
| 40–49 | 45 387 | 26.3 | 4381 | 24.8 |
| 50–52 | 88 730 | 51.5 | 8986 | 50.8 |
| 53 | 17 327 | 10.0 | 1872 | 10.6 |
| 54–62 | 17 984 | 10.4 | 2061 | 11.6 |
| Missing data | 2994 | 1.7 | 398 | 2.2 |
| Ponderal index, kg/m3 | ||||
| <24.0 | 21 675 | 12.6 | 2155 | 12.2 |
| 24.0–29.9 | 125 108 | 72.6 | 12 711 | 71.8 |
| ≥30.0 | 22 473 | 13.0 | 2409 | 13.6 |
| Missing data | 3166 | 1.8 | 423 | 2.4 |
| Head circumference, cmg | ||||
| 20–32 | 6722 | 5.4 | 692 | 5.4 |
| 33–34 | 34 552 | 27.7 | 3318 | 25.9 |
| 35–36 | 52 113 | 41.7 | 5284 | 41.2 |
| 37–38 | 15 291 | 12.2 | 1756 | 13.7 |
| 39–45 | 1090 | 0.9 | 145 | 1.1 |
| Missing data | 15 134 | 12.1 | 1624 | 12.7 |
| Birth weight by gestational age | ||||
| SGA | 5291 | 3.1 | 553 | 3.1 |
| AGA | 160 125 | 92.9 | 16 334 | 92.3 |
| LGA | 3323 | 1.9 | 454 | 2.6 |
| Missing data | 3683 | 2.1 | 357 | 2.0 |
| Total | 172 422 | 100.0 | 17 698 | 100.0 |
AGA, appropriate for gestational age.
Only births since 1997, 1987, 1999, and 1982 or later included for Denmark, Finland, Norway, and Sweden, respectively.
Only births since 1997, 1990, 1999, and 1999 or later included for Denmark, Finland, Norway, and Sweden, respectively.
Only births since 2004, 2004, and 1992 or later included for Denmark, Finland, and Sweden, respectively.
Only births until 2004 included for Finland.
Only births since 1995, 1990, 1988, and 1995 or later included for Denmark, Finland, Norway, and Sweden, respectively.
Mean birth weight among cases and controls (males/females): 3614/3483 g and 3580/3456 g.
Only births since 1997, 2004, 1978 and 1973 or later included for Denmark, Finland, Norway, and Sweden, respectively.
The ORs of selected childhood cancers according to measures of fetal growth are presented in Table 3. Risks of all childhood cancers combined increased with increasing birth weight (Ptrend ≤ .001), observed in all age groups (Fig 3). Among children diagnosed before 1 year of age, those who were LGA were at increased risk (OR: 1.6 [95% CI: 1.1–2.2]).
TABLE 3.
ORs and 95% CIs of Major Childhood Cancers According to Measures of Fetal Growth, Nordic Countries, 1967–2010
| Growth Measure | Wilms Tumor | Retinoblastoma | CNS Tumor | Bone Tumor | Connective/Soft Tissue Tumor | Lymphoma | ALL | AML | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Birth weight, g | ||||||||||||||||||
| 500–1999 | 1.6 | 0.83–3.3 | 1.1 | 0.43–2.9 | 1.1 | 0.81–1.5 | 1.6 | 0.69–3.7 | 0.78 | 0.34–1.8 | 1.1 | 0.58–2.0 | 0.75 | 0.51–1.1 | 0.50 | 0.17–1.5 | 1.0 | 0.86–1.2 |
| 2000–2499 | 1.1 | 0.65–1.8 | 1.0 | 0.53–2.0 | 1.1 | 0.88–1.4 | 0.93 | 0.48–1.8 | 0.91 | 0.53–1.6 | 1.2 | 0.80–1.8 | 0.90 | 0.69–1.2 | 0.99 | 0.55–1.8 | 1.0 | 0.90–1.1 |
| 2500–2999 | 0.92 | 0.71–1.2 | 0.70 | 0.47–1.0 | 1.1 | 0.97–1.2 | 0.96 | 0.71–1.3 | 0.92 | 0.68–1.3 | 0.98 | 0.79–1.2 | 0.91 | 0.81–1.0 | 0.90 | 0.66–1.2 | 1.0 | 0.94–1.1 |
| 3000–3499 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 3500–3999 | 1.1 | 0.93–1.3 | 1.0 | 0.79–1.3 | 1.1 | 0.99–1.1 | 1.1 | 0.85–1.3 | 1.2 | 1.0–1.5 | 1.0 | 0.87–1.2 | 1.1 | 1.0–1.2 | 1.0 | 0.83–1.2 | 1.1 | 1.1–1.1 |
| 4000–4499 | 1.5 | 1.3–1.9 | 1.1 | 0.79–1.4 | 1.1 | 1.0–1.2 | 1.2 | 0.91–1.6 | 1.3 | 0.99–1.6 | 1.3 | 1.1–1.5 | 1.2 | 1.1–1.4 | 1.1 | 0.89–1.5 | 1.2 | 1.1–1.3 |
| 4500–6000 | 1.8 | 1.3–2.6 | 1.5 | 0.95–2.5 | 1.3 | 1.1–1.5 | 1.4 | 0.89–2.2 | 1.4 | 0.90–2.1 | 1.2 | 0.86–1.6 | 1.5 | 1.3–1.8 | 1.3 | 0.85–2.0 | 1.4 | 1.3–1.5 |
| Birth length, cm | ||||||||||||||||||
| 40–49 | 0.90 | 0.73–1.1 | 0.92 | 0.68–1.2 | 0.97 | 0.89–1.1 | 1.0 | 0.80–1.3 | 1.1 | 0.86–1.4 | 0.98 | 0.82–1.2 | 0.98 | 0.89–1.1 | 1.1 | 0.86–1.4 | 0.98 | 0.93–1.0 |
| 50–52 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 53 | 0.93 | 0.72–1.2 | 1.2 | 0.87–1.7 | 0.92 | 0.82–1.0 | 1.1 | 0.81–1.5 | 0.99 | 0.75–1.3 | 1.2 | 0.99–1.5 | 0.99 | 0.88–1.1 | 1.2 | 0.88–1.5 | 1.0 | 0.96–1.1 |
| 54–62 | 1.1 | 0.86–1.5 | 1.2 | 0.81–1.7 | 1.1 | 0.95–1.2 | 0.83 | 0.58–1.2 | 1.3 | 0.97–1.8 | 1.1 | 0.92–1.4 | 0.89 | 0.78–1.0 | 0.81 | 0.58–1.1 | 1.0 | 0.96–1.1 |
| Ponderal index, kg/m3 | ||||||||||||||||||
| <24.0 | 1.2 | 0.93–1.5 | 1.2 | 0.87–1.7 | 0.98 | 0.89–1.1 | 1.2 | 0.87–1.6 | 0.97 | 0.73–1.3 | 1.0 | 0.85–1.3 | 0.97 | 0.87–1.1 | 1.1 | 0.82–1.4 | 1.0 | 0.96–1.1 |
| 24.0–29.9 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| ≥30.0 | 1.0 | 0.83–1.3 | 0.76 | 0.55–1.1 | 1.0 | 0.91–1.1 | 1.2 | 0.95–1.6 | 1.0 | 0.80–1.3 | 0.84 | 0.70–1.0 | 1.0 | 0.91–1.1 | 1.1 | 0.84–1.4 | 0.99 | 0.94–1.0 |
| Head circumference, cm | ||||||||||||||||||
| 20–32 | 0.84 | 0.52–1.4 | 1.1 | 0.57–1.9 | 0.94 | 0.78–1.1 | 1.2 | 0.70–2.1 | 0.86 | 0.49–1.5 | 0.88 | 0.61–1.3 | 0.97 | 0.78–1.2 | 1.4 | 0.83–2.2 | 0.96 | 0.87–1.1 |
| 33–34 | 0.96 | 0.77–1.2 | 1.0 | 0.74–1.3 | 1.0 | 0.94–1.1 | 1.0 | 0.77–1.3 | 1.1 | 0.88–1.4 | 1.0 | 0.84–1.2 | 0.93 | 0.84–1.0 | 0.85 | 0.66–1.1 | 0.97 | 0.92–1.0 |
| 35–36 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 37–38 | 0.97 | 0.75–1.3 | 1.2 | 0.84–1.7 | 1.1 | 1.0–1.3 | 0.89 | 0.61–1.3 | 1.1 | 0.84–1.6 | 1.1 | 0.91–1.4 | 1.0 | 0.91–1.2 | 0.93 | 0.67–1.3 | 1.1 | 1.0–1.1 |
| 39–45 | 0.83 | 0.31–2.2 | 0.81 | 0.24–2.8 | 1.7 | 1.2–2.3 | 0.51 | 0.12–2.2 | 0.68 | 0.20–2.3 | 1.1 | 0.53–2.1 | 1.0 | 0.68–1.5 | 0.50 | 0.15–1.7 | 1.2 | 0.98–1.4 |
| Birth weight by gestational age | ||||||||||||||||||
| SGA | 1.3 | 0.78–2.0 | 1.1 | 0.53–2.1 | 0.95 | 0.77–1.2 | 1.4 | 0.84–2.4 | 1.0 | 0.58–1.8 | 0.91 | 0.60–1.4 | 1.2 | 0.96–1.5 | 1.8 | 1.1–3.1 | 1.1 | 1.0–1.2 |
| AGA | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| LGA | 2.1 | 1.2–3.6 | 0.77 | 0.33–1.8 | 1.1 | 0.85–1.4 | 1.0 | 0.50–2.1 | 2.1 | 1.1–4.4 | 0.78 | 0.47–1.3 | 1.2 | 0.91–1.5 | 1.1 | 0.56–2.1 | 1.1 | 0.94–1.2 |
ORs were adjusted for gestational age, birth weight, maternal age, and parity. AGA, appropriate for gestational age.
FIGURE 3.

ORs and 95% CIs of all cancers combined according to birth weight and age, Nordic countries, 1967–2010. ORs were adjusted for gestational age, maternal age, and parity.
Risks of several tumor types increased with increasing birth size. Risk of connective/soft tissue tumors increased with increasing birth weight (Ptrend = .006) (Table 3). Risk of ALL was elevated in children with birth weight >4000 g, in all age groups (Fig 4) and in both genders. Children with birth weight >4500 g also were at increased risk of CNS tumors. In addition, children born LGA were at increased risk of Wilms tumors (OR: 2.1 [95% CI: 1.2–3.6]) and connective/soft tissue tumors (OR: 2.1 [95% CI: 1.1–4.4]). In contrast, AML risk was increased among children born SGA (OR: 1.8 [95% CI: 1.1–3.1]). The analyses including categorical variables did not indicate a U-shaped relation between birth weight and childhood cancer, except for Wilms and bone tumors. The inclusion of a quadratic term for weight in the regression model in relation to these tumors was not significant, however.
FIGURE 4.

ORs and 95% CIs of ALL according to birth weight and age, Nordic countries, 1967–2010. ORs were adjusted for gestational age, maternal age, and parity.
Children diagnosed between 10 and 14 years of age, with a birth weight >4000 g, were at increased risk of lymphoma (OR: 1.6 [95% CI: 1.2–2.0]). In addition, the risk of Wilms tumors was increased in children with birth weight >4000 g but only in girls. Girls with birth weight >4500 g were at highest risk compared with the reference (3000–3499 g) group (OR: 2.5 [95% CI: 1.5–4.0]).
For CNS tumors, risk increased with increasing head circumference (Ptrend < .001) among children diagnosed in their first year of life (Fig 5). Those whose head circumference was >39 cm at birth were at highest risk compared with the reference (35–36 cm) group (OR: 4.7 [95% CI: 2.5–8.7]). Excluding children born with hydrocephalus reduced the OR somewhat (OR: 3.6 95% CI: 1.8–7.1]). Gender- and country-specific analyses yielded similar results, except for Norway, where children with head circumference >39 cm at birth and were diagnosed at ages 1 to 4 years also were at increased risk. We observed no trends involving head circumference for the other cancer subtypes.
FIGURE 5.
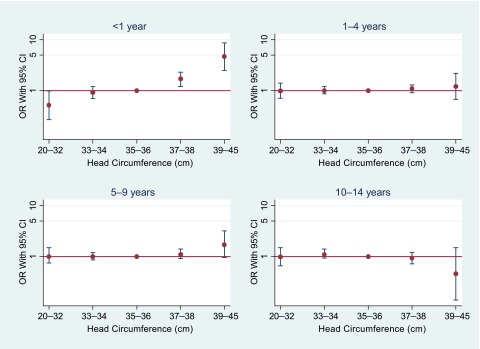
ORs and 95% CIs of CNS tumors according to head circumference and age, Nordic countries, 1967–2010. ORs were adjusted for gestational age, birth weight, maternal age, and parity.
Further adjustments for maternal BMI and smoking did not change the risk estimates appreciably. Although the combined cancer risk was slightly increased among children born SGA after adjustment for maternal smoking (OR: 1.4 [95% CI: 1.1–1.8]), information on maternal BMI and smoking was available only for a relatively small proportion of the study population (16% and 15%, respectively). In addition, adjustment for maternal diabetes did not change the risk estimates appreciably.
Discussion
This large, population-based, case-control study nested within national birth registries in 4 Nordic countries found increased risks for several childhood tumors in relation to measures of fetal growth. Risk of connective/soft tissue tumors increased with increasing birth weight, and children with high birth weight were at increased risk for ALL, Wilms tumors (girls only), and CNS tumors. Children who were LGA at birth were at increased risk for Wilms tumors and connective/soft tissue tumors. In contrast, AML risk was elevated among children born SGA. In addition, an increased risk for CNS tumors with increasing head circumference was observed in children who were aged <1 year at diagnosis.
This study took advantage of the opportunity to cross-link population-based medical birth registries with cancer registries in Denmark, Finland, Norway, and Sweden, to explore associations between measures of fetal growth and relatively rare childhood cancers.
Among the strengths of our study were the large number of childhood cancer cases and linkage of comprehensive and compulsory databases with reliable information that was collected in a similar manner in each country.12,17–20 We were also able to adjust for important maternal and gestational confounders in the analyses, including gestational age, maternal age and parity, and, in some cases, maternal BMI, smoking, and diabetes. Migration in and out of the 4 countries during the study period was rather low. Because the study was population-based selection bias was unlikely. Information on fetal growth measures and other variables in the birth registries was registered before and independent of the diagnostic data from the cancer registries, thus eliminating recall and reporting biases.
The study was limited by lack of details on other possibly important confounders, which were unavailable in the health registries used. Examples include information on infectious disease history and different environmental exposures. Another concern is that multiple comparisons in our study could have created an increased risk of false-positive results. We performed subgroup analyses for 8 different cancer types in addition to all childhood cancer, for both genders and 4 age groups. However, we only have results for groups that have previously been found in other studies.
In recent years, there has been increasing interest in identifying etiologic factors that may act during the perinatal period. In several studies, anthropometric measurements at birth have been linked to different childhood cancers. Leukemia is 1 of the most common malignancies affecting children, and growing evidence suggests that childhood leukemia originates in utero. A 2009 meta-analysis of 31 studies on birth weight and childhood leukemia showed an association between high birth weight and increased risk for leukemia overall and for ALL.4 For AML, a U-shaped association was suggested, with the risk elevated at both high and low extremes of birth weight. A recent British study using data from the National Registry of Childhood Tumors demonstrated that the increased risks for ALL with increasing birth weight were most pronounced in cases with high hyperdiploid karyotypes and those positive for t(1;19) translocation.21 We found an increased risk with high birth weight for ALL (>4000 g) and AML (>4500 g), although the latter association was not statistically significant. Although no increased risk was observed between AML and birth weight <3000 g, an increased risk was seen among SGA newborns.
Epidemiologic studies examining the association between birth weight and lymphomas have had conflicting results.22,23 A 2012 meta-analysis, encompassing 2 cohort and 7 case-control studies, found no statistically significant associations between birth weight and lymphoma in general or between birth weight and major lymphoma categories (non-Hodgkin’s and Hodgkin’s lymphoma).5 A national Swedish cohort study, overlapping partly with the Swedish data in our study, reported that increased fetal growth was associated with non-Hodgkin’s lymphoma in early life, independent of gestational age and other perinatal factors.24 We found an increased risk of all lymphomas combined with high birth weight (>4000 g) in children diagnosed at age 10 to 14 years.
High birth weight (>4000 g) generally has been associated with a 30% to 50% increased risk of Wilms tumors,7,25,26 although Nordic data (1985–2006) have shown that the effects of high birth weight and being LGA were restricted to girls.7 Our findings confirmed the earlier observations of an increased risk of Wilms tumors at high birth weights (>50% increased risk at birth weights >4000 g compared with the reference group [3000–3499 g]) and being LGA (>100% increased risk) in girls. In data from the Children’s Oncology Group in the United States, the association between high birth weight (>4000 g) and Wilms tumors was strongest among patients with perilobar nephrogenic rests, present in 17% of the girls and 9% of the boys.25
The results for birth weight and CNS tumors in children have been conflicting. An Australian study on fetal growth and risk of childhood CNS tumors found little evidence of an overall association, using relatively novel measures of fetal growth (proportion of optimal birth weight, length, and weight for length).23 In contrast, a Nordic study (using part of the current data set) found a U-shaped relation between birth weight and CNS tumors. LGA and SGA were also associated with risk; however, the latter was of borderline statistical significance only.9 In our expanded data set, we found an increased risk for CNS tumors in children with birth weight >4500 g but no associations with SGA or LGA.
There is little evidence in any study, including ours, for an association between childhood cancers and very low birth weight, except for the strong association with hepatoblastoma.27 However, we observed an increased risk for AML among children born SGA.
The biological mechanisms underlying the association between high birth weight and childhood cancer are not well established but are likely to include growth factors. In particular, insulin-like growth factor (IGF)-I and IGF-II have been linked to increased birth weight and length,28,29 and IGF-I has been shown to inhibit apoptosis and enhance tumor growth.30 There has also been speculation that a high birth weight, with larger organs, implies a larger number of stem cells, thus increasing the total number of replicating cells at risk for malignant transformation.4,31 Overgrowth conditions are believed to be associated with increased childhood cancer risk as well. The risk of tumors in individuals with Beckwith-Wiedemann syndrome, 1 of the most common overgrowth conditions, is ∼5% to 10%, with Wilms tumor being the most frequent cancer.32
IGF2 (which encodes IGF-II) is an imprinted gene, normally expressed only from the paternal allele. When loss of imprinting occurs, biallelic expression may lead to enhanced fetal growth. IGF2 resides on the short arm of chromosome 11, which also harbors the Wilms tumor genes 1 and 2. Genetic and epigenetic alterations on the short arm of chromosome 11 are often involved in sporadic Wilms tumors.33
The observed association between growth factors and increased total number of stem cells, and thus replicating cells, has also been implicated in the etiology of leukemia.4 Furthermore, overexpression of IGF2 has been associated with AML and ALL, suggesting a role of epigenetic alterations in the association between high birth weight and risk of leukemia. In our study, lack of a significant association between high birth weight and AML might be due to the low number of incident cases compared with ALL cases. The point estimates, however, were similar for the association between high birth weight and both ALL and AML.
Previous Nordic studies, which partly overlap with ours, have shown that head circumference is positively associated with childhood CNS tumors.8,9 The strongest effect was observed in the youngest age groups and persisted up to age 10 years. We also found an increasing risk with increasing head circumference but only among children diagnosed within the first year of life (except for the Norwegian data). This increased risk within the first year was observed in both boys and girls, as well as in all 4 Nordic countries. The early age dependency of the association favors the presence of an undiagnosed brain tumor at birth. However, congenital brain tumors are rare, accounting for only 0.5% to 4% of all pediatric brain tumors.34,35
Conclusions
We observed increased risks for several childhood tumors in relation to measures of fetal growth, suggesting that important determinants of tumorigenesis may arise in utero. Biological mechanisms should be further explored to explain these associations.
Glossary
- ALL
acute lymphoid leukemia
- AML
acute myeloid leukemia
- CI
confidence interval
- CNS
central nervous system
- IGF
insulin-like growth factor
- LGA
large for gestational age
- OR
odds ratio
- SGA
small for gestational age
Footnotes
Dr Bjørge made substantial contributions to the study concept and design, analysis and interpretation of data, and drafting of the initial manuscript; Drs Sørensen, Grotmol, Stephansson, Gissler, Tretli, and Troisi made substantial contributions to the study concept and design, interpretation of data, and revising of the manuscript for important intellectual content; Dr Engeland made substantial contributions to the study concept and design, analysis and interpretation of data, and revising of the manuscript for important intellectual content; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This research was funded by the National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364(9451):2097–2105 [DOI] [PubMed] [Google Scholar]
- 2.Callan AC, Milne E. Involvement of the IGF system in fetal growth and childhood cancer: an overview of potential mechanisms. Cancer Causes Control. 2009;20(10):1783–1798 [DOI] [PubMed] [Google Scholar]
- 3.Hjalgrim LL, Rostgaard K, Hjalgrim H, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst. 2004;96(20):1549–1556 [DOI] [PubMed] [Google Scholar]
- 4.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124(11):2658–2670 [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou C, Antonopoulos CN, Sergentanis TN, Panagopoulou P, Belechri M, Petridou ET. Is birth weight associated with childhood lymphoma? A meta-analysis. Int J Cancer. 2012;130(1):179–189 [DOI] [PubMed] [Google Scholar]
- 6.Samuelsen SO, Bakketeig LS, Tretli S, Johannesen TB, Magnus P. Birth weight and childhood cancer. Epidemiology. 2009;20(4):484–487 [DOI] [PubMed] [Google Scholar]
- 7.Schüz J, Schmidt LS, Kogner P, et al. Birth characteristics and Wilms tumors in children in the Nordic countries: a register-based case-control study. Int J Cancer. 2011;128(9):2166–2173 [DOI] [PubMed] [Google Scholar]
- 8.Samuelsen SO, Bakketeig LS, Tretli S, Johannesen TB, Magnus P. Head circumference at birth and risk of brain cancer in childhood: a population-based study. Lancet Oncol. 2006;7(1):39–42 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt LS, Schüz J, Lähteenmäki P, et al. Fetal growth, preterm birth, neonatal stress and risk for CNS tumors in children: a Nordic population- and register-based case-control study. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1042–1052 [DOI] [PubMed] [Google Scholar]
- 10.Gissler M, Louhiala P, Hemminki E. Nordic Medical Birth Registers in epidemiological research. Eur J Epidemiol. 1997;13(2):169–175 [DOI] [PubMed] [Google Scholar]
- 11.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268 [PubMed] [Google Scholar]
- 12.Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736 [DOI] [PubMed] [Google Scholar]
- 13.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition Cancer. 2005;103(7):1457–1467 [DOI] [PubMed] [Google Scholar]
- 14.Socialstyrelsen 2011. Cancer Incidence in Sweden 2010. Socialstyrelsen 2011
- 15.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79(6):440–449 [PubMed] [Google Scholar]
- 16.Kirkwood BR, Sterne JAC, eds. Essential Medical Statistics. 2nd ed. Oxford, UK: Blackwell Science Ltd; 2003:160–162 [Google Scholar]
- 17.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33 [DOI] [PubMed] [Google Scholar]
- 18.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(suppl 7):42–45 [DOI] [PubMed] [Google Scholar]
- 19.Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231 [DOI] [PubMed] [Google Scholar]
- 20.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33(4):365–369 [DOI] [PubMed] [Google Scholar]
- 21.O’Neill KA, Bunch KJ, Vincent TJ, Spector LG, Moorman AV, Murphy MF. Immunophenotype and cytogenetic characteristics in the relationship between birth weight and childhood leukemia. Pediatr Blood Cancer. 2012;58(1):7–11 [DOI] [PubMed] [Google Scholar]
- 22.Schüz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes Control. 2007;18(6):655–663 [DOI] [PubMed] [Google Scholar]
- 23.Milne E, Laurvick CL, Blair E, de Klerk N, Charles AK, Bower C. Fetal growth and the risk of childhood CNS tumors and lymphomas in Western Australia. Int J Cancer. 2008;123(2):436–443 [DOI] [PubMed] [Google Scholar]
- 24.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and family risk factors for non-Hodgkin lymphoma in early life: a Swedish national cohort study. J Natl Cancer Inst. 2012;104(12):923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels JL, Pan IJ, Olshan AF, Breslow NE, Bunin GR, Ross JA, Children’s Oncology Group . Obstetric history and birth characteristics and Wilms tumor: a report from the Children’s Oncology Group. Cancer Causes Control. 2008;19(10):1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puumala SE, Soler JT, Johnson KJ, Spector LG. Birth characteristics and Wilms tumor in Minnesota. Int J Cancer. 2008;122(6):1368–1373 [DOI] [PubMed] [Google Scholar]
- 27.Spector LG, Puumala SE, Carozza SE, et al. Cancer risk among children with very low birth weights. Pediatrics. 2009;124(1):96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007–2025 [DOI] [PubMed] [Google Scholar]
- 29.Vatten LJ, Nilsen ST, Odegård RA, Romundstad PR, Austgulen R. Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Pediatrics. 2002;109(6):1131–1135 [DOI] [PubMed] [Google Scholar]
- 30.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518 [DOI] [PubMed] [Google Scholar]
- 31.Albanes D, Winick M. Are cell number and cell proliferation risk factors for cancer? J Natl Cancer Inst. 1988;80(10):772–774 [DOI] [PubMed] [Google Scholar]
- 32.Rahman N. Mechanisms predisposing to childhood overgrowth and cancer. Curr Opin Genet Dev. 2005;15(3):227–233 [DOI] [PubMed] [Google Scholar]
- 33.Satoh Y, Nakadate H, Nakagawachi T, et al. Genetic and epigenetic alterations on the short arm of chromosome 11 are involved in a majority of sporadic Wilms’ tumours. Br J Cancer. 2006;95(4):541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamji MF, Vassilyadi M, Lam CH, Montes JL, Farmer JP. Congenital tumors of the central nervous system: the MCH experience. Pediatr Neurosurg. 2009;45(5):368–374 [DOI] [PubMed] [Google Scholar]
- 35.Manoranjan B, Provias JP. Congenital brain tumors: diagnostic pitfalls and therapeutic interventions. J Child Neurol. 2011;26(5):599–614 [DOI] [PubMed] [Google Scholar]