Abstract
Background
The objective of this work was to determine the cost-effectiveness of temozolomide compared with that of radiotherapy alone in the adjuvant treatment of newly diagnosed glioblastoma. Temozolomide is the only chemotherapeutic agent to have demonstrated a significant survival benefit in a randomized clinical trial. Our analysis builds on earlier work by incorporating caregiver time costs and generic temozolomide availability. It is also the first analysis applicable to the US context.
Methods
A systematic literature review was conducted to collect relevant data. Transition probabilities were calculated from randomized controlled trial data comparing temozolomide plus radiotherapy with radiotherapy alone. Direct costs were calculated from charges reported by the Mayo Clinic. Utilities were obtained from a previous cost-utility analysis. Using these data, a Markov model with a 1-month cycle length and 5-year time horizon was constructed.
Results
The addition of brand Temodar and generic temozolomide to the standard radiotherapy regimen was associated with base-case incremental cost-effectiveness ratios of $102 364 and $8875, respectively, per quality-adjusted life-year. The model was most sensitive to the progression-free survival associated with the use of only radiotherapy.
Conclusions
Both the brand and generic base-case estimates are cost-effective under a willingness-to-pay threshold of $150 000 per quality-adjusted life-year. All 1-way sensitivity analyses produced incremental cost-effectiveness ratios below this threshold. We conclude that both the brand Temodar and generic temozolomide are cost-effective treatments for newly diagnosed glioblastoma within the US context. However, assuming that the generic product produces equivalent quality of life and survival benefits, it would be significantly more cost-effective than the brand option.
Keywords: brain tumor, cost-effectiveness, glioblastoma, health-technology assessment, temozolomide
Each year there are ∼13 000 deaths and 22 000 new cases of malignant brain and CNS tumors in the United States.1 According to the 2011 report of the Central Brain Tumor Registry of the United States, gliomas accounted for 80% of all malignant brain and CNS tumors, while glioblastomas alone accounted for ∼40%.2 Gliomas are graded by the World Health Organization (WHO) according to their prognosis and histological appearance. High-grade gliomas (HGGs) include anaplastic astrocytomas (WHO grade III) and glioblastomas (WHO grade IV). Without any treatment, a diagnosis of glioblastoma can imply a life expectancy of less than a year.3–5 With modern treatment, 2-year survival is roughly 25% and 5-year survival is roughly 10%.6
The treatment of newly diagnosed glioblastoma begins with surgical resection and histological confirmation of the diagnosis. Adjuvant radiotherapy has been shown to extend overall survival (OS) from 3–4 months to around 9–10 months.4 Prior to 2005, the use of chemotherapy in the adjuvant setting was controversial. Some had suspected that nitrosourea drugs might be effective treatments owing to their lipophilicity and subsequent ability to cross the blood–brain barrier.7 Throughout the 1970's and 1980's numerous clinical trials evaluated the benefit of nitrosourea drugs such as carmustine, lomustine, dacarbazine, and a procarbazine/CCNU/vincristine regimen, in the adjuvant setting for the treatment of newly diagnosed glioblastoma.8–11 However, there has been no randomized controlled trial that has demonstrated a significant survival benefit associated with the use of a nitrosourea-based chemotherapy regimen in the adjuvant setting. The use of nitrosoureas in this setting gained some popularity in the US; however, the practice was avoided in Europe.7,12 A 2002 meta-analysis of HGG outcomes demonstrated a 5 percentage point increase in 2-year survival (from 15% to 20%) associated with the use of a chemotherapeutic agent in addition to radiotherapy in the adjuvant setting relative to radiotherapy alone.7 While this evidence may give some general support to the use of adjuvant chemotherapy, it should be noted that the studies in the meta-analysis included grade III glioma patients as well as grade IV glioblastoma patients and several different nitrosourea drug regimens.
In 2005 the FDA approved temozolomide for the treatment of adults newly diagnosed with glioblastoma in the adjuvant setting concomitant with radiotherapy and as maintenance therapy.13 Temozolomide is an oral alkylating agent that resembles many of the nitrosoureas with respect to both structure and mechanism of action. Aside from its superior efficacy, temozolomide's advantages over nitrosoureas include its ability to be taken orally and an improved adverse-effect profile. Temozolomide's FDA approval was primarily the result of a pivotal clinical trial conducted by Stupp et al.12 That study demonstrated a median OS benefit of 2.5 months and a median progression-free survival (PFS) benefit of 1.9 months associated with the use of temozolomide versus radiotherapy alone.
Study Objective
This cost-utility analysis evaluates the incremental cost-effectiveness ratio (ICER), expressed as the monetary costs per additional quality-adjusted life-year (QALY), gained from the incorporation of temozolomide into the traditional adjuvant treatment regimen. It used a US societal perspective and modeled all costs and benefits over a time horizon of 5 years. The model compared temozolomide + radiotherapy with radiotherapy alone. Such information should prove invaluable to clinicians and policymakers when guiding adjuvant treatment.
Methods
Model, Patients, and Treatment
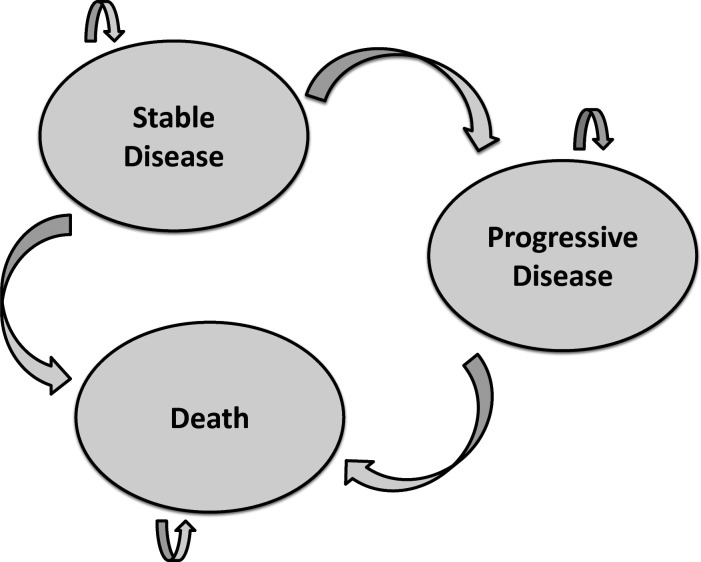
A Markov model was constructed using Microsoft Excel. The model contained 3 health states: stable disease, progressive disease, and death (Fig. 1). One thousand hypothetical patients began their model simulation with stable disease. These hypothetical patients were assumed to have the same distribution of baseline characteristics reported by Stupp et al.12 At the end of each month, patients had a given probability of transitioning to another health state or remaining in their current health state. No backward transitions were permitted (patients were not allowed to transition back to stable disease after progressing). Stable disease was divided into 4 different phases of treatment: month 1, month 2, months 3–7, and subsequent months, on the basis of the different doses of temozolomide recommended by the temozolomide prescribing information.
Fig. 1.
The Markov model. The Markov model contains 3 possible health states: stable disease, progressive disease, and death. All hypothetical patients began with stable disease. At the end of each model cycle (1 mo), patients had a defined probability of transitioning to another health state or remaining in their current health state. No backward transitions (improvements) were permitted.
Transition Probabilities
Median OS and median PFS were obtained from Stupp et al,6 whose study was an update to an earlier paper,12 following the same cohort of patients beyond 5 years. Athanassiou et al14 also conducted a similar randomized controlled trial; however, Stupp and colleagues observed significantly more patients (573 vs 110) for a longer period of time (60 mo vs 18 mo).6,14 From these medians, the monthly probability of each allowable transition within the model was calculated. The decreasing exponential approximation of life expectancy (DEALE) method was used to calculate these monthly probabilities.15,16 The DEALE method assumes that patients have a constant hazard of death throughout the time period being modeled. While restrictive, this assumption was justified in this case because the patients' life expectancy was so short.
Direct Costs
The cost of surgery was ignored because this model focused solely on guiding adjuvant treatment decisions. The costs of physician visits, laboratory tests, imaging, and caregiver support were applied equally to both groups in all phases of stable and progressive disease. The costs of temozolomide therapy, radiotherapy, and adverse-event prophylaxis were applied differently in each phase of stable disease and progressive disease to accurately reflect the dosing of temozolomide recommended in its prescribing information (Table 1).
Table 1.
Input costs
| SD Month 1 | SD Month 2 | SD Months 3–7 | SD Subsequent Months | PD | Death | |
|---|---|---|---|---|---|---|
| Radiotherapy + temozolomide | ||||||
| Temodara | $4620 | $1650 | $2019 | $0 | ||
| Generic temozolomide | $1155 | $413 | $505 | $0 | ||
| Radiotherapy | $1424 | $0 | $0 | $0 | ||
| AE prophylaxisb | $136 | $136 | $136 | $0 | ||
| Physician visits | $646 | $646 | $646 | $646 | ||
| Lab and imaging | $1337 | $1337 | $1337 | $1337 | ||
| Caregiver timec | $433 | $433 | $433 | $433 | ||
| Total | $8596 | $4203 | $4572 | $2417 | $5608 | $0 |
| Radiotherapy | ||||||
| Radiotherapy | $1424 | $0 | $0 | $0 | ||
| Physician visits | $646 | $646 | $646 | $646 | ||
| Lab and imaging | $1337 | $1337 | $1337 | $1337 | ||
| Caregiver timec | $433 | $433 | $433 | $433 | ||
| Total | $3840 | $2417 | $2417 | $2417 | $6699 | $0 |
Abbreviations: SD, stable disease; PD, progressive disease; AE, adverse event.
All costs are reported as 2011 US dollars per month.
aThe base-case prices of a 100-mg capsule and a 20-mg capsule were $110 and $22, respectively. These were taken from the Veterans Affairs Federal Supply Schedule. Generic price was estimated by reducing these prices by 75%.
bIt was assumed that patients using temozolomide would receive continual prophylaxis against opportunistic infections, nausea, constipation, and headache.
cInformal caregiver time was previously estimated to be 10 h/wk. This estimate, along with the median hourly compensation of a home health aide ($10.83), was used to calculate the value of caregiver time.
Charges associated with the treatment of HGGs were published by the Mayo Clinic in 1996.17 To our knowledge, these are the only published estimates of the cost of various health care services involved in glioblastoma treatment within the United States. The charges were separated into a number of categories: surgery, radiotherapy, chemotherapy, laboratory tests, imaging, outpatient physician visits, inpatient physician visits, inpatient hospital services (labeled “other” in the original publication), and ancillary charges. Chemotherapy charges likely reflected the use of nitrosoureas and were ignored in our analysis. The other charges were converted to costs using a cost-to-charge ratio (assumed to be 0.75 in the base-case analysis, but this was varied in sensitivity analysis) and inflated to 2011 US dollars using the medical services consumer price index. These costs were then divided by the median PFS, in months, of the population being treated in the Mayo Clinic report to obtain the expected cost for each service per progression-free month. The median PFS in the Mayo Clinic study was significantly longer than that in the Stupp et al studies6,12 (10.5 mo vs 6.9 mo) because the former included grade III as well as grade IV glioma patients. It is possible that because of this, these direct cost figures may be underestimated. All of the direct cost estimates are varied in sensitivity analysis.
Base-case Temodar prices were obtained from the Veterans Affairs Federal Supply Schedule.18 The Federal Supply Schedule offers a more realistic source of drug prices than the average wholesale price, which would be highly influenced by rebates and other unobservable factors. The average price of generic medications in the US is roughly 75% lower than the average price of branded medications.19 Therefore, the future price of generic temozolomide was estimated by reducing each of the brand Temodar unit prices by 75%.
The required dose of temozolomide was calculated according to its prescribing information using US mean height and weight to calculate mean body surface area. During month 1 of stable disease treatment, patients receiving temozolomide got a dosage of 75 mg/m2/day concomitantly with radiotherapy. During month 2, patients receiving temozolomide began maintenance therapy at a dosage of 150 mg/m2/day for 5 days. According to the temozolomide prescribing information, if patients have not experienced a serious adverse reaction, it is recommended that the maintenance dosage be increased to 200 mg/m2/day for 5 days per month at the beginning of month 3. Stupp et al12 reported that only 67% of patients qualified for this increased maintenance dose. Therefore, the model assumed that only 67% of patients received this increased maintenance dose during months 3–7. After month 7, if patients remained in the stable disease health state, it was assumed that they received no treatment but continued to incur other costs.
It was assumed that patients using temozolomide would receive adverse-event prophylaxis against opportunistic infections, nausea, headaches, and constipation. In reality, although prophylaxis against opportunistic infection is essential, most patients do not require continual prophylaxis against nausea, headaches, and constipation. This assumption greatly simplified the model and, as demonstrated by the sensitivity analysis, had a very minimal impact on the ICER.
Indirect Costs
Caregiver time was included as an indirect cost. Because this model took a societal perspective, the cost of the disease and its treatment borne by other members of society (nonpatients) had to be considered. Hayman et al.20 estimated the mean time informal caregivers of elderly cancer patients spent providing assistance with activities of daily living (ADLs) to be 10 hours per week. According to the Bureau of Labor Statistic's National Compensation Survey, the mean compensation rate for a home health aide in the US is $10.83 per hour.21 This implies that the time cost associated with informal caregiving for cancer patients is $433 per month. There is no literature specifically quantifying time spent by informal caregivers for glioblastoma patients. Hayman et al20 included caregivers for elderly patients suffering from a variety of cancers. The patients receiving temozolomide in this model are generally younger than the elderly patients included in the study by Hayman et al.20 Assuming that elderly cancer patients generally require more care than do middle-aged cancer patients, we could be overestimating caregiver time costs. However, there are also several other indirect costs that are not directly related to ADLs, such as transportation costs and symptom management. These costs were not included in the analysis by Hayman et al,20 which could indicate that this is an underestimate of caregiver time costs. Ultimately, the net effect of these potential biases is difficult to determine. The cost of caregiver time was also varied in sensitivity analyses.
Cost of Progressive Disease
Stupp et al6 reported the various salvage treatments (a second surgery, a second round of radiotherapy, a second round of chemotherapy) sought after patients were found to have progressive disease. Cost per month in progression was calculated based on these utilization data combined with the costs associated with each treatment from the Mayo Clinic publication described earlier. Patients with progressive disease were assumed to have the same laboratory, imaging, physician visit, and caregiving costs as those with stable disease. Inpatient hospitalization costs, which were excluded from the calculation of cost per progression-free month, were included in the estimated cost of a month with progressive disease. Because Stupp and colleagues did not report any specific salvage chemotherapy regimens used, salvage chemotherapy costs were assumed to be equal to the first round of adjuvant temozolomide costs.
Health State Utilities
Garside et al22 published the only estimates of utility associated with glioblastoma health states. Garside et al designed a similar Markov model to evaluate the use of temozolomide and carmustine wafers (separate analyses) in the adjuvant treatment of newly diagnosed glioblastoma. As part of the UK Value of Health Project, healthy volunteers from the general UK population were given standard gambles in an attempt to elicit UK societal preferences for a variety of health states. Unfortunately, at the time of the temozolomide evaluation, relevant utilities had been elicited from only 36 individuals. A number this small is not likely to be representative of the UK population and is even less likely to be representative of the US population, but these are the only estimates of utility to date. These utility values were used in the base-case analysis and were varied in sensitivity analyses (Table 2).
Table 2.
Input health state utilities
| Stable disease | |
| Temozolomide + radiotherapy | 0.743 |
| Temozolomide | 0.733 |
| Radiotherapy | 0.824 |
| No treatment | 0.887 |
| Progressive disease | 0.731 |
| Death | 0 |
All health state utilities were elicited from healthy UK volunteers using standard gambles and reported by Garside et al.22
Results
Using all base-case parameters, the use of Temodar produced an additional 1.8 months in overall life expectancy, 0.93 quality-adjusted life-months, and 0.078 QALYs at an additional cost of $7962 per patient. These additional costs included the acquisition of Temodar, adverse-event prophylaxis, and any additional treatment that occurred as a result of Temodar-treated patients living longer. Dividing the mean incremental lifetime costs ($7962) by the mean incremental lifetime QALYs (0.078), we calculated the base-case ICER to be $102 364 per QALY. The use of generic temozolomide produced equivalent benefits at a mean incremental cost of $690 per patient, resulting in a base-case ICER of $8875 per QALY.
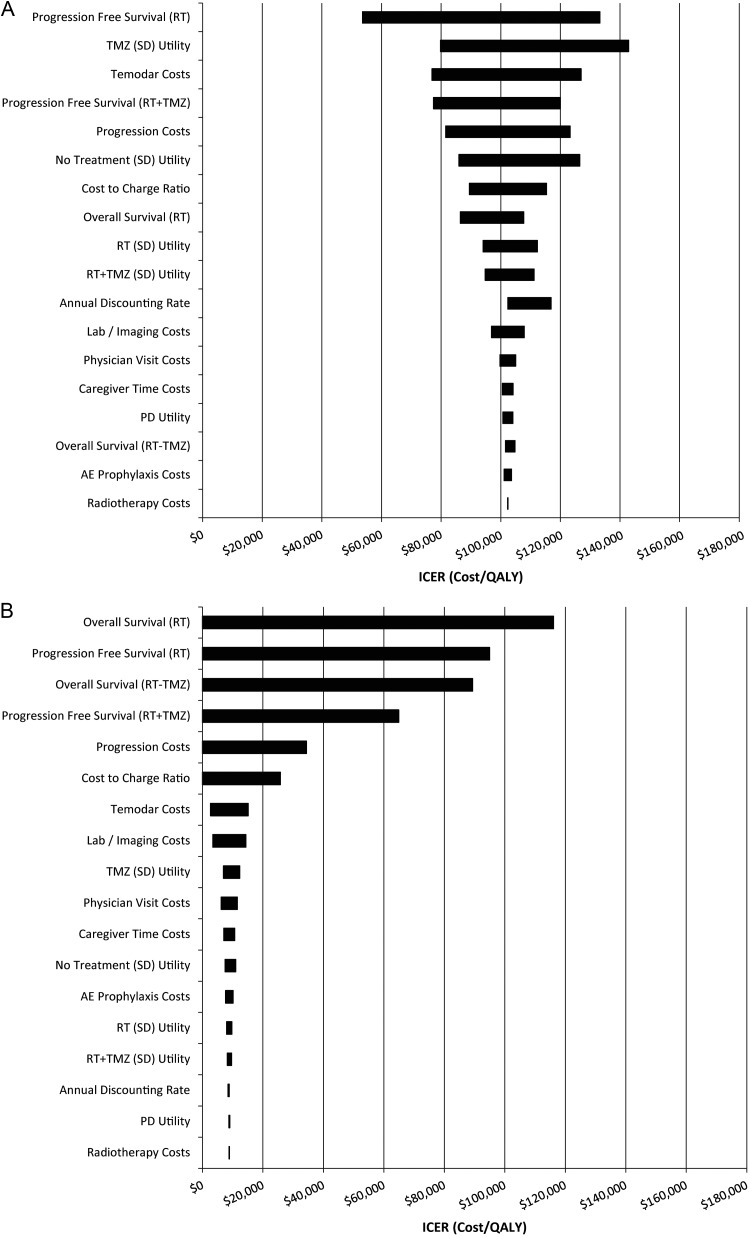
One-way sensitivity analysis revealed that the PFS of patients who received radiotherapy only was the biggest driver of model results. However, also significant were the utility associated with temozolomide's maintenance phase of stable disease treatment, the utility associated with the “no treatment” phase of stable disease (after patients had completed concomitant and maintenance phases but remained stable), the cost of progressive disease, and the cost of temozolomide (Table 3A and B). The resulting tornado diagrams are displayed in Fig. 2A and B. Because the base-case ICER for generic temozolomide was so low, several of the 1-way sensitivity analyses produced lower-bound ICERs that were negative. In this case, a negative ICER simply indicates dominance. Using these new parameter values, generic temozolomide was not only cost-effective, but also cost-saving—it produced positive QALY gains and resulted in lower costs on average. However, because negative ICERs can sometimes be ambiguous (either the numerator or the denominator can be negative), they are conventionally not displayed. Instead, we simply indicate “dominant” where appropriate in Table 3B and Fig. 2B.
Table 3A.
One-Way Sensitivity Analysis (Brand Temodar)
| Parameter | Range | ICER (Cost Per QALY, Discounted) | Change in ICER (%) |
|---|---|---|---|
| Overall Survival (RT + TMZ) | −2 Weeks | $104,765 | 2.35 |
| + 2 Weeks | $101,592 | −0.75 | |
| Progression Free Survival (RT + TMZ) | −2 Weeks | $119,955 | 17.18 |
| + 2 Weeks | $77,473 | −24.32 | |
| Overall Survival (RT) | −2 Weeks | $107,710 | 5.12 |
| + 2 Weeks | $86,475 | −15.60 | |
| Progression Free Survival (RT) | −2 Weeks | $53,606 | −47.63 |
| + 2 Weeks | $133,240 | 30.16 | |
| Temodar Costs | Both Doses −20% | $76,959 | −24.82 |
| Both Doses +20% | $126,975 | 24.02 | |
| + 6 Cycles | $137,354 | 34.18 | |
| Radiotherapy Costs | −20% | $102,311 | −0.05 |
| +20% | $102,414 | 0.05 | |
| AE Prophylaxis Costs | −20% | $101,110 | −1.23 |
| +20% | $103,609 | 1.22 | |
| Physician Visit Costs | −20% | $99,705 | −2.60 |
| +20% | $105,024 | 2.60 | |
| Lab / Imaging Costs | −20% | $96,874 | −5.36 |
| +20% | $107,861 | 5.37 | |
| Caregiver Time Costs | −20% | $100,569 | −1.75 |
| +20% | $104,142 | 1.74 | |
| Progression Costs | −20% | $123,263 | 20.42 |
| +20% | $81,509 | −20.37 | |
| RT (SD) Utility | −10% | $94,065 | −8.11 |
| +10% | $112,284 | 9.69 | |
| RT + TMZ (SD) Utility | −10% | $111,161 | 8.59 |
| +10% | $94,775 | −7.41 | |
| TMZ (SD) Utility | −10% | $142,862 | 39.56 |
| +10% | $79,798 | −22.04 | |
| No Treatment (SD) Utility | −10% | $85,955 | −16.25 |
| +10% | $126,488 | 23.24 | |
| PD Utility | −10% | $104,067 | 1.66 |
| +10% | $100,716 | −1.61 | |
| Annual Discounting Rate | 5% | $106,292 | 3.84 |
| 10% | $116,850 | 14.15 | |
| Cost to Charge Ratio | 0.5 | $115,245 | 12.58 |
| 1 | $89,483 | −12.58 |
RT - Radiotherapy; TMZ - Temozolomide; AE - Adverse Event; SD - Stable Disease; PD - Progressive Disease; ICER - Incremental Cost-Effectiveness Ratio; QALY - Quality Adjusted Life-Year
Table 3B.
One-Way Sensitivity Analysis (Generic Temozolomide)
| Parameter | Range | ICER (Cost Per QALY, Discounted) | Change in ICER (%) |
|---|---|---|---|
| Overall Survival (RT + TMZ) | −2 Weeks | Dominant | N/A |
| +2 Weeks | $32,659 | 267.99 | |
| Progression Free Survival (RT + TMZ) | −2 Weeks | $35,782 | 303.18 |
| +2 Weeks | Dominant | N/A | |
| Overall Survival (RT) | −2 Weeks | $38,793 | 337.10 |
| +2 Weeks | Dominant | N/A | |
| Progression Free Survival (RT) | −2 Weeks | Dominant | N/A |
| +2 Weeks | $45,821 | 416.29 | |
| Temodar Costs | Both Doses -20% | $2,642 | −70.23 |
| Both Doses +20% | $15,107 | 70.22 | |
| +6 Cycles | $20,552 | 131.57 | |
| Radiotherapy Costs | −20% | $8,826 | −0.55 |
| +20% | $8,927 | 0.59 | |
| AE Prophylaxis Costs | −20% | $7,625 | −14.08 |
| +20% | $10,124 | 14.07 | |
| Physician Visit Costs | −20% | $6,220 | −29.92 |
| +20% | $11,539 | 30.02 | |
| Lab/Imaging Costs | −20% | $3,389 | −61.81 |
| +20% | $14,377 | 61.99 | |
| Caregiver Time Costs | −20% | $7,084 | −20.18 |
| +20% | $10,657 | 20.08 | |
| Progression Costs | −20% | $26,082 | 193.88 |
| +20% | Dominant | N/A | |
| RT (SD) Utility | −10% | $8,017 | −9.67 |
| +10% | $9,730 | 9.63 | |
| RT + TMZ (SD) Utility | −10% | $9,634 | 8.55 |
| +10% | $8,219 | −7.39 | |
| TMZ (SD) Utility | −10% | $13,360 | 39.27 |
| +10% | $6,923 | −21.99 | |
| No Treatment (SD) Utility | −10% | $7,449 | −16.07 |
| +10% | $10,976 | 23.67 | |
| PD Utility | −10% | $9,022 | 1.66 |
| +10% | $8,732 | −1.61 | |
| Annual Discounting Rate | 5% | $8,427 | −5.05 |
| 10% | $7,407 | −16.54 | |
| Cost to Charge Ratio | 0.5 | $21,756 | 145.14 |
| 1 | Dominant | N/A |
RT - Radiotherapy; TMZ - Temozolomide; AE - Adverse Event; SD - Stable Disease; PD - Progressive Disease; ICER - Incremental Cost-Effectiveness Ratio; QALY - Quality Adjusted Life-Year; N/A – Not Applicable. Dominance indicates that the use of generic temozolomide produced positive QALY gains and lowered costs.
Fig. 2.
One-way sensitivity analysis tornado diagrams. (A) Brand Temodar. (B) Generic temozolomide. All survival estimates were varied by 2 wk. All cost estimates were varied by 20%. All utility estimates were varied by 10%. Annual discounting rates included 3% (base-case), 5%, and 10%. Cost-to-charge ratios included 0.5, 0.75 (base-case), and 1. Negative ICER estimates indicate that the treatment was dominant and were truncated at zero to avoid ambiguity. Abbreviations: RT, radiotherapy; TMZ, temozolomide; SD, stable disease; PD, progressive disease.
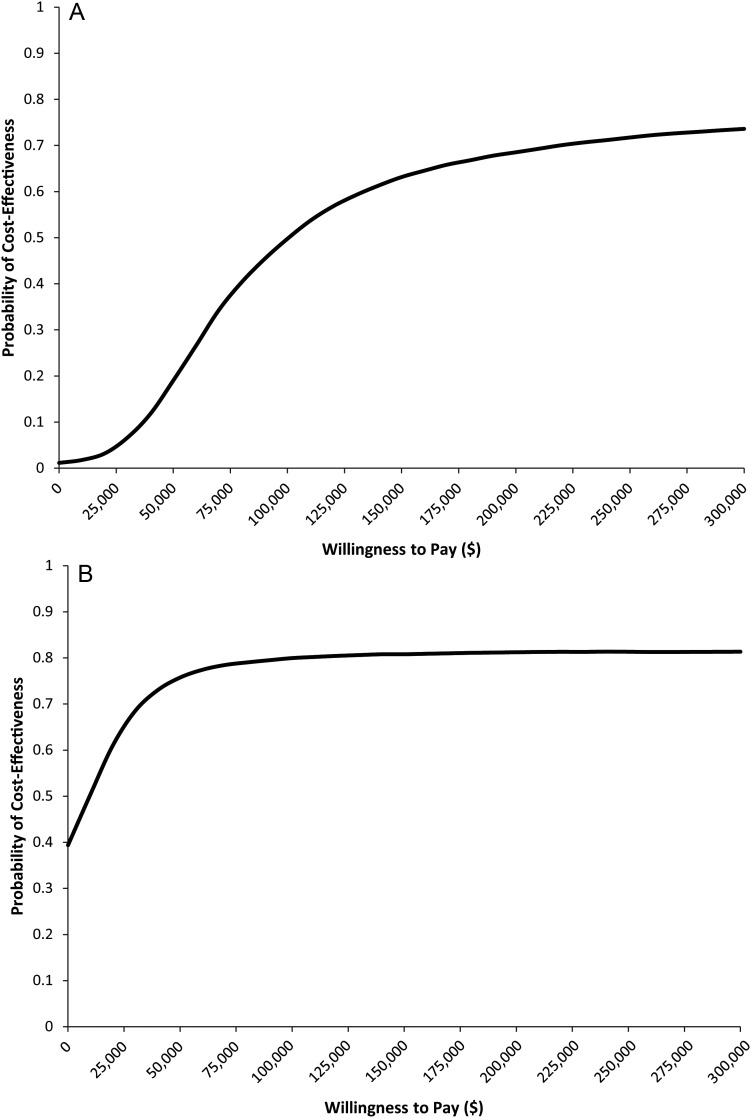
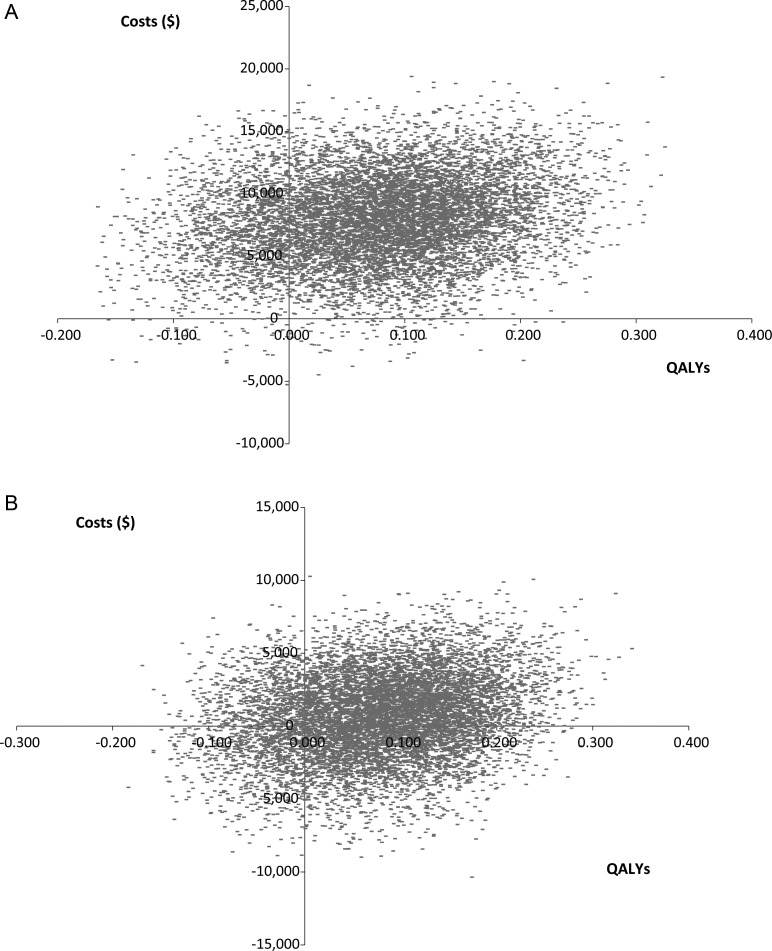
Probabilistic sensitivity analysis based on a Monte Carlo simulation (10 000 simulations) was done. The resulting cost-effectiveness acceptability curves are displayed in Fig. 3A and B. The resulting ICER scatterplots are displayed in Fig. 4A and B. Using a threshold of $150 000 per QALY, 63% of the Temodar simulations and 80% of the generic temozolomide simulations produced cost-effective ICERs. The maximum probabilities of cost-effectiveness at an infinite willingness-to-pay were 81.8% and 82.0% for Temodar and generic temozolomide, respectively.
Fig. 3.
Cost-effectiveness acceptability curves. (A) Temodar brand. (B) Generic temozolomide. A Monte Carlo simulation was used to estimate the probability of cost-effectiveness as a function of the societal willingness-to-pay for a QALY. Under a willingness-to-pay threshold of $150 000 per QALY, 63% of the simulations produced cost-effective ICERs.
Fig. 4.
Simulated ICER scatterplots. (A) Temodar brand. (B) Generic temozolomide. The costs incurred and QALYs gained from 10 000 model simulations are plotted. The majority of the resulting cloud occurs in quadrant 1 of the cost-effectiveness plane, indicating that use of temozolomide produces additional QALYs at an additional cost.
Discussion
We are aware of 3 previous cost-effectiveness analyses of temozolomide's use in the adjuvant treatment of newly diagnosed glioblastoma. Garside and colleagues22 conducted a cost-utility analysis from the perspective of the UK National Health Service. They estimated a base-case ICER of £35 861 (∼US$56 000) per QALY.22 Per the National Institute for Clinical Excellence Guidelines for Manufacturers and Sponsors, indirect costs, such as caregiver time, were not included. It should also be noted that direct costs in the UK single-payer health care system and the US fragmented health care system are often dramatically different. These discrepancies are the result of different utilization patterns and different unit costs. The direct comparison of cost-effectiveness analyses conducted within the context of different health care systems has been widely discouraged.23,24
During the Stupp et al clinical trial,12 a subset of treatment centers agreed to collect economic data in addition to the required clinical data. Lamers et al25 used these data to conduct another cost-effectiveness analysis. Fifteen treatment centers in 5 countries (Austria, Switzerland, Germany, Canada, and the Netherlands) participated in the economic evaluation. None of the US treatment centers participated. Using the Dutch unit costs, the researchers calculated an ICER of €37 361 per life-year.
Unfortunately, because neither of the quality of life instruments administered during the clinical trial—the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-BN20—is preference weighted, the researchers were unable to express the calculated ICER as a cost per QALY. Instead, they calculated the incremental cost per life-year gained without adjustment for the quality of life. Because these patients had less than perfect quality of life, the cost per life-year gained will always be lower than the cost per QALY. It is also worth noting that the researchers used a lower discounting rate for the life-years gained than for the costs of treatment. This will also lower the ICER relative to our estimates, which all employ an equal discounting rate for costs and benefits. Differential discounting has been rejected by the majority of academic journals and government agencies, including the US Public Health Service Panel on Cost-Effectiveness in Medicine.26
More recently, Wu and colleagues27 constructed another Markov model to evaluate the cost-effectiveness of temozolomide in several subgroups of patients. Among the “overall cohort” (comparable to the patients studied in clinical trials and simulated in our analysis), the base-case ICER was estimated to be $87 940 per QALY. Wu et al27 assumed a Chinese societal perspective and did not include indirect costs.
Based on a 1982 cost-effectiveness analysis of renal dialysis for patients with chronic renal failure, many modern cost-effectiveness analyses using a US societal perspective inappropriately continue to assume a cost per QALY willingness-to-pay threshold for cost-effectiveness no greater than $50 000 per QALY.28–31 Recently, economists and clinicians have begun to call that figure into question.32,33 Estimates with more theoretical and empirical support have ranged from $100 000 to $200 000 per QALY.34–38 The WHO has endorsed a threshold of 3 times the gross domestic product of the country in which the intervention is to be implemented.39 In the United States, this represents a threshold of roughly $150 000 per QALY.
Limitations
All direct medical costs other than the cost of temozolomide were calculated from charges reported by the Mayo Clinic in 1996. Although the calculated costs were adjusted to 2011 US dollars, changing practice patterns may make this conversion imperfect. However, calculating costs this way is still preferable to using cost data from outside the US, which would almost certainly be distorted by different unit prices and practice patterns.23,24
The modeled dosing of temozolomide was based on the approved temozolomide prescribing information; however, clinicians in the US sometimes use 12 cycles of temozolomide instead of the approved 6. Although our goal was to make this analysis applicable to the US context, we could not accurately model 12 cycles of temozolomide in the base-case analysis because the clinical trials, from which we derived all of the survival data, used 6 cycles. However, if we assume that the 12-cycle regimen produced equivalent survival as the 6-cycle regimen, then we can adjust our model so that the costs of stable disease treatment in months 8–12 were equivalent to those of months 3–7. This produces new ICERs of $137 354 per QALY and $20 552 per QALY for Temodar and generic temozolomide, respectively (Table 3A and B). Both of these ICERs are <$150 000 per QALY and thus could be considered more cost-effective than using only radiotherapy. However, they are both higher than the respective brand and generic base-case ICERs, indicating that under these assumptions, 6 cycles of adjuvant temozolomide is still more cost-effective than 12 cycles of adjuvant temozolomide.
Assuming that the ratio of OS to PFS is unchanged (∼2:1), a 12-cycle regimen of Temodar would be more cost-effective than a 6-cycle regimen of Temodar if it produced an additional 2 months of OS and 1 month of PFS. Under the same assumption, a 12-cycle regimen of generic temozolomide would be more cost-effective than a 6-cycle regimen of generic temozolomide if it produced an additional 2 weeks of OS and 1 week of PFS. However, we are unaware of any clinical or survival evidence to support more than 6 cycles of temozolomide.
It was assumed that all patients using temozolomide would be receiving continual prophylaxis against opportunistic infection, nausea, headache, and constipation. This can be viewed as an overestimate of the true costs associated with adverse-event prophylaxis. However, as the sensitivity analysis demonstrated, the model was extremely insensitive to the cost of adverse-event prophylaxis. A 20% reduction in adverse-event prophylaxis costs decreased the ICER by 1.23%.
It was also assumed that informal caregivers of patients with glioblastoma spend an average of 10 h per week assisting patients with ADLs. As previously mentioned, multiple factors could make this an overestimate or underestimate of the true caregiver time commitment. It is therefore reassuring that a 20% increase or decrease in caregiver time costs was associated with a <2% increase or decrease, respectively, in the ICER.
Probably the most important assumption concerns the applicability of utility values elicited from a small sample of UK citizens to the general US population. Both previous cost-effectiveness analyses have used these data because they represent the only source of elicited preferences for relevant health states. However, these estimates seem to demonstrate a significant quality of life decrement associated with the use of temozolomide (roughly equivalent to the decrement associated with disease progression). This finding is at odds with the quality of life data measured during the Stupp et al12 clinical trial and reported separately by Taphoorn and colleagues,40 who found no statistically significant difference in quality of life between the temozolomide + radiotherapy group and the radiotherapy alone group using the EORTC QLQ-C30 and QLQ-BN20 instruments. Given these discrepancies, sensitivity analyses around the utility estimates were of particular importance. It was found that increasing the utility associated with temozolomide's use in the maintenance phase of stable disease treatment (the period of greatest exposure to temozolomide) by 10% decreased the brand Temodar ICER to $79 798 per QALY.
Conclusion
This paper describes the first cost-utility analysis of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma to use a US societal perspective, include indirect costs, and estimate the impact of generic temozolomide availability. Both the brand and generic base-case ICERs were cost-effective using a willingness-to-pay threshold appropriate for a US societal context ($150 000 per QALY). One-way sensitivity analyses showed that the ICERs remained cost-effective as input parameters were varied to reflect theoretically reasonable uncertainty.
Funding
This work received no specific funding.
Conflict of interest statement. None declared.
References
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503. doi: 10.1038/ncpneuro0289. quiz 491 p following 516. [DOI] [PubMed] [Google Scholar]
- 2.Central Bra Tumor Registry of the United States. 2011. CBTRUS Statistical Report : Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 20042007. Hinsdale, IL: . http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf . Accessed 27 May 2013.
- 3.Chamberlain MC, Kormanik PA. Practical guidelines for the treatment of malignant gliomas. West J Med. 1998;168(2):114–120. [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MD, Alexander E, Jr., Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen K, Hagen S, Kollevold T, et al. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer. 1981;47(4):649–652. doi: 10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 8.Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67(2):121–132. [PubMed] [Google Scholar]
- 9.Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52(6):997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71(1):1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 11.Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19(2):509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 12.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11(19 Pt 1):6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 14.Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23(10):2372–2377. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 15.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the ‘DEALE’). I. Validation of the method. Am J Med. 1982;73(6):883–888. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 16.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the ‘DEALE’). II. Use in medical decision-making. Am J Med. 1982;73(6):889–897. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein MD, Cascino TL, Harmsen WS. High-grade astrocytomas: resource use, clinical outcomes, and cost of care. Mayo Clin Proc. 1996;71(10):936–944. doi: 10.1016/S0025-6196(11)63766-X. [DOI] [PubMed] [Google Scholar]
- 18.Department of Veterans Affairs. Drug pharmaceutical prices. http://www.pbm.va.gov/drugpharmaceuticalprices.asp. (accessed May 27, 2013) [Google Scholar]
- 19.Assistant Secretary for Planning and Evaluation DoHaHS. Expanding the Use of Generic Drugs. 2010. [Google Scholar]
- 20.Hayman JA, Langa KM, Kabeto MU, et al. doi: 10.1200/JCO.2001.19.13.3219. Estimating the cost of informal caregiving for elderly patients with cancer. J Clin Oncol. Jul 1 2001;19(13):3219–3225. [DOI] [PubMed] [Google Scholar]
- 21.Bureau of Labor Statistics DoOES. The National Compensation Survey. 2012; http://www.bls.gov/oes/current/oes311011.htm , 2013. [Google Scholar]
- 22.Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(45):iii–iv. doi: 10.3310/hta11450. ix–221. [DOI] [PubMed] [Google Scholar]
- 23.Drummond MF, Bloom BS, Carrin G, et al. Issues in the cross-national assessment of health technology. Int J Technol Assess Health Care. 1992;8(4):671–682. doi: 10.1017/s0266462300002361. [DOI] [PubMed] [Google Scholar]
- 24.Greiner W, Schoffski O. The transferability of international economic health-economic results to national study questions. Health Econ Prev Care. 2000;1(2):94–102. [Google Scholar]
- 25.Lamers LM, Stupp R, van den Bent MJ, et al. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme: a report from the EORTC 26981/22981 NCI-C CE3 Intergroup Study. Cancer. 2008;112(6):1337–1344. doi: 10.1002/cncr.23297. [DOI] [PubMed] [Google Scholar]
- 26.Gold MRSJ, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- 27.Wu B, Miao Y, Bai Y, et al. Subgroup economic analysis for glioblastoma in a health resource-limited setting. PLoS ONE. 2012;7(4):e34588. doi: 10.1371/journal.pone.0034588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung KC, Oda T, Saddawi-Konefka D, Shauver MJ. An economic analysis of hand transplantation in the United States. Plast Reconstr Surg. 2010;125(2):589–598. doi: 10.1097/PRS.0b013e3181c82eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak EJ, Silverstein MD, Bozic KJ. The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Joint Surg Am. 2007;89(11):2389–2397. doi: 10.2106/JBJS.F.01109. [DOI] [PubMed] [Google Scholar]
- 30.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253–1259. doi: 10.2106/JBJS.D.02152. [DOI] [PubMed] [Google Scholar]
- 31.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 32.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283(7):889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 33.Newby LK, Eisenstein EL, Califf RM, et al. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med. 2000;342(11):749–755. doi: 10.1056/NEJM200003163421101. [DOI] [PubMed] [Google Scholar]
- 34.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 35.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146(4):473–481. [PMC free article] [PubMed] [Google Scholar]
- 36.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 37.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12(1):80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 38.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development. Geneva, Switzerland: Author; 2001. [Google Scholar]
- 40.Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]