Abstract
Background
The Agency for Healthcare Research and Quality (AHRQ) patient safety indicators (PSIs) and the Centers for Medicare and Medicaid Services (CMS) hospital acquired conditions (HACs) are used to evaluate the safety and quality of health care. We determined the incidence rates of PSIs and HACs among brain tumor patients in the Nationwide Inpatient Sample database (NIS).
Methods
We queried the NIS for all hospitalizations involving a brain tumor. We determined the incidence rates of various PSIs and HACs among these patients by searching the hospital records for codes in the International Classification of Diseases, 9th Revision indicating each PSI or HAC.
Results
Among the 501 908 hospitalizations involving a brain tumor in the NIS, there were 102 046 occurrences of an AHRQ PSI, with 16% of patients experiencing one or more AHRQ PSI. Among brain tumor patients treated without surgery, 17.2% experienced ≥1 PSI. Among brain tumor patients treated with surgery, 9.8% experienced ≥1 PSI. The most common PSIs were postoperative respiratory failure, deep vein thrombosis, and sepsis. The total number of HACs associated with brain tumor patients was 13 778, with 2.63% of patients experiencing ≥1 HAC. Among brain tumor patients treated without surgery, 3.0% experienced ≥1 HAC. Among brain tumor patients treated with surgery, 7.4% experienced ≥1 HAC. The most common HACs were falls and trauma and pressure ulcers. Increasing comorbidity score was associated with increased likelihood of almost all PSIs and HACs.
Conclusion
These data may be used to determine individual institutional improvements or success by comparison.
Keywords: brain tumor, hospital acquired condition, patient safety indicators, pay for performance
In 1999, the Institute of Medicine (IOM) sparked controversy with its report titled To Err Is Human. In this report, the IOM highlighted that health care in the United States appeared to be far behind in ensuring basic safety compared with other high-risk industries.1,2 The IOM report stated that errors caused between 44 000 and 98 000 deaths every year in US hospitals and over 1 million injuries.2 In a follow-up report titled The Quality Chasm, the IOM called for an ambitious 50% reduction in medical errors.
A new national agenda for health care was subsequently put in action by government agencies that oversee the health care industry. The Agency for Healthcare Research and Quality (AHRQ),3 part of the US Department of Health and Human Services (http://www.ahrq.gov), created a list of indicators that represent inpatient complications or adverse events that are potentially avoidable. These patient safety indicators (PSIs) are reported by many hospitals to the University Health Consortium, an alliance of 116 academic medical centers and 276 of their affiliates, to determine hospital performance. In addition to publicly available rankings based on PSIs, hospitals are incentivized to reduce inpatient complications by the Centers for Medicare and Medicaid Services (CMS) pay-for-performance program. The CMS determined a list of hospital acquired conditions (HACs) for which hospitals will not receive additional reimbursement.3
The impact of these measures to ensure patient safety and quality is still unknown and continues to generate controversy. Measuring the success or failure of these programs will require surveys of large patient populations, such as the data available through the Nationwide Inpatient Sample database (NIS). These metrics are especially relevant to brain tumor patients who are at high risk for morbidity and mortality due to their underlying diagnoses. These metrics are now being used to determine the safety and quality of care being delivered by health care systems and providers and will be linked directly to reimbursement. Therefore, it is important to determine the national rates for these metrics in brain tumor patients to better understand the benchmarks specifically for this patient population. It is also important to know what factors influence the likelihood of a brain tumor patient developing a PSI or HAC during hospitalization. To better understand these metrics in hospitalized brain tumor patients, we determined the incidence rates of PSIs and HACs among patients with a diagnosis of a brain tumor in the NIS.
Materials and Methods
We queried the NIS, part of the AHRQ's Healthcare Cost and Utilization Project, for all hospitalizations between 2002 and 2010 involving a brain tumor. These data included all hospitalizations with codes from the International Classification of Diseases, 9th Revision (ICD-9) for a brain tumor (eg, 191.0-0.9, 225.0-0.2, 225.9, 198.3, 192.1, 239.6, 237.1, 237.5-0.6, 227.3-0.4). To identify patients who had undergone surgery, we used brain tumor surgery ICD-9 codes (011.3, 011.4, 040.1, 015.1, 015.3, 015.9). The incidence rate of a particular PSI or HAC among these hospitalizations was determined by summing the number of hospital records that included the ICD-9 code or codes indicating the presence of each PSI or HAC (Table 1). The NIS is the largest all-payer hospital inpatient database and contains data approximating a 20% stratified sample of US hospitals.
Table 1.
The PSIs and HACs used to search the NIS
| Complication, PSIs | ICD-9 |
|---|---|
| Anesthetic complication | |
| Misplaced endotracheal tube | E876.3 |
| Adverse effects in therapeutic use, other CNS depressants and anesthetics | E938.1-9 |
| Poisoning by other CNS depressants and anesthetics | 968.1-4, 968.7, E855.1 |
| Pressure ulcer | 707.0, 707.00-9 |
| Foreign body retained after procedure/surgery | 998.4, 998.7, E871.0, E871.3-9 |
| Iatrogenic pneumothorax | 512.1 |
| CVL infection | |
| Prior to October 1, 2007 | 996.62 |
| October 1, 2007–October 1, 2011 | 999.31 |
| After October 1, 2011 | 999.31, 999.32 |
| Postoperative hip fracture | 820.00-3, 820.09-13, 820.19-22, 820.30-32, 820.8-9 |
| Physiologic and metabolic derangements | |
| Secondary diabetes with ketoacidosis | 249.10-1, 249.20-1, 249.30-1 |
| Diabetes with ketoacidosis | 250.10-3 |
| Diabetes with hyperosmolarity | 250.20-3 |
| Diabetes with other coma | 250.30-3 |
| Postoperative hemorrhage | 998.11-2 |
| Postoperative respiratory failure | |
| Acute respiratory failure | 518.51, 518.53, 518.81, 518.84 |
| Mechanical ventilation for 96 consecutive hours or more | 967.2 |
| Mechanical ventilation | 967.0-1 |
| Reintubation | 960.4 |
| DVT | 451.11, 451.19, 451.2, 451.81, 451.9, 453.40-2, 453.8-9 |
| PE | 415.1, 415.11, 415.13, 415.19 |
| Sepsis | 038.0, 038.1, 038.10-2, 038.19, 038.2-3, 785.52, 785.59, 998.0, 998.00, 998.02, 038.40-4, 038.49, 038.8-9, 995.91-2 |
| Postoperative wound dehiscence | 546.1 |
| Accidental puncture or laceration | E870.0-9, 998.2 |
| Transfusion reaction | 999.6, 999.60-3, 999.69, 999.7, 999.70-4, E876.0 |
| Complications, HACs | ICD-9 |
| Foreign object retained after surgery | 998.4, 998.7 |
| Air embolism | 999.1 |
| Blood incompatibility | 999.60-3, 999.69 |
| Pressure ulcer stages III–IV | 707.23-4 |
| Falls and trauma | |
| Fracture | 800-829 |
| Dislocation | 830-839 |
| Intracranial injury | 850-854 |
| Crushing injury | 925-929 |
| Burn | 940-949 |
| Other injuries | 991-994 |
| Catheter-associated UTI | 996.64, 112.2, 590.10-1, 590.2-3, 590.80-1, 595.0, 597.0, 599.0 |
| Manifestations of poor glycemic control | 250.10-13, 250.20-23, 251.0, 249.10-11, 249.20-21 |
| Surgical site infection | |
| Mediastinitis, following coronary artery bypass grafting | 519.2, 36.10-19 |
| Following certain orthotopic procedures (spine, neck, shoulder, elbow) | 996.67, 998.59, 81.01-08, 81.23-24, 81.31-38, 81.83, 81.85 |
| Following bariatric surgery for obesity (laparoscopic gastric bypass, gastroenterostomy, laparoscopic gastric restrictive surgery) | 539.01, 539.81, 998.59, 44.38, 44.39 44.95 |
| DVT and PE following certain procedures (total knee replacement, hip replacement) | 415.11, 415.13, 415.19, 453.40-42, 00.85-87, 81.51-52, 81.54 |
Statistical Methods
We used the SAS v9.3 statistical software package to calculate means, standard deviations, and frequencies for all patient and hospital characteristics and to estimate all PSI and HAC incidence rates. The NIS is a sample of US hospitals stratified by region, location/teaching status, bedsize category, and ownership. We followed methods outlined in NIS documentation (http://www.hcup-us.ahrq.gov) to account for this stratification in our estimates of the total number of brain tumor hospitalizations for the years 2002–2010 and the national incidence and incidence rates of each indicator during that period among these patients.
We performed multivariable analysis of all indicators that had an observed incidence rate of >0.1%. An overall comorbidity score was generated for use as a covariate by summing the number of Elixhauser comorbidities4 recorded in the NIS for each hospitalization. We used generalized estimating equations (SAS PROC GENMOD, v9.3) to assess possible associations between patient and hospital characteristics and AHRQ and CMS indicators. In all models, the presence of the indicator (yes or no) was taken as the outcome variable. The patient's age, gender, and comorbidity score and the hospital type (teaching vs nonteaching), region (Northeast, Midwest, South, or West), and bedsize (small, medium, or large) were included as covariates. Results for hospital region were largely uninformative, showing no clear patterns, and were omitted. For all models, we graphically assessed the relationship between the probability of having the indicator and age, comorbidity score, and hospital bedsize. We used these results and the quasi-likelihood information fit criterion for generalized estimating equation models to determine whether any particular covariate modification (typically, adding a quadratic term in age or treating bedsize as an ordered rather than a nominal variable) resulted in a better fit than the baseline model. We assumed a binary distribution for the outcome variable, with a logit link function. To account for the clustering of observations on hospitals, we treated hospital as a repeated factor and assumed an exchangeable working correlation.
Results
From 2002 to 2010, there were 501 908 hospitalizations in the NIS with ICD-9 codes for brain tumor (191.0-0.9, 225.0-0.2, 225.9, 198.3, 192.1, 239.6, 237.1, 237.5-0.6, 227.3-0.4). Patient demographics and hospital characteristics are shown in Table 2. Patients who had surgery were significantly younger with lower comorbidity scores compared with those who did not have surgery. They were also more likely to receive their care at a teaching hospital. Importantly, they were much less likely to have a PSI or HAC during their hospitalization.
Table 2.
Patient and hospital characteristics for brain tumor patient hospitalizations (n = 501 908)
| Overall | Without Surgery (n = 421 672) | With Surgery (n = 80 236) | P*** | |
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| (mean ± SD) | 58.3 ± 19.2 | 59.4 ± 19.1 | 52.6 ± 18.6 | <.0001* |
| Gender | ||||
| Female | 270 628 (54.1%) | 228 488 (54.3%) | 42 140 (52.9%) | <.0001** |
| Male | 230 035 (45.9%) | 192 551 (45.7%) | 37 484 (47.1%) | |
| Comorbidity score | ||||
| (mean ± SD, median) | 2.1 ± 1.6, 2.0 | 2.2 ± 1.6, 2.0 | 1.5 ± 1.4, 1.0 | <.0001* |
| Hospital type | ||||
| Nonteaching | 204 698 (41.0%) | 188 069 (44.8%) | 16 629 (20.8%) | <.0001** |
| Teaching | 294 671 (59.0%) | 231 475 (55.2%) | 63 196 (79.2%) | |
| Hospital bedsize | ||||
| Small | 51 053 (10.2%) | 45 854 (10.9%) | 5199 (6.5%) | <.0001** |
| Medium | 104 138 (20.9%) | 91 068 (21.7%) | 13 070 (16.4%) | |
| Large | 344 178 (68.9%) | 282 622 (67.4%) | 61 556 (77.1%) | |
| Indicator rates | ||||
| PSIs, n | 102 046 (20.3%) | 92 275 (21.9%) | 9771 (12.2%) | <.0001* |
| HACs, n | 13 778 (2.7%) | 13 161 (3.1%) | 617 (0.77%) |
*t-test; **chi-square test; *** comparing with and without surgery.
Among these hospitalizations, there were 102 046 occurrences of an AHRQ PSI, with a total of 80 344 patients (16.0%) experiencing ≥1 AHRQ PSI. The most common PSIs were postoperative respiratory failure (estimated national rate [ENR] = 5.97%, 95% confidence interval [CI] = 5.90%, 6.03%), deep vein thrombosis (DVT) (ENR = 3.91%, 95% CI = 3.85%, 3.96%), sepsis (ENR = 3.86%, 95% CI = 3.81%, 3.91%), and pulmonary embolism (PE) (ENR = 2.39%, 95% CI = 2.34%, 2.43%) (Table 3).
Table 3.
Incidence (with 95% CI) of AHRQ PSIs for all brain tumor patients (NIS n = 501 908; estimated national admissions 2002–2010 = 2 470 342)
| Indicator | Incidence in NIS | Est. National Incidence 2002–2010 | ENR | 95% CI for Rate | LOS w/o Indicator, mean (SD); median (range) | LOS with Indicator, mean (SD); median (range) | P for LOS Comparison |
|---|---|---|---|---|---|---|---|
| AHRQ indicators | |||||||
| Anesthetic complications— therapeutic | 14 | 67 | 0.0027% | [0.0010%, 0.0044%] | 6.5 (7.9); 4.0 (1, 365) | 8.6 (7.5); 6.0 (2, 27) | .200 |
| Anesthetic complications— poisoning | 1 | 5 | 0.0002% | [0%, 0.0007%] | 6.5 (7.9); 4.0 (1, 365) | 3.0 (NA); 3.0 (3, 3) | NA |
| Pressure ulcer | 9870 | 48 548 | 1.97% | [1.84%, 2.09%] | 6.4 (7.5); 4.0 (1, 343) | 12.8 (17.0); 8.0 (1, 365) | <.0001 |
| Foreign body | 29 | 145 | 0.0059% | [0.0036%, 0.0081%] | 6.5 (7.9); 4.0 (1, 365) | 11.7 (7.9); 10.0 (1, 28) | <.0001 |
| Iatrogenic pneumothorax | 2663 | 13 075 | 0.529% | [0.503%, 0.556%] | 6.5 (7.8); 4.0 (1, 365) | 10.9 (10.4); 8.0 (1, 157) | <.0001 |
| Central venous line infection | 2825 | 13 832 | 0.560% | [0.522%, 0.597%] | 6.4 (7.7); 4.0 (1, 365) | 15.7 (19.1); 10.0 (1, 301) | <.0001 |
| Postoperative hip fracture | 2419 | 11 888 | 0.481% | [0.452%, 0.510%] | 6.5 (7.9); 4.0 (1, 365) | 7.6 (7.5); 6.0 (1, 206) | <.0001 |
| Postop physiologic derangement—secondary diabetes with ketoacidosis | 60 | 301 | 0.0122% | [0.0088%, 0.0156%] | 6.5 (7.9); 4.0 (1, 365) | 5.9 (4.0); 5.0 (1, 23) | .242 |
| Postop physiologic derangement—diabetes with ketoacidosis | 695 | 3427 | 0.139% | [0.126%, 0.151%] | 6.5 (7.9); 4.0 (1, 365) | 7.9 (8.9); 5.0 (1, 94) | <.0001 |
| Postop physiologic derangement—diabetes with hyperosmolarity | 353 | 1730 | 0.0700% | [0.0620%, 0.0781%] | 6.5 (7.9); 4.0 (1, 365) | 7.6 (9.0); 5.0 (1, 113) | <.0001 |
| Postop physiologic derangement—diabetes with other coma | 73 | 357 | 0.0144% | [0.0110%, 0.0179%] | 6.5 (7.9); 4.0 (1, 365) | 6.5 (7.1); 5.0 (1, 47) | .845 |
| Postoperative hemorrhage | 1278 | 6331 | 0.256% | [0.234%, 0.278%] | 6.5 (7.8); 4.0 (1, 365) | 13.1 (13.0); 9.0 (1, 129) | <.0001 |
| Postoperative respiratory failure | 29 952 | 147 425 | 5.97% | [5.81%, 6.13%] | 6.1 (7.0); 4.0 (1, 343) | 12.3 (14.9); 8.0 (1, 365) | <.0001 |
| Deep vein thrombosis | 19 610 | 96 795 | 3.92% | [3.77%, 4.06%] | 6.3 (7.6); 4.0 (1, 365) | 10.4 (12.3); 7.0 (1, 287) | <.0001 |
| Pulmonary embolism | 11 974 | 59 102 | 2.39% | [2.32%, 2.47%] | 6.4 (7.8); 4.0 (1, 365) | 8.8 (10.3); 6.0 (1, 287) | <.0001 |
| Sepsis | 19 373 | 95 278 | 3.86% | [3.72%, 4.00%] | 6.3 (7.3); 4.0 (1, 365) | 12.1 (15.7); 8.0 (1, 301) | <.0001 |
| Postop wound dehiscence | 0 | 0 | 0% | NA | 6.5 (7.9); 4.0 (1, 365) | NA | NA |
| Accidental puncture or laceration | 856 | 4191 | 0.170% | [0.149%, 0.190%] | 6.5 (7.8); 4.0 (1, 365) | 10.3 (12.5); 6.0 (1, 125) | <.0001 |
| Transfusion reaction | 1 | 6 | 0.0002% | [0%, 0.0007%] | 6.5 (7.9); 4.0 (1, 365) | 28.0 (NA); 28.0 (28, 28) | NA |
The total number of HACs associated with brain tumor patients was 13 778, with 13 187 patients (2.63%) experiencing ≥1 HACs. The most common HACs were falls and trauma (ENR for any type of fall/trauma = 2.09%, 95% CI = 2.05%, 2.13%) and stage III–IV pressure ulcers from 2008 to 2010 (ENR = 0.384%, 95% CI = 0.357%, 0.414%) (Table 4).
Table 4.
Incidence (with 95% CI) of HACs for brain tumor patients (n = 501 908)
| Indicator | Incidence in NIS | Est. National Incidence 2002–2010 | ENR | 95% CI for Rate | LOS w/o Indicator, mean (SD); median (range) | LOS With Indicator, mean (SD); median (range) | P for LOS Comparison |
|---|---|---|---|---|---|---|---|
| Hospital acquired conditions | |||||||
| Foreign object retained after surgery | 29 | 145 | 0.0059% | [0.0036%, 0.0081%] | 6.5 (7.9); 4.0 (1, 365) | 11.7 (7.9); 10.0 (1, 28) | <.0001 |
| Air embolism | 11 | 55 | 0.0022% | [0.0008%, 0.0036%] | 6.5 (7.9); 4.0 (1, 365) | 13.5 (15.5); 8.0 (1, 53) | .031 |
| Blood incompatibility | 0 | 0 | 0% | NA | 6.5 (7.9); 4.0 (1, 365) | NA | NA |
| Pressure ulcer stages III–IV (y 2008–2010 only, n = 181 457) | 697 | 3500 | 0.387% | [0.348%, 0.426%] | 6.2 (7.2); 4.0 (1, 310) | 12.3 (16.8); 8.0 (1, 194) | <.0001 |
| Falls and trauma—fracture | 7769 | 38 306 | 1.55% | [1.49%, 1.62%] | 6.5 (7.9); 4.0 (1, 365) | 7.2 (7.4); 5.0 (1, 206) | <.0001 |
| Falls and trauma—dislocation | 217 | 1087 | 0.0440% | [0.0379%, 0.0501%] | 6.5 (7.9); 4.0 (1, 365) | 9.1 (11.2); 6.0 (1, 77) | .0008 |
| Falls and trauma—intracranial injury | 2470 | 12 175 | 0.493% | [0.463%, 0.523%] | 6.5 (7.9); 4.0 (1, 365) | 6.6 (7.9); 4.0 (1, 112) | .435 |
| Falls and trauma—crushing injury | 10 | 49 | 0.0020% | [0.0008%, 0.0032%] | 6.5 (7.9); 4.0 (1, 365) | 5.2 (3.3); 4.0 (2, 13) | .997 |
| Falls and trauma—burn | 246 | 1208 | 0.0489% | [0.0424%, 0.0555%] | 6.5 (7.9); 4.0 (1, 365) | 7.7 (10.1); 5.0 (1, 86) | .070 |
| Falls and trauma—other injuries | 235 | 1155 | 0.0468% | [0.0406%, 0.0529%] | 6.5 (7.9); 4.0 (1, 365) | 7.3 (11.2); 4.0 (1, 131) | .994 |
| Catheter-associated urinary tract infection | 132 | 644 | 0.0261% | [0.0212%, 0.0310%] | 6.5 (7.9); 4.0 (1, 365) | 11.2 (12.0); 8.0 (1, 91) | <.0001 |
| Vascular catheter-associated infection (y 2007–2010 only, n = 238 693) | 777 | 3871 | 0.325% | [0.289%, 0.361%] | 6.2 (7.2); 4.0 (1, 310) | 14.4 (17.0); 9.0 (1, 253) | <.0001 |
| Manifestations of poor glycemic control—diabetic ketoacidosis | 695 | 3427 | 0.139% | [0.126%, 0.151%] | 6.5 (7.9); 4.0 (1, 365) | 7.9 (8.9); 5.0 (1, 94) | <.0001 |
| Manifestations of poor glycemic control—nonketotic hyperosmolar coma | 353 | 1730 | 0.0700% | [0.0620%, 0.0781%] | 6.5 (7.9); 4.0 (1, 365) | 7.6 (9.0); 5.0 (1, 113) | <.0001 |
| Manifestations of poor glycemic control—hypoglycemic coma | 13 | 64 | 0.0026% | [0.0012%, 0.0040%] | 6.5 (7.9); 4.0 (1, 365) | 5.7 (4.2); 4.0 (2, 16) | .916 |
| Manifestations of poor glycemic control—secondary diabetes with ketoacidosis | 33 | 167 | 0.0068% | [0.0041%, 0.0094%] | 6.5 (7.9); 4.0 (1, 365) | 5.8 (4.3); 5.0 (1, 23) | .632 |
| Manifestations of poor glycemic control—secondary diabetes with hyperosmolarity | 27 | 134 | 0.0054% | [0.0032%, 0.0076%] | 6.5 (7.9); 4.0 (1, 365) | 6.4 (3.6); 6.0 (1, 18) | .092 |
| Surgical site infection, mediastinitis, following coronary artery bypass graft | 0 | 0 | 0% | NA | 6.5 (7.9); 4.0 (1, 365) | NA | NA |
| Surgical site infection following certain orthopedic procedures | 19 | 93 | 0.0038% | [0.0020%, 0.0055%] | 6.5 (7.9); 4.0 (1, 365) | 32.9 (19.1); 29.0 (10, 86) | <.0001 |
| Surgical site infection following bariatric surgery for obesity | 0 | 0 | 0% | NA | 6.5 (7.9); 4.0 (1, 365) | NA | NA |
| Deep vein thrombosis | 45 | 227 | 0.0092% | [0.0060%, 0.0123%] | 6.5 (7.9); 4.0 (1, 365) | 18.2 (30.6); 11.0 (1, 206) | <.0001 |
Patients Treated Without Surgery
Of the 501 908 brain tumor patients found in the NIS, 421 672 (84%) were treated without surgery. The total number of PSIs in brain tumor patients treated without surgery was 92 275, with a total of 72 485 patients (17.2%) experiencing ≥1 PSI. The number of HACs in brain tumor patients treated without surgery was 13 161, with 12 596 (3.0%) experiencing ≥1 HAC.
Patients Treated With Surgery
Of the 501 908 brain tumor patients found in the NIS, 80 236 (16%) were treated with surgery. The number of PSIs in brain tumor patients treated with surgery was 9771, with 7859 patients (9.79%) experiencing ≥1 PSI. HACs in brain tumor patients treated with surgery occurred 617 times, with 591 (7.4%) experiencing ≥1 HAC.
Length of Stay
For PSIs, almost all of the indicators resulted in increased length of stay (LOS), except for anesthetic complications, postoperative physiologic derangement, postoperative wound dehiscence, and transfusion reaction (Table 3). Most of the increases of LOS were only by a couple of days. However, pressure ulcer, foreign body, central venous line (CVL) infection, postoperative hemorrhage and respiratory failure, and sepsis were associated with twice as long LOS.
HACs associated with increased LOS included retained foreign object, air embolism, pressure ulcers, falls and trauma, catheter-associated urinary tract infection (UTI), vascular catheter-associated infection, manifestation of poor glycemic control, surgical site infections following orthopedic procedures, and DVT (Table 4). Almost all of these were associated with a doubling of LOS except for surgical site infection following certain orthopedic procedures, which was associated with an impressive 5× increase in LOS.
Effect of Age and Gender on Incidence of PSIs and HACs
For PSIs, increasing age in brain tumor patients was associated with a linearly increasing risk of having a pressure ulcer (odds ratio [OR] × 1.015 for each additional year of age, P < .0001), iatrogenic pneumothorax (OR × 1.010 for each additional year of age, P < .0001), and postoperative hip fracture (OR × 1.06 for each additional year of age, P < .0001) (Supplementary Table S1). PSIs that linearly decreased with aging included CVL infection (OR × 0.957 for each additional year of age, P < .0001), postoperative physiologic derangement (OR × 0.982 for each additional year of age, P < .0001), and sepsis (OR × 0.994 for each additional year of age, P < .0001). The risk of postoperative respiratory failure was estimated to decrease exponentially with age (P < .0001) in brain tumor patients. DVT risk increased exponentially until the age of 58 and then decreased exponentially with age (P < .0001). Similarly, PE risk increased exponentially until the age of 56 and then decreased exponentially with age (P < .0001).
For HACs, increasing age in brain tumor patients was associated with linearly increased risk of stage III–IV pressure ulcers (OR × 1.010 for each additional year of age, P < .01) and catheter-associated UTI (OR × 1.020 for each additional year of age, P = .0014) (Supplementary Table S2). Vascular catheter-associated infection risk decreased linearly with increasing age (OR × 0.953, P < .0001), as did manifestations of poor glycemic control (OR × 0.984, P < .0001). Falls and trauma risk increased exponentially with age (P < .0001).
For PSIs, male gender was associated with increased likelihood of iatrogenic pneumothorax (OR = 1.12, P < .01), postoperative respiratory failure (OR = 1.24, P < .0001), DVT (OR = 1.13, P < .0001), PE (OR = 1.20, P < .0001), and sepsis (OR = 1.17, P < .0001) (Supplementary Table S3). Male gender was associated with a decreased likelihood of CVL infection (OR = 0.85, P < .0001), postoperative hip fracture (OR = 0.52, P < .0001), postoperative physiologic derangement (OR = 0.85, P < .01), pressure ulcers (OR = 0.82, P = .02), falls and trauma (OR = 0.71, P < .0001), and manifestations of poor glycemic control (OR = 0.86, P = .02).
Effect of Comorbidity Score on PSIs and HACs
Increasing comorbidity score was estimated to increase risk of all PSIs and HACs studied in multivariable analysis (those with incidence rates >0.1%). For PSIs, the increase in risk was linear for pressure ulcer (OR × 1.43 for each additional comorbidity, P < .0001), iatrogenic pneumothorax (OR × 1.07, P < .0001), postoperative hip fracture (OR × 1.25, P < .0001), postoperative hemorrhage (OR × 1.12, P < .0001), postoperative respiratory failure (OR × 1.39, P < .0001), DVT (OR × 1.24, P < .0001), and PE (OR × 1.36, P < .0001) (Table 5, Figures 1–6). Increasing comorbidity score was associated with exponential increases in CVL infection, postoperative physiologic derangement, and sepsis (P < .0001 for all).
Fig. 2.
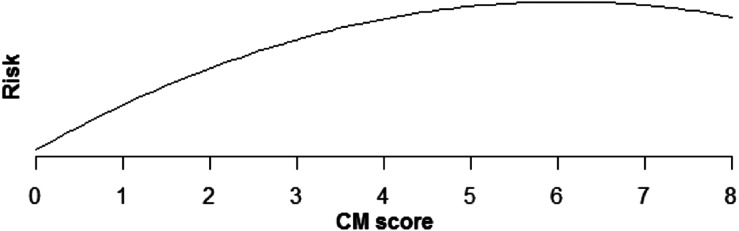
Relationship of postoperative physiologic derangement risk and CM score.
Fig. 3.
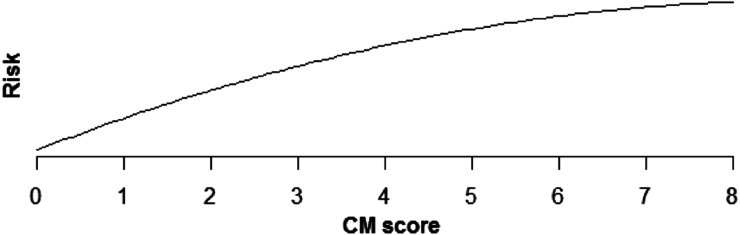
Relationship of sepsis risk and CM score.
Fig. 4.
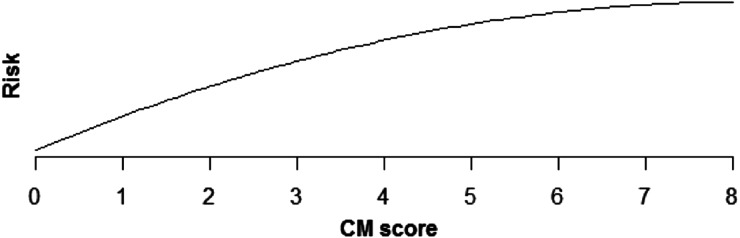
Relationship of pressure ulcer risk and CM score.
Fig. 5.
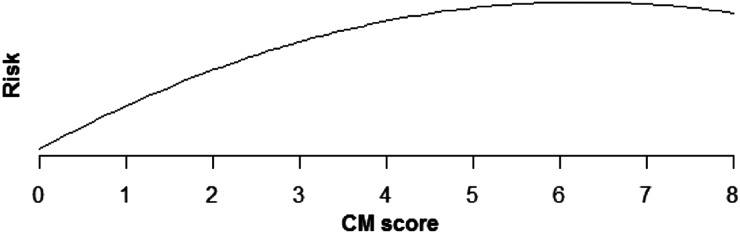
Relationship of falls and trauma risk and CM score.
Table 5.
Effect of comorbidity score (CM) on various indicators for brain tumor patients
| Indicator | For linear relationships (no graphic), or for having indicator multiplied by X for each unit increase in comorbidity score |
|---|---|
| AHRQ indicators | |
| Pressure ulcer | X = 1.43, CI = [1.40, 1.46], P < .0001 |
| Iatrogenic pneumothorax | X = 1.07, CI = [1.04, 1.09], P < .0001 |
| Central venous line infection | Risk is estimated to increase quickly for low CM scores but more slowly for higher scores (P > .0001) (Fig 1). |
| Postoperative hip fracture | X = 1.25, CI = [1.22, 1.28], P < .0001 |
| Postop physiologic derangement, any | Risk is estimated to increase quickly for low CM scores, but more slowly for higher scores (P > .0001) (Fig 2). |
| Postoperative hemorrhage | X = 1.12, CI = [1.08, 1.16], P < .0001 |
| Postoperative respiratory failure | X = 1.39, CI = [1.38, 1.41], P < .0001 |
| Deep vein thrombosis | X = 1.24, CI = [1.23, 1.25], P < .0001 |
| Pulmonary embolism | X = 1.36, CI = [1.35, 1.38], P < .0001 |
| Sepsis | Risk is estimated to increase quickly for low CM scores, but more slowly for higher scores (P > .0001) (Fig 3). |
| Accidental puncture or laceration | P > .05 |
| Hospital acquired conditions | |
| Pressure ulcer stages III–IV | Risk is estimated to increase quickly for low CM scores, but more slowly for higher scores (P > .0001) (Fig 4). |
| Falls and trauma, any | Risk is estimated to increase quickly for low CM scores, but more slowly for higher scores (P > .0001) (Fig 5). |
| Vascular catheter-associated infection | Risk is estimated to increase quickly for low CM scores, but more slowly for higher scores (P > .0001) (Fig 6). |
| Manifestations of poor glycemic control, any | X = 1.51, CI = [1.47, 1.54], P < .0001 |
| Catheter-associated UTI | X = 1.19, CI = [1.09, 1.30], P = .0002 |
Fig. 1.
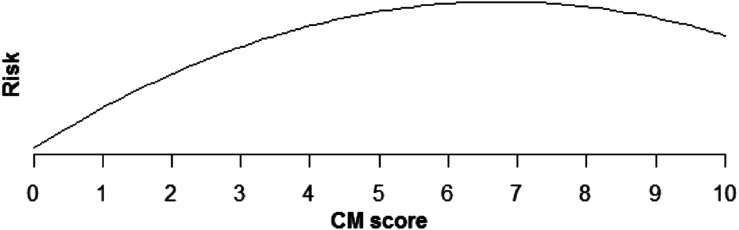
Relationship of CVL infection risk and CM score.
Fig. 6.
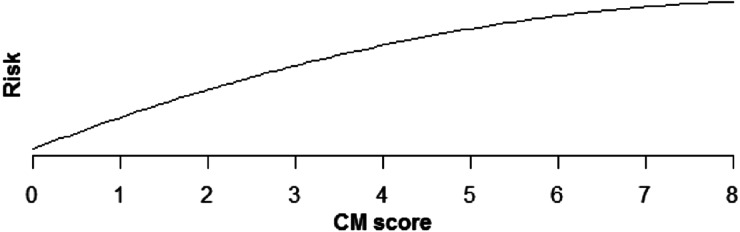
Relationship of vascular catheter-associated infection risk and CM score.
For HACs, increasing comorbidity score was associated with linearly increased risk of manifestations of poor glycemic control (OR × 1.51, P < .0001) and catheter-associated UTI (OR × 1.19, P < .001). The risks of pressure ulcers, falls and trauma, and vascular catheter-associated infections were estimated to increase exponentially with increasing comorbidity score (P < .0001).
Hospital Type and Size Effect on PSIs and HACs
The likelihood of being diagnosed with a PSI or HAC was higher for patients admitted to nonteaching hospitals for postoperative hip fractures (OR = 1.38, P < .0001), postoperative physiologic derangement (OR = 1.17, P = .03), sepsis (OR = 1.19, P < .0001), falls and trauma (OR = 1.10, P < .001), and manifestations of poor glycemic control (OR = 1.17, P = .04) (Supplementary Table S4). The risks of pressure ulcer, postoperative hemorrhage, postoperative respiratory failure, DVT, and accidental puncture were estimated to increase with hospital bedsize (Supplementary Table S5).
Discussion
PSIs and HACs are extremely common in brain tumor patients, especially those treated without surgery. The relatively low rates of PSIs and HACs in surgically treated brain tumor patients reflects the ability of surgeons to appropriately choose low-risk patients for surgical treatment. Surgical patients were more likely to be treated at teaching institutions. Not surprisingly, postoperative respiratory failure, DVT, and sepsis were among the most common PSIs/HACs identified. As expected, increasing comorbidities were associated with increased incidence of almost all PSIs and HACs. Overall, the rates of PSIs and HACs in the NIS were comparable to rates from other databases.5
Metrics like PSIs and HACs are sets of administrative data-based indicators used to identify in-hospital patient safety events.6 Movements to track these metrics have their roots in the IOM's definition of patient safety: “freedom from accidental injury caused by medical care.”2 This definition has been expanded to include “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim. Errors can include problems in practice, products, procedures, and systems.”7
Due to implications of the incidence of PSIs/HACs for hospitals and physicians regarding reimbursement and ratings, significant effort is now being invested in accurately coding patient preadmission risk profiles and preventing inpatient harm events. Standardized procedural protocols have been highly successful in reducing the number of patient harm events.8–10
Despite changes in identification of higher-risk patients and standardization of treatment, PSIs and HACs continue to be reported in high numbers.11,12 In response to this reality, the Partnership for Patients was launched in 2011 by the US Secretary of Health and Human Services. The Partnership for Patients is an effort designed to reduce patient harm events by 40% in 2013 compared with 2010 and to reduce preventable hospital readmissions within 30 days by 20% in 2013 compared with 2010.1,2 The effects of these types of initiatives on a national scale will take years to determine. In the meantime, individual institutions will be able to use databases such as that of the University Health Consortium and the NIS for relative comparisons of PSI and HAC rates.
Study Limitations
Limitations of this study include its retrospective nature and potential for selection bias. The NIS also has the potential for coding errors and variability in coding.
Our data are limited because the NIS does not allow distinction between preadmission conditions and hospital acquired diagnoses. Therefore, some of these reported PSIs and HACs may have been present on admission. This may be much more likely for certain conditions (eg, physiologic and metabolic derangements, sepsis, DVT) compared with others (eg, anesthetic complication, transfusion reaction).
Brain tumor patients are at high risk for experiencing a PSI or HAC. This reality seems to be recognized by neurosurgeons, explaining the association of nonsurgical treatment of brain tumors and elevated rates of PSIs and HACs. These data are important when counseling patients about the diagnosis and treatment of brain tumors. Importantly, these values provide baselines for hospital comparisons and quality standards.
Conclusion
Patient safety and delivery of quality care is an important part of our national health care agenda. Systems are being put in place to monitor and ensure patient safety. Our results demonstrate baseline national rates of PSIs and HACs in brain tumor patients. These data may be used to determine individual institutional improvements or success by comparison. Further prospective studies are required to accurately determine the true incidence of these patient safety metrics in a neurosurgical population.
Supplementary Material
Conflict of interest statement. None declared.
Funding
None declared.
Supplementary Material
References
- 1.Stelfox HT, Palmisani S, Scurlock C, Orav EJ, Bates DW. The “To Err Is Human” report and the patient safety literature. Qual Saf Health Care. 2006;15:174–178. doi: 10.1136/qshc.2006.017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. 2000. [PubMed]
- 3.Centers for Medicare and Medicaid Services. Hospital-acquired conditions (HAC) in acute inpatient prospective payment system (IPPS) hospitals. 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/downloads/hacfactsheet.pdf . Accessed 29 June 2013. [Google Scholar]
- 4.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Rivard PE, Elwy AR, Loveland S, Zhao S, Tsilimingras D, Elixhauser A, et al. Applying patient safety indicators (psis) across health care systems: achieving data comparability and methodology. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality; 2005. [PubMed] [Google Scholar]
- 6.McDonald KM, Romano PS, Geppert J, et al. AHRQ Report no. 02-0038; 2002 Measures of Patient Safety Based on Hospital Administrative Data—The Patient Safety Indicators. [Google Scholar]
- 7.Quality Interagency Coordination Force Doing what counts for patient safety: federal actions to reduce medical errors and their impact. 2012. http://archive.ahrq.gov/quic/report/mederr2.htm . Published 2012. Accessed 30 June 2013.
- 8.Pronovost PJ, Marsteller JA, Goeschel CA. Preventing bloodstream infections: a measurable national success story in quality improvement. Health Aff (Millwood) 2011;30:628–634. doi: 10.1377/hlthaff.2011.0047. [DOI] [PubMed] [Google Scholar]
- 9.Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–248. March 4. [PubMed] [Google Scholar]
- 10.Rahman M, Whiting JH, Fauerbach LL, Archibald L, Friedman WA. Reducing ventriculostomy-related infections to near zero: the eliminating ventriculostomy infection study. Jt Comm J Qual Patient Saf. 2012;38:459–464. doi: 10.1016/s1553-7250(12)38061-6. [DOI] [PubMed] [Google Scholar]
- 11.Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134. doi: 10.1056/NEJMsa1004404. [DOI] [PubMed] [Google Scholar]
- 12.McCannon J, Berwick DM. A new frontier in patient safety. JAMA. 2011;305:2221–2222. doi: 10.1001/jama.2011.742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


