Abstract
Background
Meningiomas often harbor an immune cell infiltrate that can include substantial numbers of T and B cells. However, their phenotype and characteristics remain undefined. To gain a deeper understanding of the T and B cell repertoire in this tumor, we characterized the immune infiltrate of 28 resected meningiomas representing all grades.
Methods
Immunohistochemistry was used to grossly characterize and enumerate infiltrating lymphocytes. A molecular analysis of the immunoglobulin variable region of tumor-infiltrating B cells was used to characterize their antigen experience. Flow cytometry of fresh tissue homogenate and paired peripheral blood lymphocytes was used to identify T cell phenotypes and characterize the T cell repertoire.
Results
A conspicuous B and T cell infiltrate, primarily clustered in perivascular spaces, was present in the microenvironment of most tumors examined. Characterization of 294 tumor-infiltrating B cells revealed clear evidence of antigen experience, in that the cardinal features of an antigen-driven B cell response were present. Meningiomas harbored populations of antigen-experienced CD4+ and CD8+ memory/effector T cells, regulatory T cells, and T cells expressing the immune checkpoint molecules PD-1 and Tim-3, indicative of exhaustion. All of these phenotypes were considerably enriched relative to their frequency in the circulation. The T cell repertoire in the tumor microenvironment included populations that were not reflected in paired peripheral blood.
Conclusion
The tumor microenvironment of meningiomas often includes postgerminal center B cell populations. These tumors invariably include a selected, antigen-experienced, effector T cell population enriched by those that express markers of an exhausted phenotype.
Keywords: B cell, meningioma, T cell, tumor-infiltrating lymphocytes
Meningiomas are brain tumors that arise from the meninges surrounding the brain and spinal cord and are among the most common adult neoplasms of the CNS.1 Although 80% of meningiomas are slow-growing nonmalignant World Health Organization (WHO) grade I tumors,2 they may still cause harm via mass effect on neighboring structures. Favorable outcomes for those WHO grade I tumors can often be achieved by complete surgical resection. However, the atypical meningiomas, WHO grades II and III, can have more devastating effects.
Despite optimal management that includes surgical resection and radiation treatment, patients with grade II and grade III tumors have 5-year survival rates of 78.5% and 44%, respectively, and corresponding 10-year survival rates of 53.3% and 14.2%.3 Unfortunately, there is no consensus for treatment strategies, and after several surgeries, treatment options are often limited. Thus, treatment strategies beyond radiation and surgery for these patients are needed.4,5 One such strategy that is being explored in other primary brain tumors, such as gliomas, is immunotherapy.6,7 Currently, our understanding of the relationship between meningiomas and the immune system is limited. By further exploring these interactions, we aim to determine whether immunotherapeutic strategies could be used to target meningiomas.
Several studies in non-CNS tumors, such as lung cancer, melanoma, ovarian cancer, and colon cancer, indicate that the presence of lymphocytes can be a critical determinant of clinical prognosis.7–11 Furthermore, it has been suggested that the types and distribution patterns of these infiltrating lymphocytes can be more powerful prognostic indicators than the previously used pathological criteria for tumor staging12 or oncogene expression.13 For example, a high tumor infiltrating CD8+/regulatory T cell (Treg) ratio is often correlated with long-term survival.14 However, high levels of T cell response and antibody production can also be present in progressively growing tumors, indicating that an immune response does not necessarily result in immune protection.15 The interaction between tumors and the immune system is complex, and its outcome cannot be predicted from the size of the infiltrate alone.
Immune cell infiltrates of meningiomas can include variable numbers of T cells, B cells, plasma cells, and macrophages.16–20 Although the presence of immune cells in meningiomas has been well delineated at a descriptive level, many of the characteristics of these cells remain unknown. Understanding whether such cells demonstrate antigen experience, have developed into activated effector cells, or display exhausted phenotypes is one of the first steps toward understanding their role in tumor biology. In the present work, we began to determine the role of the immune cells that populate the tumor parenchyma in meningiomas.
Materials and Methods
Clinical Specimens
Meningioma specimens were obtained from 28 patients undergoing neurosurgical craniotomies for clinical indications unrelated to the current work. The sources of tissue included Columbia University Medical Center, Massachusetts General Hospital Cancer Center Tumor Bank, and Yale School of Medicine. For meningiomas 025, 027, 029, 032, 033, and 034, peripheral blood samples were obtained from the same patients at the time of the surgery. The clinical demographics are summarized in Table 1. Sections from all of the specimens were snap-frozen in optimal cutting temperature (OCT) compound immediately following surgical removal. Many of the specimens that were large enough were also dissected so that segments could be fixed in paraformaldehyde and embedded in paraffin. Sections from several fresh specimens were reserved for flow cytometry-based analysis. Fluorescence-activated cell sorted naïve and antigen-experienced B cells from normal healthy subjects were used as controls. Institutional review boards at each facility approved this study, and informed consent was obtained from all subjects when required.
Table 1.
Meningioma specimen demographics and mean TIL counts
| Tumor ID | Grade (WHO) | T Cells (n) | B Cells (n) | Plasma Cells (n) |
|---|---|---|---|---|
| 3104 | I | 3 | 0 | 0.1 |
| 3113 | I | 1.7 | 0.2 | 1.3 |
| 3115 | I | 9 | 0.2 | 0 |
| 3125 | I | 8.4 | 0.2 | 0 |
| 3129 | I | 0.1 | 2.3 | 0 |
| 103111 | I | 0.1 | 0 | 0 |
| 2739 | I | 2.3 | 0.1 | 0 |
| 4217 | I | 3.2 | 0 | 0 |
| 001 | I | 3.4 | 1.8 | 1.2 |
| 002 | I | 1.7 | 0.3 | 0.6 |
| 004 | I | 9.2 | 1.4 | 0.2 |
| 025 | I | 2.4 | 4.2 | 0 |
| 032 | I | 1 | 0.3 | 0.67 |
| 033 | I | 0.3 | 0 | 0.3 |
| 034 | I | 0.3 | 0 | 0 |
| 027 | I | 5.1 | 0.3 | 0 |
| 029 | II | 1.4 | 0.2 | 0 |
| 2111 | II | 2.2 | 0 | 0 |
| 3106 | II | 1.1 | 0 | 0 |
| 3123 | II | 2.4 | 0.2 | 0 |
| 3152 | II | 4.2 | 0 | 0 |
| 3118 | II | 9.7 | 0.3 | 0 |
| 1343 | II | 2.9 | 0 | 0 |
| 1408 | II | 2.1 | 0.2 | 0 |
| 15016 | II | 5.9 | 0.1 | 0 |
| 2628 | III | 3 | 0.2 | 0 |
| 3097 | III | 0.6 | 0 | 0 |
| 3119 | III | 2.7 | 0 | 0.1 |
Immunohistochemistry
Immunohistochemistry was performed on either paraformaldehyde-fixed paraffin-embedded tissues or OCT-embedded frozen tissues as previously described.21 Murine monoclonal anti-human CD20 (Clone L26, Dako Cytomation) was used at a dilution of 1:250, anti-human CD3 (Clone F7.2.38, Dako Cytomation) at 1:50, and anti-human CD138 (Clone MI15, Dako Cytomation) at 1:50. Primary antibodies were applied for 1 h at room temperature. Slides were washed in Tris-HCl (50 mM, pH 7.4) and incubated with anti-mouse secondary antibodies for 1 h at room temperature. After further washing, the slides were developed using diaminobenzidine chromogen and counterstained with hematoxylin. Tumor-infiltrating lymphocytes (TILs) were enumerated under 400X magnification within 9 fields.
Immunoglobulin Variable Region Cloning
Immunoglobulin (Ig) variable region of heavy chain (VH) libraries were constructed from frozen tumor specimens (8 μm thickness) and from defined control subsets that included fluorescence-activated cell sorted naïve (CD19+CD27−) and antigen-experienced (CD19 + CD27+) B cells derived from the peripheral blood mononuclear cells (PBMCs) of normal healthy subjects. Total mRNA was extracted using the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. From the total RNA, cDNA was synthesized and human Ig variable region genes were amplified according to the protocol described by Wang and Stollar,22 with minor modifications described by our group.21
Immunoglobulin VH Sequence Analysis
The Ig VH sequences were analyzed using the ImmunoGeneTics/V-Query and Standardization (IMGT/V-Quest) tool (www.imgt.org)23 to assess isotype distribution, somatic hypermutation, and clonal expansion. The first 10 primer encoded codons of framework region 1 (FR1) were excluded from this analysis. Allelic polymorphism was not considered in the assessment, because the Ig variable gene alleles have very few such nucleotide substitutions,24 and the IMGT database includes various alleles for alignment. Chimeric molecules arising from PCR amplification artifacts were not included in any analyses.25 The variable region cloning procedure also captures the 5′ end of the Ig constant region, allowing the Ig isotype and thereby extent of isotype switching to be determined. By aligning each sequence to the most homologous germline segments (V, D, and J), the nucleotide mutations and amino acid replacements were counted. Clonal expansion and intraclonal variants were determined as previously described.21
Clonal Expansion and Intraclonal Variation
Clones were identified through the invariably unique sequences of their third complementarity determining region (CDR3). Identical sequences from nonconsecutive tissue sections defined clonal expansion, whereas identical sequences within one tissue section were considered to be the product of PCR amplification rather than expanded clones, as the two cannot be reliably distinguished. This highlights the need for the preparation of distinct tissue libraries to properly evaluate clonal expansion. If 2 or more sequences had an identical CDR3 and differed by at least 2 somatic mutations or at least 1 amino acid replacement in the VH region, they were considered to be derived from clonally related B cells, which we termed clonal variants. The PCR enzyme used here introduces <0.6 base changes into the amplified variable regions, which are ∼360 bp in length.26 Thus, we considered variants to be present when at least 2 bases were different. Statistical analysis of the sequencing data was performed with the χ2 test and Student's t-test using GraphPad Prism v6.0 software.
Quantification of Antigen-Driven Selection Strength
After using IMGT/V-Quest to determine the germline genes and the junction regions for each sequence, we divided the sequences into clones. For meningioma 004 (95 sequences from serial sections), we were left with 1 clone of 6 sequences, 5 of size 4, 3 of size 3, and 4 of size 2. The rest were mono sequence clones. After the sequences were divided into clones and a germline was assigned for each clone, we used BASELINe27 in a focused binomial test formulation28,29 to quantify the strength of antigen-driven selection acting on the sequenced B cells. Due to uncertainty in the germline assignment of the D segment and surrounding N and P additions,30 we used only mutations in the V and J regions for the selection analysis.
Immunophenotyping and T Cell Receptor Variable β Repertoire Analysis by Flow Cytometry
Fresh single-cell suspensions from meningiomas were obtained by Percoll gradient centrifugation after mechanical dispersion through nylon mesh cell strainers in Roswell Park Memorial Institute media. Matched fresh PBMCs were isolated by Ficoll-Hypaque gradient centrifugation. For immunophenotyping analysis, flow cytometric analysis was performed with the following anti-human surface and intracellular antibodies: anti-CD4-V450 (Clone RPA-T4, BD Bioscience), anti-CD8-V500 (Clone RPA-T8, BD Bioscience), anti-Tim-3-PE (Clone F38-2E2, BioLegend), anti-PD-1-PerCp-Cy5.5 (Clone EH12.1, BD Bioscience), anti-CD45RA-APC (Clone HI100, BD Bioscience), anti-CD45RO-APC-H7 (Clone UCHL1, BD Bioscience), anti-CD25-Alexa700 (Clone BC96, BioLegend), and anti-Foxp3-FITC (Clone PCH101, eBioScience). The T cell receptor variable β domain (TCR Vβ) repertoire in tumor-infiltrating T cells (TIL-Ts) and whole blood was determined using the TCR Vβ repertoire kit (IOTest Beta Mark, Beckman Coulter) according to the manufacturer's protocol. Cells were stained simultaneously with anti-CD3-APC (Clone UCHT1, BD Bioscience), anti-CD4-PE-Cy7 (Clone SK3, BD Bioscience), anti-CD8-PerCP-Cy5.5 (Clone SK1, BioLegend), and 1 of 8 sets of antibody directed against 3 distinct TCR Vβ families. In this manner, TCR usage from a total of 24 distinct Vβ families (about 70% coverage of normal human TCR Vβ repertoire) was detected. All flow cytometric analyses were conducted on a LSR Fortessa instrument (BD Bioscience), and FlowJo 9.4.11 software was used for analysis. To analyze differences in Vβ family usage, we calculated P-values using Pearson's χ2 test to determine whether the observed TIL-T Vβ family frequency matched that expected from paired whole blood, controlling for tube and total count number. The Bonferroni correction was applied to control for family-wise error rate.
Results
Immunohistochemistry of Tumor-Infiltrating Lymphocytes
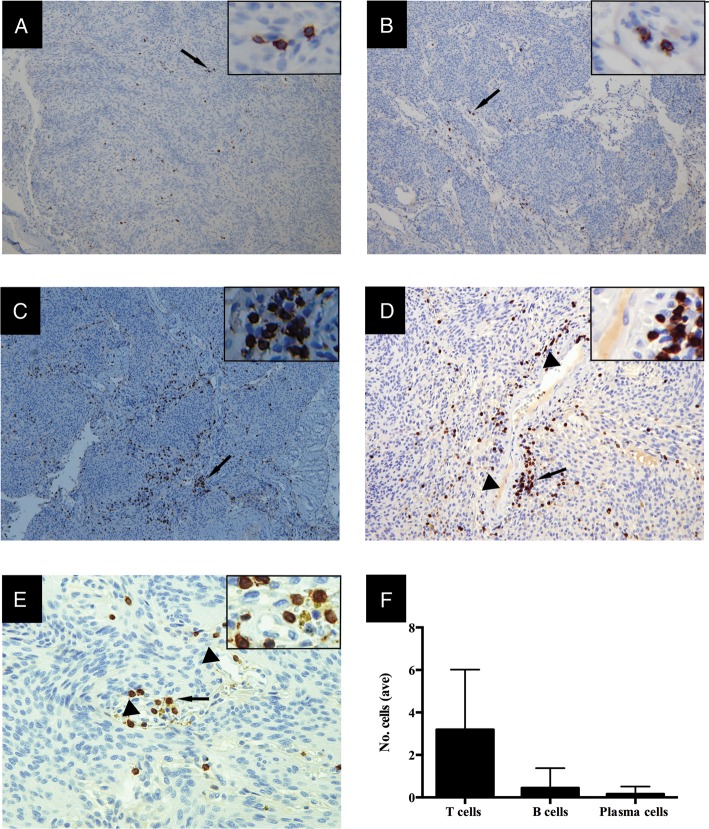
All meningioma specimens were first examined for the presence of an immune cell infiltrate using immunohistochemical markers for B cells (CD20+; Fig. 1A), plasma cells (CD138+; Fig. 1B), and T cells (CD3+; Fig. 1C–E). The frequency of cells was determined from the immunohistochemistry slides. Considerable variation among the specimens was observed. B cells were found in 17/28 (61%) of the tumors (Table 1). The density of the B cell infiltrate was sparse in most specimens (Fig. 1A and F). However, several had a more defined and conspicuous infiltrate with clustering in the perivascular space. Antibody-producing plasma cells were observed in 8/28 (28.5%) of the tumors (Table 1). The plasma cell density was very sparse, often more diffuse than that of B cells and present in fewer specimens (Fig. 1B and F). T cells were seen in all 28 specimens, and their density was greater than that of B cells (Fig. 1A–C and Table 1). T cells often assembled in clusters in the perivascular space (Fig. 1D and E) but were occasionally seen in the tumor parenchyma in the absence of such clusters. To determine how the immune cell infiltrate was distributed throughout the entire tumor, one specimen with a robust infiltrate of both B and T cells (004) was serial sectioned along its z-axis to build a 3-dimensional reconstruction. The density, distribution patterns, and phenotype of the TILs were homogeneous throughout the entire tumor, indicating that no particular region provided a preferential environment for the infiltrate. We then compared the WHO grade of all 28 specimens with characteristics of the infiltrate. Neither the frequency nor the type of cells in the infiltrate correlated with tumor grade (not shown).
Fig. 1.
Meningiomas harbor B, plasma, and T cell infiltrates within the tumor microenvironment. Immunohistochemistry demonstrated the presence of CD20+ B cells (A), CD138+ antibody-secreting plasma cells (B), and CD3+ T cells (C). T cells often assembled in clusters (arrow) in perivascular spaces (arrow head) (D and E). The images shown are representative of the specimens that had a visible infiltrate under a 100X (A–C), 200X (D), and 400X (E) field. The inset is a magnification of the arrow-indicated site. The mean cell counts (within 9 fields per specimen under a 400X field) included all the specimens examined (F). Error bars indicate SEM.
Molecular Characterization of Tumor-Infiltrating B Cell Immunoglobulin Variable Regions
Having identified tumor-infiltrating B cells (TIL-Bs) in most of the meningiomas we examined, we next constructed Ig VH libraries from the snap-frozen and serially sectioned meningioma specimens to assess whether the TIL-Bs had the characteristic features of an antigen-driven response. This analysis was based on the Ig VH, as its sequence complexity is significantly greater than that observed in the light chain variable region. Isotype switching, somatic mutation and amino acid replacement, and clonal expansion with intraclonal variation provide clear evidence of an antigen-driven B cell response; thus, each of these characteristics was assessed.
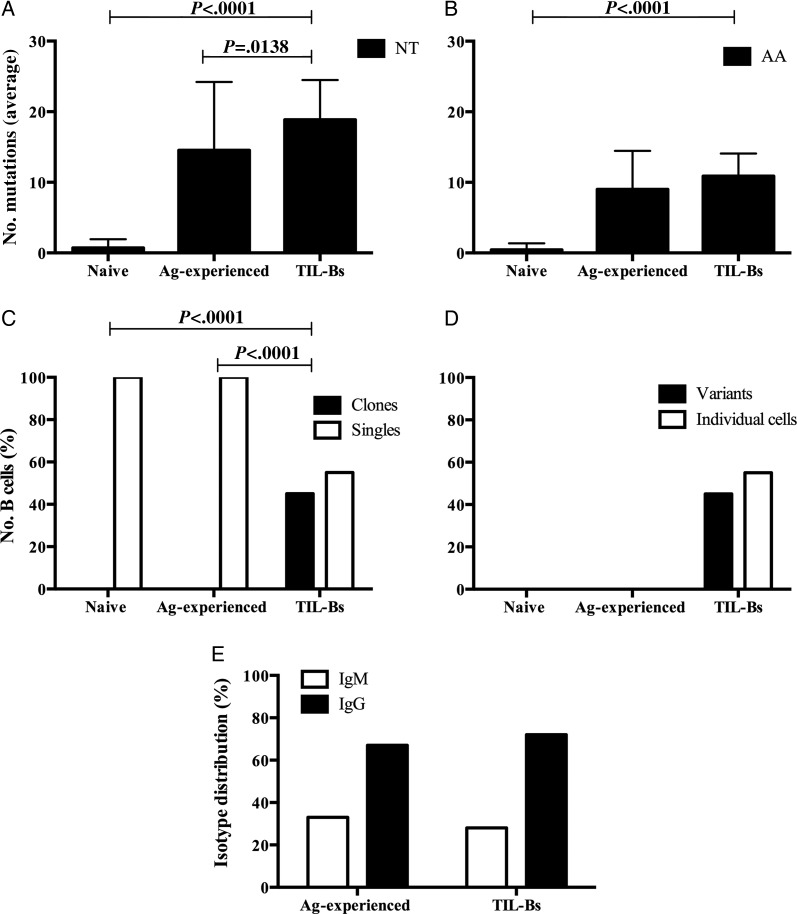
B cells derived from nontumor sources were compared with those derived from the meningiomas. In TIL-Bs, there was abundant somatic mutation. On average, each Ig VH of ∼400 nucleotides had a mean of 19 nucleotide mutations (relative to the germline allele), resulting in 11 amino acid substitutions in TIL-Bs. There were even more nucleotide mutations in TIL-Bs than in antigen-experienced B cells from healthy subjects (mean = 15 nucleotide mutations) (P = .0138, Student's t-test; Fig. 2A). The amino acid mutation values (mean = 9 amino acid substitutions) were consistent with those of sorted antigen-experienced B cells (not significant, Student's t-test; Fig. 2B), while sorted naïve B cells showed few somatic mutations (<1 on average), as expected (P < .0001, Student's t-test; Fig. 2A and B). No VH gene was amplified from the control reactions that did not contain RNA. No sequence overlap between different tumor specimens was found. These results indicate that no contamination or cross-contamination between tumors occurred. Our immunohistochemistry-based study indicated that several tumors did not harbor B cells, but the molecular analysis confirmed their presence in the same specimens. This discrepancy is likely due to the lower sensitivity of the immunohistochemistry performed with frozen specimens.
Fig. 2.
The immunoglobulin repertoire of TIL-Bs and peripheral B cells. TIL-Bs (294 sequences), fluorescence-activated cell sorted naïve B cells (34 sequences), and fluorescence-activated cell sorted antigen (Ag)-experienced B cells (45) were analyzed for evidence of antigen-driven characteristics. (A and B) Extent of somatic mutation and amino acid (AA) replacement (average number), NT: nucleotide. Error bars indicate SEM; (C) clonal expansion. Black columns represent the percentage of clonally expanded sequences, and white columns represent percentages of the other analyzed sequences that were not clonally expanded sequences (singles); (D) intraclonal variants. Black columns represent the percentage of B cells within the expanded clones that were variants (having undergone somatic mutation), and white columns represent the percentage of B cells within the expanded clones that were not variants (individual B cells); (E) heavy chain isotype distribution (%) in fluorescence-activated cell sorted antigen-experienced B cells and TIL-Bs. Statistical differences are shown when significant.
Meningioma 004 provided a representative example of the characteristics of TIL-Bs in meningiomas. Ig VH libraries were constructed from 3 nonconsecutive sections. Interestingly, we found clonally related TIL-Bs across these distinct sections, indicating either that clonal expansion and migration occurred within the tumor or that these clones evolved in a lymph node and then migrated to multiple regions within the tumor. Clonal expansion and intraclonal variation were typical among the B cells that infiltrated the meningioma microenvironment. Among a total of 294 sequenced TIL-Bs from 22 meningioma specimens, 46% were members of B cell clones, and the remaining 54% were unrelated to one another. On average, each meningioma that we examined contained 3 B cell clones (families) and 6 B cells that were members of clones, of which 5 were clonal variants. Overall, among the clonally expanded TIL-Bs, 80% could be identified as variants, based on their distinct somatic mutation patterns relative to their clonal family members. The frequency of a specific B cell clone in the peripheral blood of normal individuals is about 1/20 000;31 this indicates that the B cells we examined from the tumors were not derived from blood. Furthermore, we observed no clonal expansion within the sorted naïve or antigen-experienced B cell populations we studied (P < .0001, χ2 test; Fig. 2C and D).
Given that naïve B cells are defined by their expression of IgM, we examined the isotype distribution of the TIL-Bs and sorted antigen-experienced B cells. As expected, sorted antigen-experienced B cells had largely undergone class switching to the IgG isotype and were thus not different from those derived from the tumor (not significant, χ2 test; Fig. 2E), suggesting that the TIL-Bs were also antigen experienced.
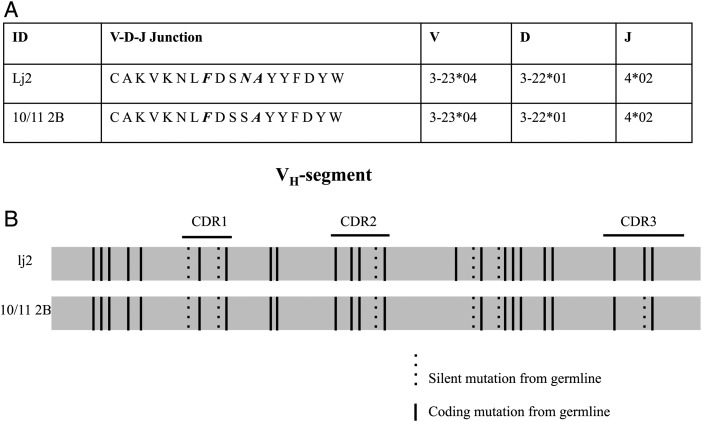
Figure 3A and B show more detailed analyses of 2 IgG sequences that demonstrate the clonal expansion and intraclonal diversity that were typical in these TIL-B populations. The two TIL-Bs (lj2 and 10/11 2 B) were both detected in meningioma 004. Both silent and replacement mutations were found throughout the variable regions, including CDR3, compared with the germline VH allele. The two TIL-Bs shared the same mutation pattern in FR1, CDR1, FR2, and CDR2. The FR3 region of lj2 contained 1 additional amino acid substitution. Interestingly, at one locus in the CDR3 region, lj2 contained 2 point mutations (from agt to aac), resulting in an amino acid substitution (from S to N), while 10/11 2 B contained 1 point mutation (from agt to agc), without an amino acid substitution. This overlapping mutation pattern demonstrates that these B cells are the progeny of the same parent cell, which indicates that a process of antigen-driven maturation took place, either within the meningioma environment or in a lymph node.
Fig. 3.
Clonal expansion and intraclonal diversity of a B cell clone isolated from a meningioma. (A) Alignment of CDR3 protein sequences, as well as V-D-J gene segment use, of clonally related TIL-Bs. Amino acid differences are italicized and in bold compared with the CDR3 region encoded by the germline allele. (B) Variable gene segments were aligned at the nucleotide level for 2 clonally related TIL-Bs. Solid vertical lines represent coding mutations that resulted in amino acid replacement, and dashed lines represent silent mutations, compared with the most homologous germline segment.
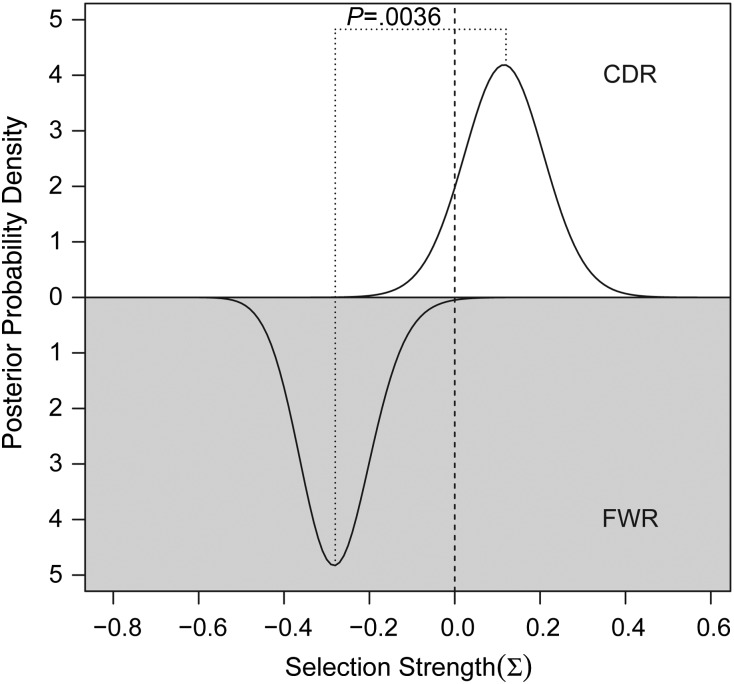
To further confirm that the TIL-Bs were antigen driven, we employed an algorithm (BASELINe) that detected selection by analyzing mutation patterns in experimentally derived Ig sequences. Using BASELINe, we observed negative selection in the framework regions and slightly positive/neutral selection in the complementary determining regions (Fig. 4). The difference between the selection estimates in the different regions was highly significant (P = .0036), in agreement with normal antigen-specific B cells. Collectively, these results indicate that TIL-Bs had undergone activation, Ig class switching, somatic hypermutation, and clonal expansion, all of which are hallmarks of antigen exposure.
Fig. 4.
Quantification of antigen-driven selection strength using BASELINe. The top half of the plot shows the estimated selection strength in the complementary determining regions (CDR), while the bottom part provides an estimate for the framework regions (FWR). Negative sigma values indicate negative selection, while positive values indicate positive selection. In the meningioma 004 sequences shown here, we observed negative selection in the framework regions and slightly positive/neutral selection in the complementary determining regions. The difference between the selection estimates in the different regions is highly significant (P = .0036), in agreement with normal antigen-specific B cells.
Phenotypes and Frequencies of Tumor-Infiltrating T Cells
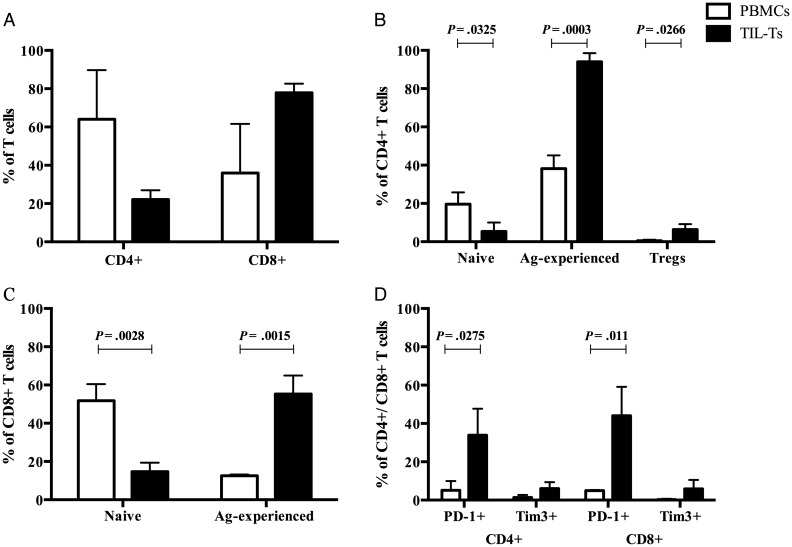
Having demonstrated that most TIL-Bs were antigen experienced, we asked whether tumor-infiltrating T cells TIL-Ts also exhibited the characteristics of an antigen-driven response. We used flow cytometry to further assess the phenotypes and frequencies of TIL-Ts and compared those to PBMCs from the same patients. First, we investigated the distribution of CD4+ and CD8+ cells within the T cell populations of tumor and matched peripheral blood. Then, naïve and antigen-experienced subsets were examined based on the expression of CD45RA and CD45RO. We found that there were more CD8+ T cells than CD4+ T cells in the meningiomas, while this ratio was reversed in matched PBMCs (Fig. 5A). That is, TIL-Ts had a lower frequency of CD4+ cells and a higher frequency of CD8+ cells compared with PBMCs, but the differences were not significant (Student's t-test; Fig. 5A). Among TIL-Ts expressing CD4 or CD8, there was a significantly higher percentage of cells with a memory/effector phenotype (CD45RO+CD45RA−) and a significantly lower frequency of naïve cells (CD45RO−CD45RA+) compared with CD4+ and CD8+ PBMCs (P < .05, Student's t-test; Fig. 5B and C). The T cell infiltrate was also enriched in CD4+ CD25+ Foxp3+ Tregs relative to matched PBMCs (P < .05, Student's t-test; Fig. 5B). We next examined the negative regulatory or immune checkpoint molecules programmed death-1 (PD-1) and T cell Ig and mucin protein-3 (Tim-3) expression on TIL-Ts.32 These molecules, often observed on TIL-Ts, are associated with an exhausted phenotype.33 Compared with PBMCs in both CD4+ and CD8+ TIL-Ts, we found a significant increase in the expression of PD-1 (P < .05, Student's t-test) and a trend of increased expression of Tim-3, but no significance difference (Student's t-test) (Fig. 5D). These collective changes in the TIL-T populations suggested that they experienced antigen-driven activation, which was consistent with our findings for TIL-Bs.
Fig. 5.
Flow cytometric analysis of TIL-Ts and matched PBMCs. TIL-Ts (025, 027, 029) and matched PBMCs were first gated by CD4+ and CD8+ T cell subsets. Then the surface markers CD45RA, CD45RO, PD-1, Tim-3, CD25, and the intracellular marker Foxp3 were used to demarcate particular T cell subsets. (A) Frequency of CD4+ and CD8+ T cell subsets. (B) Frequency of naïve (CD45RA+CD45RO-), antigen (Ag)-experienced memory/effector (CD45RA−CD45RO+) and Treg (CD25+Foxp3+) populations within CD4+ T cell subsets. (C) Frequency of naïve (CD45RA+CD45RO−), antigen-experienced memory/effector (CD45RA−CD45RO+) populations within CD8+ T cell subsets. (D) Frequency of T cells displaying an exhausted phenotype (PD-1+ and Tim-3+) within the CD4+ and CD8+ T cell subsets. Error bars represent SEMs. Statistical differences are shown when significant.
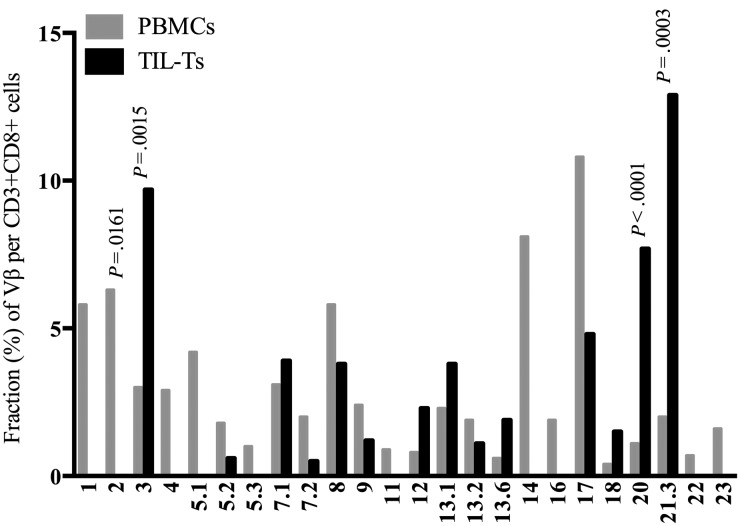
We used an additional, complementary approach to determine whether the tumor microenvironment included an immune repertoire that differed from that in the periphery. The T cell repertoire, assessed by TCR Vβ usage, from both the TILs and periphery was determined. The CD4+ and CD8+ subsets were examined separately. In agreement with other reports, the T cell repertoire varied among individual patients,34 so we focused on comparing the repertoire of TIL-Ts and PBMCs from the same patient. The TCR Vβ repertoire expressed by CD4+ TIL-Ts generally resembled the diverse TCR Vβ profile expressed by CD4+ T cells in matched peripheral blood (Supplementary data). The usage of some Vβ families in the CD4+ subset was statistically different, but the overall profile was quite similar. The TCR repertoire of CD8+ T cells in the TILs was, however, quite different from that in matched peripheral blood. Relative to matched peripheral blood, a number of families were considerably overrepresented in the TIL-Ts, while some were less apparent or not represented at all (Fig. 6 and Supplementary data). The CD8+ TCR Vβ repertoire from meningioma 032 is shown as a representative case and highlights these differences well (Fig. 6). Here, 3 families (Vβ3, 20, and 21.3) were preferentially expressed within the TILs, such that they represented more than half of the TCR Vβ repertoire. However, in the paired periphery, these families accounted for only 8% of the repertoire. Particular families were substantially diminished in the TILs. For example, the Vβ2 family was overexpressed in the peripheral blood (6.3%) of tumor 032 but was absent from the TILs (P < .05, χ2 test; Fig. 6).
Fig. 6.
TCR Vβ repertoire of a meningioma T cell infiltrate and paired blood. An informative example of the expansions and omissions within the tumor T cell repertoire compared with those of the peripheral blood is shown. In this example, the TCR Vβ usage within the CD3+CD8+ T cell population from meningioma 032 and matched peripheral blood are compared. Statistical differences are shown when significant.
Discussion
TILs are harbored by many solid tumors, including melanoma, colon cancers, breast cancers, lung cancer, intracranial germinomas, and meningiomas.21,35–37 The precise nature of the role TILs play remains to be thoroughly described. TILs appear to have a tumoricidal capacity, as their adoptive transfer participates in the resolution of established cancer in murine tumor models38 and successful regression of human neoplasms.39 However, inflammation can also play the opposite role by promoting tumor progression.40 This can be achieved by selecting for tumor cells that are capable of survival in the context of an immune response (immunoediting)41 or by contributing to conditions within the tumor microenvironment that facilitates tumor outgrowth.15,42
Although TILs have been intensely studied in a number of tumors, fewer studies have been focused on meningiomas. This may be due to the fact that meningiomas are often considered benign neoplasms and are more easily treated than gliomas and medulloblastomas, resulting in favorable outcomes. However, their recurrence rates vary 7%–25% for WHO grade I, 29%–52% for WHO grade II, and 50%–94% for WHO grade III,43 the latter being associated with a 1.5-year median survival.44 Moreover, benign meningiomas can still cause significant problems due to mass effects and limited surgical access inherent to their proximity to the skull base. Given that immunotherapy is emerging as a conceivable treatment approach and that the TILs of meningiomas have not been thoroughly characterized, a more complete understanding of the TIL repertoire is needed. Accordingly, we focused our study on further defining the phenotypes of the immune cell infiltrate in a series of meningiomas.
To begin, immunohistochemistry was used to identify the presence of B and T cells within meningioma tissues, and then the number of TIL subsets was counted to quantify the infiltrate. The organization of the infiltrate along with its distribution throughout the tumor was also assessed. We undertook a molecular characterization of the B cell antigen receptor repertoire to test whether these cells had previously encountered antigen. Flow cytometry was performed to determine which T cell subsets were present and whether they displayed phenotypic markers associated with tumor evasion of cellular immunity. Finally, a flow cytometry-based TCR Vβ chain usage assay allowed us to determine the profile of the T cell repertoire in these tumors. Comparing the results found in the tumor with those from matched blood considerably strengthened all of the flow cytometry-based studies; we were able to observe enrichment of cell types in the tumor that would not have been appreciated by studying the tumor-derived cells alone.
A discernible T and B cell infiltrate was present in the microenvironment of most meningiomas examined; these tumors often harbored B cells and invariably included a T cell infiltrate. However, heterogeneity between tumors, both in terms of cell type and frequency, was a consistent finding. B cell Ig variable regions revealed clear evidence of antigen experience. The CD8+ T cell frequency was higher than CD4+, a ratio that was reversed in the periphery of the subjects we studied. Tregs, capable of suppressing immune responses, were enriched in the tumor. Although the T cells were antigen experienced memory/effector cells and were enriched relative to the blood, many displayed a phenotype associated with exhaustion (PD-1+, Tim-3+), indicating that they may not be capable of mounting an effective response. The shape of the T cell repertoire (identified by Vβ usage) in the CD4+ and CD8+ compartments was different than that found in the peripheral blood of the same individual, suggesting that the T cells migrating and residing in the tumor were selected by a specific antigen or antigens expressed in the tumor.
Little is known about TIL-Bs, which often co-localize with T cells, enhancing T cell responses by producing antibodies, stimulatory cytokines, and chemokines and serving as local antigen presenting cells (APCs).35 Interestingly, the presence of B cells in tumors is thought to have a paradoxical effect. In tumor immunity, activated B cells may play a beneficial role in the chronic immune process by expressing the costimulatory activities needed to activate naïve CD4+ T cells and by acting as APCs capable of promoting immune responses.45 Conversely, resting B cells can inhibit T cell responses via a mechanism dependent on major histocompatibility complex class II.46 B cells need to encounter their specific antigen to activate their function. The presence of substantial B cell numbers dispersing throughout the microenvironment of many meningiomas suggests a role for an antigen-driven, antibody-mediated immune response within the meningioma tissue or nonspecific lymphocyte recruitment by inflammatory and chemotactic cytokines.
The vast majority of B cells that were present in the tumors we studied were antigen experienced, indicating that they had migrated to the tumor after maturing in a germinal center. Given that immunohistochemistry indicated that the B cell family infiltrate included a small fraction of plasma cells, we concluded that these were primarily memory B cells. Infiltrates of clonally expanded memory B cells have been observed in other solid tumors.47 These B cells had switched from IgM to the IgG isotype. The process of Ig isotype switching requires specific interactions between CD4+ T cells and B cells along with the expression of activation-induced deaminase. Ig isotype switching is a codependent process involving the B cell receptor and CD40 and CD40L (ligand). CD40, expressed on B cells, is a member of the tumor necrosis factor receptor superfamily and is critical for B cell Ig isotype switching and the formation of the germinal center. Binding to CD40L expressed on activated T cells to CD40 on B cells activates the isotype switching process. Thus, the role of CD40 and CD40L is a crucial event in T cell priming and ultimately Ig isotype switching.48 That IgG frequency was significantly higher than IgM frequency suggests that these B cells most likely undergo additional clonal expansion outside of the tumor microenvironment at a nearby site where they can interact with antigen-specific T cells and migrate to the tumor. It is possible that tumor antigens could drive this process.
TIL-Bs could mediate their effects through the production of tumor-specific antibodies. To investigate this possibility, we looked for evidence of an antigen-driven immune response within the tumor tissue by examining the nature of the B cell infiltrate in meningiomas. The molecular features of the B cell antigen receptor expressed by tumor-associated B cells were elucidated, thereby describing the characteristics of the B cell infiltrate. Although B cells appear to represent the lowest frequency of the immune cell infiltrates, they do not appear to be innocuous. Indeed, these are fully mature functional B cells. Our phenotypic characterization of the intratumoral B cell repertoire revealed that the resident B cells included (i) expanded sets of clones, (ii) intraclonal variation, (iii) Ig genes that had undergone isotype switching, and (iv) somatic mutations within these genes. These are fundamental features of antigen-driven B cell maturation requiring T cell help and CD40-CD40L interaction. Although mature, antigen-experienced B cells were present, we did not observe extranodal lymphoid-like follicles that have been shown to populate a number of other tumor types.21,49,50 These structures, present in normal lymph nodes, are the site of antigen-driven B cell affinity maturation. That such structures are absent from meningiomas suggests that the experienced B cells present in the tumor parenchyma mature elsewhere and then migrate to the tumor.
The degree of heterogeneity among the TILs in meningiomas has not been addressed. Indeed, it is possible that there could be different microenvironments. For example, it would be reasonable to consider that there could be zones of augmented TILs, specifically in the perivascular space and near the arachnoid cap. We investigated the TILs throughout the z-axis of one specimen to further investigate the possibility of heterogeneity as a cause. Immunohistochemistry did not reveal such heterogeneity, as the infiltrate was rather consistently distributed. B cell repertoires of this tumor were prepared from 3 distinct regions that were separated by at least 0.25 cm. The B cell repertoires were surprisingly consistent and did not suggest the presence of different microenvironments located throughout the tissue. Interestingly, B cells derived from the same clone (parent) were found in each distinct region. This suggests that an antigen in the tumor parenchyma recognized by this expanded B cell clone, may be the target of these cells. Although such experiments were beyond the scope of the present study, there are established approaches to investigate this possibility. These include expression of recombinant Ig51,52 and ex vivo B cell expansion53 from a dominant, tumor-associated clone. The IgG derived from these approaches could then be screened on meningioma-derived antigens.
Meningioma-derived T cells have been studied more than B cells have been. In agreement with other studies, we found T cells to be more frequent than B cells, both in terms of the magnitude of the infiltrate and the number of tumors including such cells. T cells of the CD8+ subtype within a number of solid tumors often outnumber the CD4+ T cell population.54 This phenomenon is consistent with our findings in that we invariably observed more CD8+ T cells in all 28 tumors studied. When examined for the presence of effector functions, both subsets included an enrichment of antigen-experienced memory/effector cells relative to those circulating in the same patients. This approach wherein the tumor homogenate was compared with the PBMCs of the same individual allowed us to be certain that the analysis was not erroneously focused on cells derived from the tumor vasculature but included TILs.
In addition to CD4+ and CD8+ T cells, Tregs were present in the infiltrates. Tregs have been observed in a number of solid tumors,55,56 and their presence, although less frequent than that observed in glioblastomas, has been reported in meningiomas.57 This T cell subset is responsible for suppressing the immune response and may assist the tumor in evading the immune system by affecting the antigen-presenting process of dendritic cells, directly contacting and lysing effector T cells, and secreting cytokines such as interleukin 10 and transforming growth factor β.58 The infiltrates examined also included T cells expressing Tim-3 and PD-1, indicative of T cell exhaustion. Such cells have been observed among the infiltrates of other solid tumors.59 Both the Tregs and exhausted T cells were enriched in the tumor compared with the periphery, indicative of preferential selection of particular T cell clones in the tumor but not in the circulation. The presence of these 2 cell types implies that the T cell response in meningiomas contributes to an immunosuppressive environment.
We did not observe significant associations between the characteristics of the TILs we identified and meningioma WHO grades. Future studies comprising larger cohorts, should evaluate whether such correlations exist. This is particularly relevant given that meningioma subgroups defined by several characteristics, including genetics and anatomical location, have recently been reported.60 Indeed, whether characteristics of the TILs further define these established subgroups will be valuable for prognosis, treatment response, and selection of therapeutic options. It is becoming clear that the immune composition of primary tumors is an essential prognostic factor for tumor eradication and overall survival,61 both of which represent important consequences in clinical practice.
We must also continue to investigate how a tumor is able to grow in an environment that includes a host immune response. Does this occur because it is recognizable as normal tissue and thus tolerated by immune cells, or because it evades immunosurveillance? Future immunotherapy, dependent on this understanding, may be an effective adjunct to standard therapy, reversing immunologic tolerance in the tumor microenvironment. The immune response is not entirely effective in eliminating meningiomas. Given that these tumors include an infiltrate of antigen-experienced B and T cells, it is possible that an equilibrium has been established between the tumor and the immune system.62 This balance may result in the slow growth that is characteristic of many meningiomas. More aggressive tumors may move past this equilibrium into a phase of immune evasion allowing progression and metastasis. The collective data shown here indicate that the microenvironment of meningiomas supports an active immune response that may be maintained through the expression of specific antigens. This understanding of the microenvironment, along with the future identification of such tumor antigens, provides the potential for the design, development, and clinical application of immunotherapy that one day may include directed cancer vaccines.
Supplementary Material
Funding
None declared.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We thank Dr Hongzhi Quan for assisting with the production of the figures, Amanda MacAvoy for making editorial suggestions, and Dr Chris Cotsapas for providing recommendations concerning the statistical approaches.
References
- 1.Perry A, Gutmann DH, Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70(2):183–202. doi: 10.1007/s11060-004-2749-0. [DOI] [PubMed] [Google Scholar]
- 2.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 3.Durand A, Labrousse F, Jouvet A, et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol. 2009;95(3):367–375. doi: 10.1007/s11060-009-9934-0. [DOI] [PubMed] [Google Scholar]
- 4.Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3. doi: 10.3171/FOC/2008/24/5/E3. [DOI] [PubMed] [Google Scholar]
- 5.Simon M, Bostrom J, Koch P, Schramm J. Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry. 2006;77(6):767–773. doi: 10.1136/jnnp.2005.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112(13):4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SK, Kerr KM, Chapman AD, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27(1):27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 9.Leffers N, Gooden MJ, de Jong RA, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58(3):449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svennevig JL, Lunde OC, Holter J, Bjorgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49(3):375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correale P, Rotundo MS, Botta C, et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res. 2012;18(3):850–857. doi: 10.1158/1078-0432.CCR-10-3186. [DOI] [PubMed] [Google Scholar]
- 12.Bromwich EJ, McArdle PA, Canna K, et al. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89(10):1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Bo L, Mork SJ, Nyland H. An immunohistochemical study of mononuclear cells in meningiomas. Neuropathol and Appl Neurobiol. 1992;18(6):548–558. doi: 10.1111/j.1365-2990.1992.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 17.Gi H, Nagao S, Yoshizumi H, et al. Meningioma with hypergammaglobulinemia. Case report. J Neurosurg. 1990;73(4):628–629. doi: 10.3171/jns.1990.73.4.0628. [DOI] [PubMed] [Google Scholar]
- 18.Becker I, Roggendorf W. Immunohistological investigation of mononuclear cell infiltrates in meningiomas. Acta Neuropathol. 1989;79(2):211–216. doi: 10.1007/BF00294381. [DOI] [PubMed] [Google Scholar]
- 19.Rossi ML, Cruz Sanchez F, Hughes JT, Esiri MM, Coakham HB. Immunocytochemical study of the cellular immune response in meningiomas. J Clin Pathol. 1988;41(3):314–319. doi: 10.1136/jcp.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paine JT, Handa H, Yamasaki T, Yamashita J, Miyatake S. Immunohistochemical analysis of infiltrating lymphocytes in central nervous system tumors. Neurosurgery. 1986;18(6):766–772. doi: 10.1227/00006123-198606000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Willis SN, Mallozzi SS, Rodig SJ, et al. The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol. 2009;182(5):3310–3317. doi: 10.4049/jimmunol.0803424. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Stollar BD. Human immunoglobulin variable region gene analysis by single cell RT-PCR. J Immunol Methods. 2000;244(1–2):217–225. doi: 10.1016/s0022-1759(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 23.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2001;29(1):207–209. doi: 10.1093/nar/29.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook GP, Tomlinson IM. The human immunoglobulin VH repertoire. Immunol Today. 1995;16(5):237–242. doi: 10.1016/0167-5699(95)80166-9. [DOI] [PubMed] [Google Scholar]
- 25.Ford JE, McHeyzer-Williams MG, Lieber MR. Chimeric molecules created by gene amplification interfere with the analysis of somatic hypermutation of murine immunoglobulin genes. Gene. 1994;142(2):279–283. doi: 10.1016/0378-1119(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 26.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 27.Yaari G, Uduman M, Kleinstein SH. Quantifying selection in high-throughput Immunoglobulin sequencing data sets. Nucleic Acids Res. 2012;40(17):e134. doi: 10.1093/nar/gks457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uduman M, Yaari G, Hershberg U, Stern JA, Shlomchik MJ, Kleinstein SH. Detecting selection in immunoglobulin sequences. Nucleic Acids Res. 2011;39:W499–W504. doi: 10.1093/nar/gkr413. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershberg U, Uduman M, Shlomchik MJ, Kleinstein SH. Improved methods for detecting selection by mutation analysis of Ig V region sequences. Int Immunol. 2008;20(5):683–694. doi: 10.1093/intimm/dxn026. [DOI] [PubMed] [Google Scholar]
- 30.Gaeta BA, Malming HR, Jackson KJ, Bain ME, Wilson P, Collins AM. iHMMune-align: hidden Markov model-based alignment and identification of germline genes in rearranged immunoglobulin gene sequences. Bioinformatics. 2007;23(13):1580–1587. doi: 10.1093/bioinformatics/btm147. [DOI] [PubMed] [Google Scholar]
- 31.Yamada M, Wasserman R, Reichard BA, Shane S, Caton AJ, Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24(2):213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Beemd R, Boor PP, van Lochem EG, et al. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry. 2000;40(4):336–345. doi: 10.1002/1097-0320(20000801)40:4<336::aid-cyto9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 36.Balch CM, Riley LB, Bae YJ, et al. Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch Surg. 1990;125(2):200–205. doi: 10.1001/archsurg.1990.01410140078012. [DOI] [PubMed] [Google Scholar]
- 37.Imahayashi S, Ichiyoshi Y, Yoshino I, Eifuku R, Takenoyama M, Yasumoto K. Tumor-infiltrating B-cell-derived IgG recognizes tumor components in human lung cancer. Cancer Investi. 2000;18(6):530–536. doi: 10.3109/07357900009012192. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report . N Engl J Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 40.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development . Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 41.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 42.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. ‘Malignancy’ in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238(2):67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother. 2007;30(3):323–332. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 47.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190(11):5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 48.Maclennan ICM. Germinal-Centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 49.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Re. 2003;63(12):3275–3280. [PubMed] [Google Scholar]
- 50.Coronella JA, Spier C, Welch M, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169(4):1829–1836. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Lake DF, Barbuto JA, Bernstein RM, Grimes WJ, Hersh EM. A human monoclonal antimelanoma single-chain Fv antibody derived from tumor-infiltrating lymphocytes. Cancer Res. 1995;55(16):3584–3591. [PubMed] [Google Scholar]
- 52.Pavoni E, Monteriu G, Santapaola D, et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007;7:70. doi: 10.1186/1472-6750-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Punt CJ, Barbuto JA, Zhang H, Grimes WJ, Hatch KD, Hersh EM. Anti-tumor antibody produced by human tumor-infiltrating and peripheral blood B lymphocytes. Cancer Immunol, Immunother: CII. 1994;38(4):225–232. doi: 10.1007/BF01533513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domingues PH, Teodosio C, Ortiz J, et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J Pathol. 2012;181(5):1749–1761. doi: 10.1016/j.ajpath.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Malchow S, Leventhal DS, Nishi S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humphries W, Wei J, Sampson JH, Heimberger AB. The role of tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg Clin of N Am. 2010;21(1):125–137. doi: 10.1016/j.nec.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs JF, Idema AJ, Bol KF, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009;11(4):394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunother. 2011;3(4):517–537. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69(15):1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark VE, Erson-Omay EZ, Serin A, et al. Genomic Analysis of Non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.