Abstract
Background
Ependymoma is treated with maximal surgical resection and localized radiotherapy. Minimizing unnecessary exposure to radiation is of paramount importance for young children. Proton radiotherapy (PRT) spares healthy tissues outside the target region, but reports of clinical outcomes are scarce. We report outcomes for 70 patients treated with PRT for intracranial ependymoma.
Methods
Seventy patients with localized ependymoma treated with involved-field PRT at the Massachusetts General Hospital between October 2000 and February 2011 were included.
Results
Median age at diagnosis was 38 months (range, 3 mo–20 y). Nineteen (27%) patients had supratentorial ependymoma and 51(73%) had infratentorial ependymoma. Forty-six (66%) had gross total resection (GTR), and 24 (34%) had subtotal resection (STR). At a median follow-up of 46 months, 3-year local control, progression-free survival, and overall survival were 83%, 76%, and 95%, respectively. STR was significantly associated with worse progression-free survival (54% vs 88%, P = .001) and overall survival (90% vs 97% for GTR, P = .001). In a subset of patients (n = 14), mean intelligence was 108.5 at baseline and 111.3 after mean 2.05 years of follow-up. In a larger group of patients (n = 28), overall adaptive skills were 100.1 at baseline and 100.8 after 2.21 years of follow-up. Few patients developed evidence of growth hormone deficiency, hypothyroidism, or hearing loss.
Conclusion
Outcomes for children treated with PRT compare favorably with the literature. STR correlated with inferior outcome. The young age at diagnosis and the proximity of critical structures in patients with ependymoma make PRT an ideal radiation modality.
Keywords: central nervous system, pediatric ependymoma, proton, radiation
Ependymoma represents 5%–8% of pediatric brain tumors, with most occurring in very young children.1 The best outcomes for nondisseminated disease are achieved with maximal surgical resection followed by involved-field radiation therapy (RT).2–12 Late side effects of RT are of great concern in this young population with a relatively high chance of cure. Many investigators have explored the delivery of chemotherapy to avoid or delay RT in children under the age of 3 years, with inferior results.8,9,13–16
Proton RT (PRT) is a form of particle radiation that provides highly conformal RT to the area at greatest risk of recurrence with a lower integral dose than any form of photon RT. Based on the apparent physical advantage (Fig. 1) and early outcomes in our previously published series of 17 patients, many children with ependymoma are now referred to proton centers to receive RT that is predicted to maintain rates of local control while decreasing late toxicities.17 Given the increased number of proton facilities and higher cost of PRT, it is crucial to provide clinical evidence of comparable survival outcomes and an improved side effect profile.
Fig. 1.
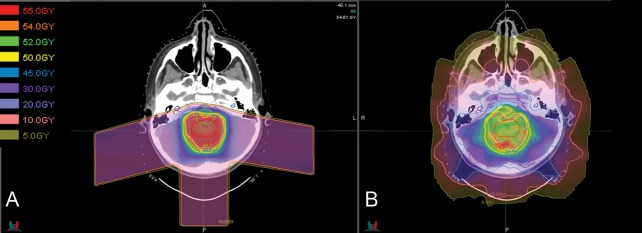
Representative (A) proton RT plan and (B) intensity-modulated RT plan for infratentorial ependymoma.
We report outcomes in a cohort of 70 patients treated with PRT for intracranial ependymoma. We report neurocognitive, endocrine, and auditory outcomes for a subset of these patients. To our knowledge, this study population is the largest series of pediatric proton patients reported in the literature, and the first to report endocrine, cognitive, and auditory outcomes after involved-field PRT.
Materials and Methods
Patient Selection
We identified all pediatric (<21 y) patients (n = 70) with intracranial ependymoma consecutively treated with conformal PRT at the Massachusetts General Hospital between October 2000 and February 2011. Demographic, tumor, and treatment characteristics were obtained from review of the medical records. The researchers performed the human investigations after approval by a local human investigations committee.
Radiation
All children underwent 3D planning with a contrast-enhanced CT scan and MRI fusion. The tumor bed and residual tumor were contoured as gross tumor volume. The clinical tumor volume was defined as a 0.5- to 1-cm extension of the gross tumor volume restricted for anatomical boundaries. Dose was prescribed in gray relative biological equivalents (Gy[RBE]) using the RBE value of 1.1. It is the policy of our institution to give a prescribed dose to the clinical tumor volume of 54 Gy(RBE) in fractions of 1.8 Gy(RBE). A higher dose of up to 60 Gy(RBE) was delivered to a smaller volume at the discretion of the treating physician for high-risk features. All patients were treated with a conformal proton plan using at least 3 fields.
Surgery, Chemotherapy, and Residual Disease
Maximal feasible resection was performed for all patients. Patients were referred for a second surgical resection when necessary. GTR was defined as no evidence of disease on immediate postoperative MRI. Near-total resection (NTR) was defined as <5 mm of enhancing tumor visible on immediate postoperative MRI. STR was defined as ≥5 mm of enhancing tumor. This designation was given after retrospective review of patient films by a single radiologist (P.C.). The most common indications for chemotherapy were residual disease after resection and patient age under 3 years.
Clinical Data
Variables from demographic, tumor, and treatment characteristics included the patient's gender, ethnicity, date of birth, date of pathological diagnosis, histological grade (based on review by Massachusetts General Hospital pathologists), receipt of chemotherapy before RT, dates of surgical procedures, extent of surgical resection, RT dose, field arrangement, dates of start and completion of RT, location of relapse if any, date of relapse, treatment of relapse, and date of last follow-up.
Endocrine and Auditory Follow-up
A subset of patients (n = 24) underwent endocrinology evaluation in follow-up. Growth hormone deficiency (GHD) was defined as below-normal levels of insulin-like growth factor 1 (IGF-1) and/or IGF-binding protein 3 (IGF BP-3), in addition to clinical diagnosis by a pediatric endocrinologist. We used normal ranges by gender and age, but not pubertal status, from assays by the Esoterix facility.18 Most recent height was obtained from the last available clinic note. Baseline height was defined as the measurement recorded within 50 days of the initiation of RT. Height percentiles were calculated using the 2000 Centers for Disease Control and Prevention age-appropriate growth charts. Hypothyroidism was defined as abnormal values of thyroid stimulating hormone (TSH) and/or free thyroxine (T4). Auditory follow-up time was defined as the time from the start of RT to the most recent evaluation by an audiologist.
Neurocognitive Assessment
All assessments were conducted by one neuropsychologist at our institution (M.P.). Testing was administered during RT, which served as the pre-PRT baseline, and after the completion of RT. Intelligence was assessed using one of the following age-appropriate, standardized measures: Bayley Scales of Infant Development–Second Edition Mental Scale, the Wechsler Preschool and Primary Scale of Intelligence–Third Edition, the Wechsler Intelligence Scale for Children–Fourth Edition, and the Wechsler Adult Intelligence Scale–Third Edition. The primary outcomes for these standardized measures are either an age-based Mental Development Index (MDI; Bayley-II) or an intelligence quotient (IQ) score (all mean = 100; SD = 15). Adaptive skills and functional independence were assessed using the Scales of Independent Behavior–Revised (SIB-R) reference (mean = 100; SD = 15), a standardized written questionnaire completed by the parents.
Statistical Analysis
Progression-free survival (PFS) was measured from the start of RT to the date of the first local, distant, or synchronous failure. Overall survival (OS) was measured from the start of RT to the date of death from disease. PFS and OS were censored for patients who had not failed or died at the date of their last follow-up, defined as the date of the most recent clinic note and/or MRI. PFS, OS, and disease-free survival were estimated by the Kaplan–Meier method, using progression, death, and local and distant recurrence as the respective failure endpoints. The log-rank test was used for univariate analysis of PFS and OS. The proportional hazards model was used in multivariate analysis to assess the independent effects of prognostic factors while controlling for confounding variables. Change in total IQ and SIB-R from baseline to follow-up was assessed for significance using a paired t-test. The change in IQ and SIB-R was compared between age groups (≤3 vs >3 y) using a repeated-measures ANOVA. We used SAS v9.2 for all data analyses except exact log rank P-values in the OS analysis, which were computed by StatXact 6.0 (Cytel Software). All P-values were based on a 2-sided hypothesis.
Results
Patient and Tumor Characteristics
Table 1 summarizes the characteristics of all the patients included in this study. Chemotherapy before radiotherapy was administered to 21 (30%) patients, 15 of whom had residual disease after resection, and 13 of whom were under 3 years of age at the time of diagnosis. The most common regimen was vincristine, carboplatin, cyclophosphamide, and etoposide. Five patients were managed with observation after a GTR (n = 3), chemotherapy alone (n = 1), and resection followed by chemotherapy (n = 1). After suffering local recurrence, all of these patients received PRT as their first radiation treatment.
Table 1.
Patient characteristics
| Patients Treated With Protons | n = 70 |
|---|---|
| Age at diagnosis | |
| Median (range) | 38 mo (3 mo– 20 y) |
| <3 y | 34 (49%) |
| Gender | |
| Female | 37 (53%) |
| Male | 33 (47%) |
| Ethnicity | |
| White | 60 (86%) |
| Hispanic | 4 (6%) |
| Black | 1 (1%) |
| Other | 5 (7%) |
| Home region | |
| New England (MA, ME, NH, CT, RI) | 27 (39%) |
| Other | 43 (61%) |
| Pre-RT chemotherapy | 21 (30%) |
| Duration of RT | |
| Median (range) | 43 days (34 d–54 d) |
| Time to RT from most recent surgery | |
| Median (range) | 49 days (30 d–9 mo) |
| Dose | |
| Median (range) | 55.8 Gy (50.4–60.0 Gy) |
| >54 Gy | 40 (57%) |
| Tumor grade | |
| Differentiated (classic) | 37 (53%) |
| Anaplastic | 33 (47%) |
| Tumor location | |
| Infratentorial | 51 (73%) |
| Supratentorial | 19 (27%) |
| Extent of resection | |
| GTR | 46 (66%) |
| STR | 23 (33%) |
| NTR | 1 (1%) |
| Number of surgeries | |
| 1 | 54 (77%) |
| 2 | 14 (20%) |
| 3 | 2 (3%) |
| Hydrocephalus | 38 (54%) |
| Shunt | 29 (76%) patients with hydrocephalus |
| Follow-up of 63 patients still alive | |
| Median (range) | 46 months (12 mo–11.7 y) |
Treatment Outcomes
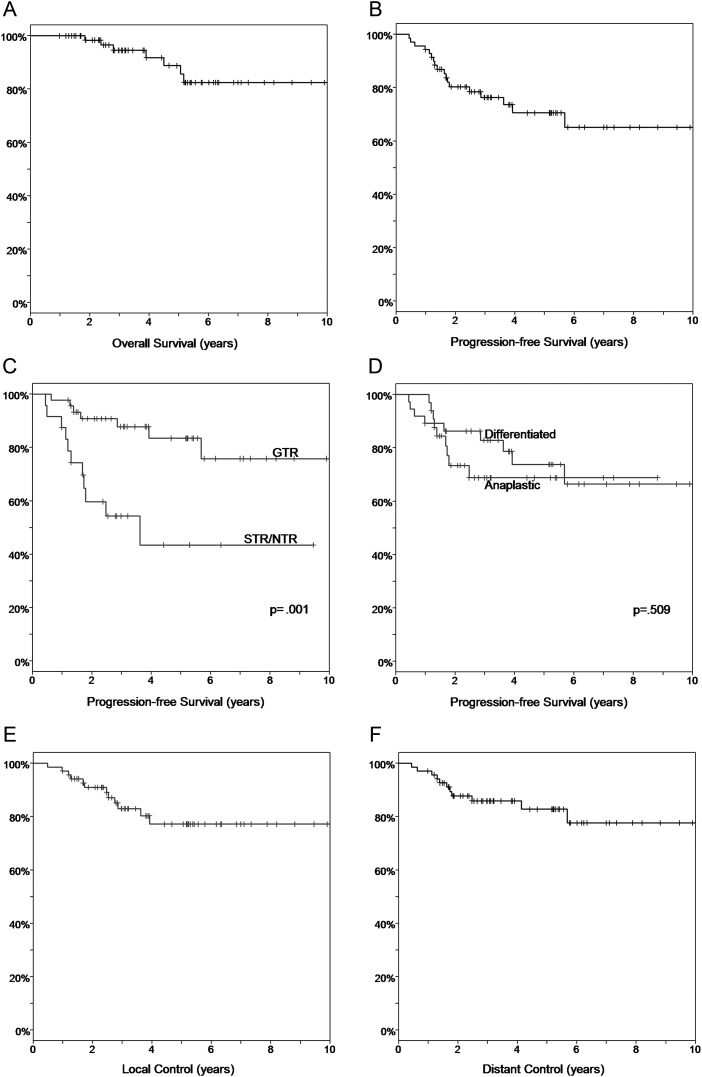
Sixty-three patients were alive and were followed for a median of 46 months (range, 1–11.7 y) after the initiation of RT. A Kaplan–Meier estimate of PFS was 76% at 3 years (Fig. 2B). Univariate analysis (Table 2) found a statistically significant difference in 3-year PFS by extent of resection (88% for GTR vs 54% for STR/NTR, P = .001; Fig. 2C) and tumor location (68% for infratentorial vs 100% for supratentorial, P = .019). Worse PFS was observed for anaplastic tumors (83% for differentiated vs 69% for anaplastic, P = .509; Fig. 2D), but the difference was not statistically significant. Other variables, including gender, age at diagnosis, ethnicity, and the use of chemotherapy, were not associated with worse PFS.
Fig. 2.
(A) PFS for all patients in the cohort. (B) OS for all patients in the cohort. (C) PFS by extent of resection (GTR vs STR/NTR). (D) PFS by histology (differentiated vs anaplastic). (E) Local control for all patients in the cohort. (F) Distant control for all patients in the cohort. Abbreviations: GTR, gross-total resection; NTR, near-total resection; OS, overall survival; PFS, progression-free survival; STR, sub-total resection.
Table 2.
Univariate analysis of survival outcomes
| Variable | PFS |
OS |
||
|---|---|---|---|---|
| 3 y (±95% CI) | P | 3 y (±95% CI) | P | |
| All patients | 76% | 95% | ||
| Age | .431 | .155 | ||
| <3 y | 69% | 92% | ||
| ≥3 y | 83% | 96% | ||
| Gender | .61 | .388 | ||
| Female | 77% | 94% | ||
| Male | 75% | 96% | ||
| Ethnicity | .576 | .697 | ||
| White | 78% | 94% | ||
| Non-White | 70% | 100% | ||
| Tumor grade | .509 | .336 | ||
| Differentiated | 83% | 93% | ||
| Anaplastic | 69% | 96% | ||
| Tumor location | .019 | .106 | ||
| Infratentorial | 68% | 93% | ||
| Supratentorial | 100% | 100% | ||
| Extent of resection | .001 | .001 | ||
| GTR | 88% | 97% | ||
| STR/NTR | 54% | 90% | ||
| Pre-RT Chemo | .594 | .464 | ||
| No | 81% | 95% | ||
| Yes | 64% | 93% | ||
Bolded type-face indicates statistical significant (P < .05).
The OS at 3 years was 95% (Fig. 2A). Seven patients died of disease at a median time of 46.8 months (range, 22.1–61.9 mo). In univariate analysis, extent of resection significantly affected survival (97% for GTR vs 90% for STR/NTR, P = .001). Tumor location showed a trend approaching significance (93% for infratentorial vs 100% for supratentorial, P = .106). Other variables, including gender, age at diagnosis, ethnicity, use of chemotherapy, and the time to start of RT, did not significantly affect OS.
Patterns of Failure
Eighteen patients experienced disease progression at a median time of 18.0 months (range, 5.3–68.1 mo). Figure 3 presents patterns of failure by tumor grade and location. Seventeen of the 18 relapsing patients had infratentorial primary tumors. Eight relapses were local, 8 were distant (5 occurred in the spine and 3 in the brain), and 2 were synchronous local and distant failures. Local and distant control rates at 3 years were 83% and 86%, respectively. At 5 years, respective local and distal control rates were 77% and 83% (Fig. 2E and F).
Fig. 3.
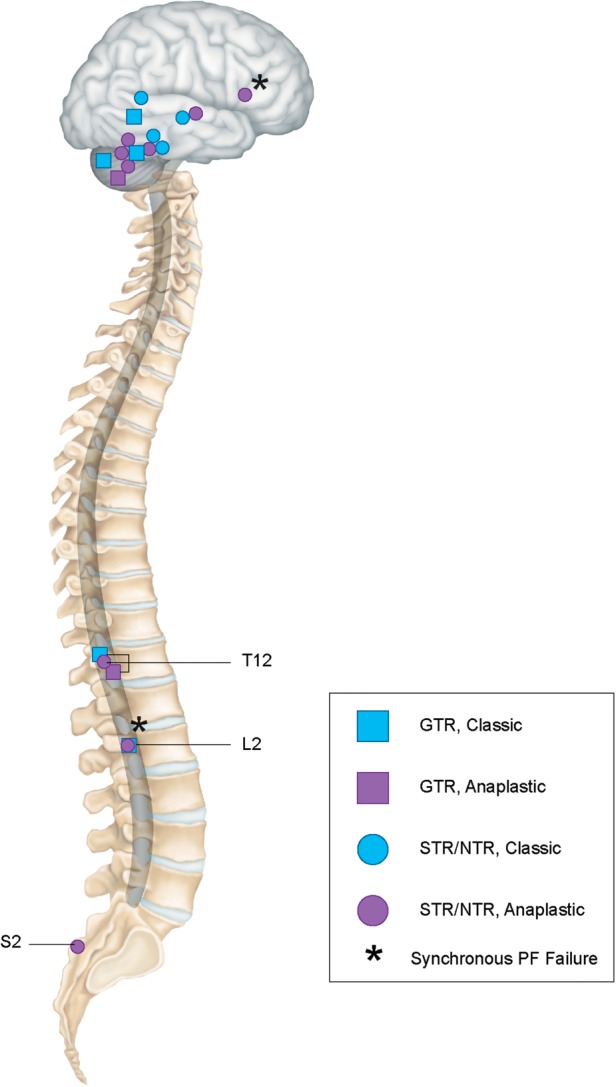
Patterns of failure: blue, gross-total resection (GTR); purple, sub-total resection/near-total resection (STR/NTR); square shape, classic histology; circle shape, anaplastic histology.
Endocrine and Auditory Outcomes
Of 32 patients (median age at diagnosis, 34 mo; range, 4–250) with median 42 months of thyroid hormone follow-up, only 1 exhibited laboratory evidence of central hypothyroidism. Of 25 patients (median age, 33 months; range, 4–250) with median 42 months of growth hormone follow-up, 2 were diagnosed with GHD and are being treated with replacement therapy. Nine additional patients demonstrated deficient levels of IGF-1 but have not received a diagnosis of GHD and are not on replacement therapy. Two of these patients had baseline IGF-1 deficiency. Two additional patients had normal values of IGF BP-3 obtained in follow-up. The growth hormone status of the remaining 5 patients was equivocal (see Appendix).
Fifty-seven patients (median age at diagnosis, 34 months; range, 4–210) had recorded heights available at a median of 41 months (range, 9 mo–9.9 y) from the start of radiotherapy. Baseline median height was in the 54th percentile (range, 0.2–99.9). Follow-up median height was in the 36th percentile (range, 0.1–99.9), and follow-up median z-score was −0.312. The change in height was highly variable among the patients, ranging from a loss of 94 percentiles to a gain of 74 percentiles. On average, the change in height was a median loss of 2.6 percentiles (P = .142). Among 68 patients, median average doses to the hypothalamus and pituitary were 2.25 Gy(RBE) (range, 0–52.2) and 0.4 Gy(RBE) (range 0–52.6), respectively.
Twenty-three patients (median age at diagnosis, 34 months; range, 6–250) underwent audiology evaluation at a median of 27 months (range, 4 mo–9.4 y) from the start of RT. Two patients with infratentorial tumors developed hearing loss attributable to RT. Both of these patients received higher doses of radiation to their cochlea than the average median dose because of tumor extension into the foramen of Luschka. Among 68 patients, the average median dose to the right cochlea was 7.1 Gy(RBE) (range, 0–54.5), and the average median dose to the left cochlea was 6.95 Gy(RBE) (range 0–51.9).
Neurocognitive Assessment
A subset of patients (n = 14) had neurocognitive testing before and after RT with a mean time interval of 2.05 years (range, 1.0–4.5). A larger subset, comprising nearly half of the cohort (n = 28), underwent assessment of adaptive skills/functional independence (SIB-R) before and after RT with a mean time interval of 2.21 years (range, 1.0–5.9). The average total MDI/IQ across all measures was 108.5 at baseline and 111.3 at follow-up (P = .475). The mean SIB-R standard score was 100.1 at baseline and 100.8 at follow-up (P = .809). Among patients aged 3 years and younger (n = 6), the mean MDI/IQ was 104.3 at baseline and 114.0 at follow-up (P = .145). In the same age group (n = 12), SIB-R was 93.3 at baseline and 96.5 at follow-up (P = .404). Among patients above the age of 3 years (n = 8), the mean IQ was 111.6 at baseline and 109.3 at follow-up (P = .618). In the same age group (n = 16), SIB-R was 105.2 at baseline and 104 at follow-up (P = .774). Comparison of change in MDI/IQ between patients aged 3 years and under and patients over the age of 3 years was not statistically significant (9.7 vs −2.4; P = .118). Similarly, comparison of change in SIB-R scores between these age groups was not statistically significant (3.2 vs −1.2; P = .449).
Other Toxicity
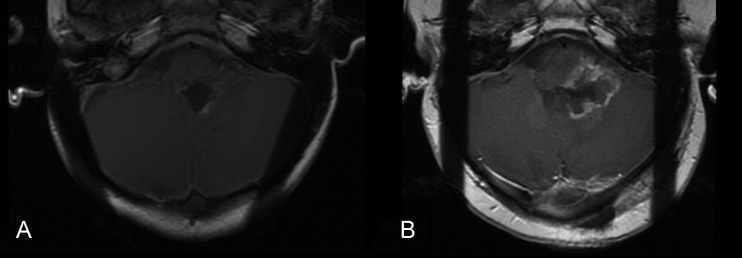
We identified no cases of secondary malignancies. There were 2 cases of cervical subluxation. Both occurred in patients who required laminectomy for cervical extension of infratentorial tumor. Postradiotherapy development of cavernomas was noted in 2 patients. One patient with residual disease adjacent to the brainstem developed necrosis of this tumor with symptoms of brainstem compression. Biopsy of the mass was performed and it was confirmed as a viable tumor with necrosis (Fig. 4). The patient later went on to have a resection of this tumor with pathology showing anaplastic ependymoma and necrosis and later developed metastatic disease. There were no cases of brainstem necrosis.
Fig. 4.
(A) Pretreatment and (B) posttreatment images for a child with residual tumor in the left foramen of Luschka showing tumor recurrence with necrosis adjacent to the brainstem.
Discussion
Our results suggest effective disease control in the treatment of ependymoma by postoperative PRT. Recent estimates of 5-year PFS and OS from other retrospective single-institution photon series have ranged from 41%–58% and 54%–71%, respectively.3,5,8,11,19 Merchant et al,10 in their landmark report on a prospective cohort of 153 patients with ependymoma, found a 5-year PFS of 74% and a 5-year OS of 85%. Our outcomes, after shorter follow-up, are comparable to this report, particularly considering that we had a lower rate of GTRs (66% vs 81.7% in the Merchant series). In addition, 5 patients in our series were treated with their initial course of RT following a relapse. We report few overall toxicities and encouraging cognitive, endocrine, and hearing outcomes. We had no cases of brainstem necrosis.
Our analysis finds prognostic value to the extent of resection, joining many previous studies that have emphasized the importance of aggressive removal of the primary tumor.2,3,6,8,10,11,15 We report a rate of GTR similar to other series, but not as high as that of Merchant et al, in part because of differences in the rate of second surgery. In the 2009 series, 66 patients (43.1%) underwent a second surgery, compared with 16 (23%) in our series. The decision to refer for additional surgery remains challenging and is often limited by anatomical constraints and the level of morbidity considered acceptable by parents and physicians. Morris et al20 recently established that >1 surgical procedure is safe for most patients.20 We advocate second-look surgery.
The role of other prognostic factors is less clear. Anaplastic histology has been shown to worsen OS and PFS in some series, including the large prospective cohort studied by Merchant et al.2,6–8,10,11 Other series, similar to our report, found worse outcomes that did not reach statistical significance.5,21 This variation in findings may result from lack of statistical power and variation in the pathological interpretation of anaplasia. Emerging genetic profiles may better characterize prognostic subgroups.2,22
We did not find worse survival outcomes among patients under the age of 3 years, a trend that was reported by older studies, but not by Merchant's study.3,5,8,19 This finding was likely related to the formerly common practice of delaying or omitting RT for this population. The usefulness of RT in children under the age of 3 years, and the limited efficacy of chemotherapy as a tool to delay RT, is well established, and postoperative conformal RT should continue as the standard of care for all ages.6,10,15,16
Similar to Merchant et al, we note that the incidence of distant failures appears to be increasing relative to the incidence of local failure, likely because of improvements in local disease control. However, while distant relapses appear more frequent, the absolute incidence does not appear to have changed. Modern series describe a cumulative incidence between 10% and 15% over 3–5 years, which is similar to historical rates of distant failure reported in older single-institution series, many of which describe patients treated with craniospinal irradiation.7,11,12
This is the first report of long-term endocrine and audiology results after focal PRT. Few of our patients developed frank GHD or central hypothyroidism, both of which are common complications of focal photon radiotherapy.23–25 Merchant et al, in a prospective study of pediatric patients receiving focal photon RT for localized brain tumors, found a correlation of dose to the hypothalamus and GHD. A mean dose below 16.1 Gy limited the risk to 50%, and a dose under 5 Gy limited the risk to below 20%.26 We delivered an average median dose of 2.25 Gy(RBE) to the hypothalamus. Although follow-up was short and not available for all patients, GHD and/or height impairment was seen in few patients. Two cases of hearing loss attributed to RT were noted. Even with PRT, tumor extension into the foramina of Luschka can require high doses to the cochlea; however, in our cohort, the average median doses delivered to the cochleae were very low.
We also provide the first report on the impact of focal PRT for ependymoma on intelligence and adaptive functioning. Relative cognitive stability after conformal photon therapy for patients with ependymoma has already been established.27–29 In addition, theoretical quantitative modeling predicted a benefit for protons from the decreased volume of temporal lobes and cerebrum receiving intermediate and high doses.30 Among a subset of patients in our cohort, we found stable overall intelligence and adaptive skills/functional independence after the completion of RT. Similar to other reports, we observed that patients under the age of 3 years had a lower baseline IQ but improved substantially over time.29 These results will require validation in larger cohorts and longer follow-up.
The major limitation of our study was the retrospective nature of the data collection. In addition, our cognitive, endocrine, and auditory data were obtained at varying schedules from subsets of patients rather than the whole cohort. The effect of the selection bias introduced by this method is unknown. Finally, growth hormone status was not evaluated using provocative testing, which made it difficult to assess its true incidence.
In summary, we report encouraging survival, cognitive, auditory, and endocrine outcomes in a large cohort of ependymoma patients treated with PRT. We demonstrate that disease control and a low rate of complications can be jointly achieved with PRT. The young age of patients at diagnosis and the close proximity of ependymoma tumors to critical brain structures make protons an attractive form of radiation treatment for this pediatric malignancy.
Supplementary Material
Funding
R.V.S. was supported as a clinical research fellow by a grant from the Doris Duke Charitable Foundation to Harvard Medical School. B.Y.Y. and a portion of this research was supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
Conflict of interest statement. N.T. was on the medical advisory board of ProCure until 2008 and has stock options in ProCure that are currently without value. N.T.'s spouse continues to serve on the medical advisory board of ProCure. Actual or potential conflicts of interest do not exist for any other author.
Supplementary Material
Appendix
The following table summarizes the status of 9 patients with equivocal growth hormone laboratory testing who had not received a diagnosis of GDH and are not on replacement therapy.
.
| Patient | Age at Follow-up | Follow-up IGF-1 (ng/mL) | Follow-up IGF BP-3 (ng/mL) | Height z-Score in Follow-up | Age at Baseline | Baseline IGF-1 (ng/mL) | Baseline IGF BP-3 (ng/mL) | Height z-Score at Baseline |
|---|---|---|---|---|---|---|---|---|
| 1 | 19.7 | Low: 219 | – | 0.136 | 13.4 | – | – | 0.198 |
| 2 | 11.6 | Low: 130 | – | −0.625 | – | – | – | – |
| 3 | 5.7 | Low: 65 | Normal: 3.8 | 0.226 | 5.3 | Normal: 141 | Normal: 4.1 | 1.176 |
| 4 | 5.8 | Low: 39 | – | −1.53 | 1.3 | – | – | 0.241 |
| 5 | 5.3 | Low: 67 | Normal: 3.7 | −0.917 | 4.4 | – | – | 1.697 |
| 6 | 5.4 | Low: 56 | – | −1.458 | 1.7 | – | – | 0.094 |
| 7 | 9.1 | Low: 114 | Normal: 3.8 | −1.404 | 8.0 | Low: 90 | Normal: 4.1 | −0.839 |
| 8 | 5.3 | Low: 52.9 | – | 2.8 | Normal: 183 | Normal: 3.3 | 0.309 | |
| 9 | 2.6 | Low: 48 | Normal: 2.6 | −1.02 | 1.29 | Low: <25 | Normal: 1.6 | −1.201 |
References
- 1.CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 ( Hsdale, IL: Author; 2012. Central Brain Tumor Registry of the United States. March 23, 2012 Revision). [Google Scholar]
- 2.Phi JH, Wang KC, Park SH, et al. Pediatric infratentorial ependymoma: prognostic significance of anaplastic histology. J Neurooncol. 2012;106(3):619–626. doi: 10.1007/s11060-011-0699-x. [DOI] [PubMed] [Google Scholar]
- 3.Perilongo G, Massimino M, Sotti G, et al. Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neuro-oncology Group. Med Pediatr Oncol. 1997;29(2):79–85. doi: 10.1002/(sici)1096-911x(199708)29:2<79::aid-mpo3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE. Three-dimensional conformal radiation therapy for ependymoma. Childs Nerv Syst. 2009;25(10):1261–1268. doi: 10.1007/s00381-009-0892-9. [DOI] [PubMed] [Google Scholar]
- 5.Mansur DB, Perry A, Rajaram V, et al. Postoperative radiation therapy for grade II and III intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2005;61(2):387–391. doi: 10.1016/j.ijrobp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Koshy M, Rich S, Merchant TE, Mahmood U, Regine WF, Kwok Y. Post-operative radiation improves survival in children younger than 3 years with intracranial ependymoma. J Neurooncol. 2011;105(3):583–590. doi: 10.1007/s11060-011-0624-3. [DOI] [PubMed] [Google Scholar]
- 7.Schild SE, Nisi K, Scheithauer BW, et al. The results of radiotherapy for ependymomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 1998;42(5):953–958. doi: 10.1016/s0360-3016(98)00350-2. [DOI] [PubMed] [Google Scholar]
- 8.Shu HK, Sall WF, Maity A, et al. Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer. 2007;110(2):432–441. doi: 10.1002/cncr.22782. [DOI] [PubMed] [Google Scholar]
- 9.Timmermann B, Kortmann RD, Kuhl J, et al. Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: results of the German prospective trials HIT 88/89 and HIT 91. Int J Radiat Oncol Biol Phys. 2000;46(2):287–295. doi: 10.1016/s0360-3016(99)00414-9. [DOI] [PubMed] [Google Scholar]
- 10.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oya N, Shibamoto Y, Nagata Y, Negoro Y, Hiraoka M. Postoperative radiotherapy for intracranial ependymoma: analysis of prognostic factors and patterns of failure. J Neurooncol. 2002;56(1):87–94. doi: 10.1023/a:1014442106111. [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Haida T, Wang MH, Finlay JL, Leibel SA. Anaplastic ependymoma: treatment of pediatric patients with or without craniospinal radiation therapy. J Neurosurg. 1997;86(6):943–949. doi: 10.3171/jns.1997.86.6.0943. [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75(3):287–299. doi: 10.1007/s11060-005-6753-9. [DOI] [PubMed] [Google Scholar]
- 14.Needle MN, Molloy PT, Geyer JR, et al. Phase II study of daily oral etoposide in children with recurrent brain tumors and other solid tumors. Med Pediatr Oncol. 1997;29(1):28–32. doi: 10.1002/(sici)1096-911x(199707)29:1<28::aid-mpo5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Timmermann B, Kortmann RD, Kuhl J, et al. Role of radiotherapy in anaplastic ependymoma in children under age of 3 years: Results of the prospective German brain tumor trials HIT-SKK 87 and 92. Radiother Oncol. 2005;77(3):278–285. doi: 10.1016/j.radonc.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Zacharoulis S, Levy A, Chi SN, et al. Outcome for young children newly diagnosed with ependymoma, treated with intensive induction chemotherapy followed by myeloablative chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;49(1):34–40. doi: 10.1002/pbc.20935. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–986. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 18.America ELSaLCo. Endocrinology Expected Values & S.I. Unit Conversion Tables. In: Services EL, editor. Calabasas Hills, CA, USA: Esoterix, LabCorp: 2010. [Google Scholar]
- 19.Jaing TH, Wang HS, Tsay PK, et al. Multivariate analysis of clinical prognostic factors in children with intracranial ependymomas. J Neurooncol. 2004;68(3):255–261. doi: 10.1023/b:neon.0000033383.84900.c1. [DOI] [PubMed] [Google Scholar]
- 20.Morris EB, Li C, Khan RB, et al. Evolution of neurological impairment in pediatric infratentorial ependymoma patients. J Neurooncol. 2009;94(3):391–398. doi: 10.1007/s11060-009-9866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldwein JW, Leahy JM, Packer RJ, et al. Intracranial ependymomas in children. Int J Radiat Oncol Biol Phys. 1990;19(6):1497–1502. doi: 10.1016/0360-3016(90)90362-n. [DOI] [PubMed] [Google Scholar]
- 22.Mack SC, Taylor MD. The genetic and epigenetic basis of ependymoma. Childs Nerv Syst. 2009;25(10):1195–1201. doi: 10.1007/s00381-009-0928-1. [DOI] [PubMed] [Google Scholar]
- 23.Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys. 1995;31(5):1165–1170. doi: 10.1016/0360-3016(95)00019-U. [DOI] [PubMed] [Google Scholar]
- 24.Merchant TE, Goloubeva O, Pritchard DL, et al. Radiation dose-volume effects on growth hormone secretion. Int J Radiat Oncol Biol Phys. 2002;52(5):1264–1270. doi: 10.1016/s0360-3016(01)02788-2. [DOI] [PubMed] [Google Scholar]
- 25.Rappaport R, Brauner R. Growth and endocrine disorders secondary to cranial irradiation. Pediatr Res. 1989;25(6):561–567. doi: 10.1203/00006450-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29(36):4776–4780. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pinto M, Conklin HM, Li C, Xiong X, Merchant TE. Investigating verbal and visual auditory learning after conformal radiation therapy for childhood ependymoma. Int J Radiat Oncol Biol Phys. 2010;77(4):1002–1008. doi: 10.1016/j.ijrobp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22(15):3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 30.Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1546–1554. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.