Abstract
Research addressing the health impacts of polychlorinated biphenyls (PCBs) has primarily focused on the effects of coplanar, or dioxin-like (DL), congeners, which is especially true for research assessing impacts in fish species. Ortho substituted non-coplanar, termed non-dioxin-like (NDL), PCBs have received less attention. In mammals, NDL PCBs enhance the activity of ryanodine receptors (RyR), calcium release channels necessary for engaging excitation-contraction (EC) coupling in striated muscle. We utilized in vitro receptor binding analysis to determine whether NDL PCB congeners detected in aquatic environments alter the activity of RyR isoform 1 (RyR1) found in the skeletal muscle of rainbow trout. Congeners 52, 95, 136, and 149 were the most efficacious leading to an increase in receptor activity that was approximately 250% greater than that found under solvent control conditions. Other environmentally relevant congeners, namely PCB 153, 151 and 101, which all contain two or more chlorines in the ortho-position, enhanced receptor activity by greater than 160% of baseline. The mono-ortho congeners or the non-ortho PCB 77 had negligible impact on the RyR1. When combined, in binary or environmentally relevant mixtures, congeners shown to enhance receptor activity appeared to display additivity and when the active PCB 95 was present with the non-active congener PCB 77 the impact on receptor activity was reduced from 250% to 230%. The important role of the RyR and the demonstrated additive nature of NDL congeners towards altering channel function calls for further investigation into the ecological implications of altered RyR function in fish with high PCB burdens.
Keywords: Non-Dioxin-Like Polychlorinated Biphenyls, Muscle Contraction, Calcium Signaling, Ryanodine Receptor, Halogenated Compound Mixtures, Relative Potency Scheme
1.0 Introduction
Polychlorinated biphenyls (PCBs) are a class of halogenated aromatic hydrocarbons representing 209 different congeners with varying placement and degrees of chlorine substitution. They were used as mixtures throughout the early part of the 20th century in a wide array of industries where their high stability and lipophilic nature made them ideal flame retardants, plasticizers, lubricants, paint additives and sealants. These characteristics, combined with their heavy usage and improper disposal, led to their accumulation in the environment and subsequently human and wildlife species (Beyer and Biziuk, 2009). Due to concerns over the health of humans and the environment, PCBs were banned in the 1970’s; however, more than forty years later they are still commonly detected in all forms of environmental media gaining global recognition as a contaminant of concern (Van den Berg et al., 1998; Faroon et al., 2003; Van den Berg et al., 2006).
In aquatic ecosystems, specifically, studies of US waterways show that PCB’s are amongst the most commonly detected contaminant classes (Stahl et al., 2009) occurring in the sediment (Hwang et al., 2006), as well as, organisms from plankton up to top predators (Howell et al., 2008; Walters et al., 2011). In fish, the most recent and comprehensive study conducted in lakes across the US, detected PCBs in 100% of the samples collected with concentrations reaching 704 and 1,200 μg kg−1 in predatory and bottom dwelling fish respectively. With mercury in first place, the PCB load represented the second leading cause for fish consumption advisories, and over 16% of fish in US lakes were predicted/estimated to contain PCB levels above what is considered safe to consume (Stahl et al., 2009; USEPA, 2009). Additionally, PCBs are well known to biomagnify across trophic levels showing high concentrations in top predatory fish including wild and farmed salmon species (Hites et al., 2004a), fish from the Great Lakes of North America (Dellinger et al., 2013), as well as, species caught in the Pacific and Atlantic Oceans (Domingo and Bocio, 2007; Hayward et al., 2007; Tanabe and Ramu, 2012).
Due to their wide spread environmental occurrence the toxic impact of PCBs has received a great deal of attention. Of the 209 different PCBs, which vary by degree and placement of the chlorine substitution, toxicity studies have primarily focused on the mode of action of coplanar congeners, especially in non-mammalian species, namely fish. The coplanar PCBs lack chlorine substitution in the ortho position and are often termed dioxin-like (DL) due to their similar action on the arylhydrocarbon receptor (AhR) ascribed to 2,3,7,8-tetrachlorodibenzodioxin (TCDD). When dioxins or DL compounds bind the AhR this leads to the nuclear localization of the AhR–ligand complex, where it interacts with the AhR nuclear translocator (ARNT). The AhR-ARNT complex binds to specific DNA response elements leading to the transcription of AhR responsive genes including the well-studied cytochrome P4501A (CYP1A) and other xenobiotic metabolism enzymes (Denison and Nagy, 2003; Köhle and Bock, 2007). This pathway is thought to be involved in mediating, at least in part, the organismal toxicity observed in fish exposed to dioxin like compounds, such as coplanar PCBs. Dioxin like toxicity endpoints in fish, have recently been reviewed (King-Heiden et al., 2012) and include altered growth and food intake, an array of developmental malformations such as cardiac edema and cranial malformations, skin lesions and impaired reproductive success.
Non-coplanar PCBs, those with two or more chlorines in the ortho position, are considered non-dioxin like (NDL) because they have little to no activity at the AhR in mammalian and non-mammalian species (Giesy and Kannan, 1998). These NDL congeners have received far less attention in regulatory considerations. They are linked to a number of toxic endpoints that are believed to be independent of the AhR pathway including altered thyroid signaling (e.g. Khan and Hansen, 2003; Brar et al., 2010; Giera et al., 2011) and developmental neurotoxicity (Pessah et al., 2010; Wayman et al., 2012a; Wayman et al., 2012b). Several indicators regarding the neurotoxic potential of PCBs include effects on the central nervous system that are correlated with altered reflexes, cognitive and motor functioning, and hearing impairments in mammalian species (Pessah et al., 2010). Here, the neurotoxic potential of PCBs is believed to be independent of the well-known effects on the AhR due to finding that show that non-coplanar, rather than DL coplanar, PCBs are enriched in the brains of individuals with impaired neurodevelopment and that studies using pure non-coplanar PCBs demonstrate developmental neurotoxicity (Pessah et al., 2010).
The direct mechanism by which NDL PCBs lead to developmental neurotoxicity remains elusive. A suggested mode of toxic action includes their ability to alter Ca2+ dynamics and Ca2+ dependent signaling through the activation of the ryanodine receptor (RyR) (Pessah et al., 2010) and potentiation of GABA-induced currents mediated by α1β2γ2L expressed in Xenopus oocytes (Fernandes et al., 2010). The RyRs are integral membrane proteins anchored within the sarcoplasmic reticulum (SR) and endoplasmic reticulum (ER) that have a well-known role in physiological and pathophysiological processes of the peripheral and central nervous systems. They are; however, most known for their role in excitation-contraction (EC) coupling in both cardiac and skeletal muscle. Here, muscle cell depolarization provides the signal to activate voltage gated L-type Ca+ channels within the transverse tubule membrane, which through direct mechanical link and/or Ca2+entry, signals the opening of RyR channels that release Ca2+ stored within the SR/ER. Ca2+ release mediated by RyR channel activation thereby amplifies Ca2+ signals necessary for fundamental biological processes including muscle contraction and neuronal growth (Pessah et al., 2010; Berridge, 2012). Therefore, the interaction of NDL PCBs with RyRs can contribute to acute impairments in muscle function and long-term declines in muscle health (Lanner, 2012), but the implications of RyR dysfunction on muscle health in fish require further investigation.
The form and function of the RyR is highly conserved across vertebrate taxa (Franck et al., 1998; Darbandi and Franck, 2009) yet the action of NDL PCBs on the channel’s activity has not been addressed outside of mammalian species. Specifically, teleosts share approximately 70% sequence homology with mammalian RyR isoforms (Franck et al., 1998; Darbandi and Franck, 2009) and it has been demonstrated that altering the function of RyRs, or related proteins in fish, leads to altered muscle function and swimming performance (Hirata et al., 2007; Seebacher et al., 2012). Together these findings suggest that NDL PCBs may activate the RyR in fish presenting a threat to the developmental and/or physiological processes in teleost species. Furthermore, studies measuring complete congener profiles in fish show that NDL PCBs far exceed concentrations of those DL congeners which are potent at the AhR (Partial congener list see Table 1; USEPA 2009) supporting the need to evaluate the risk posed by the NDL congeners in aquatic ecosystems.
Table 1.
Estimated detection levels of individual PCB congeners in predatory fish from US lakes.a
| PCB Congener | Chlorine Substitution | Sample Detection (%, N=486) | Maximum Concentration (μg kg−1) | Maximum Concentration (μM) |
|---|---|---|---|---|
| Sum of All Congeners | 100 | 704.92 | 2.16 | |
|
| ||||
| Ortho- Substituted | ||||
| 20+28 | 2,3,3′ or 2,4,4′ | 88.07 | 7.91 | 0.03 |
| 44b | 2,2′3,5′ | 93.00 | 10.70 | 0.04 |
| 52 | 2,2′,5,5′ | 96.5 | 12.50 | 0.04 |
| 66 | 2,2′,4,4′ | 94.65 | 8.00 | 0.03 |
| 95b | 2,2′3,5′,6 | 98.56 | 17.20 | 0.05 |
| 99b | 2,2′4,4′,5 | 99.59 | 24.30 | 0.07 |
| 101b | 2,2′,4,5,5′ | 99.59 | 36.50 | 0.11 |
| 110b | 2,3,3′,4′,6 | 99.79 | 31.80 | 0.10 |
| 132 | 2,2′,3,3′,4,6′ | 99.79 | 9.02 | 0.02 |
| 136 | 2,2′,3,3′,6,6′ | 89.51 | 2.67 | 0.01 |
| 149b | 2,2′,3,4′,5′,6 | 99.38 | 25.50 | 0.07 |
| 151b | 2,2′,3,5,5′,6 | 97.12 | 13.00 | 0.04 |
| 153b | 2,2′,4,4′,5,5′ | 99.79 | 101.00 | 0.28 |
| 170 | 2,2′,3,3′,4,4′,5 | 97.33 | 18.20 | 0.05 |
| 180b | 2,2′,3,4,4′,5,5′ | 99.38 | 55.80 | 0.14 |
| 183b | 2,2′,3,4,4′,5′,6 | 98.35 | 14.80 | 0.04 |
| 187 | 2,2′,3,4′,5,5′,6 | 99.38 | 39.70 | 0.10 |
| 194 | 2,2′,3,3′4,4′,5,5′ | 99.18 | 9.78 | 0.02 |
| Non-Ortho Substituted | ||||
| 77c | 3,3′4,4′ | 37.24 | 0.28 | ≪0.01 |
| 126c | 3,3′4,4′,5 | 10.49 | 0.30 | ≪0.01 |
Tissue levels based on the maximum detected concentration of individual congeners as summarized from USEPA 2009 (EPA-823-R-09-006)
Congener co-eluted with other PCBs;
Alter AhR related pathways; Van De Berg et al. 1998 and 2006
To confirm a common mode of toxic action across vertebrate taxa we assessed whether NDL PCB congeners alter the activity of the RyR found in skeletal muscle, namely RyR isoform 1 (RyR1), of the rainbow trout (Oncorhynchus mykiss). We assessed the individual toxicity of 20 different PCB congeners (Table 1) with varying degrees of chlorine substitution, as well as, the impact of binary and complex environmentally relevant PCB mixtures. Specific congeners of interest were chosen based on several criteria. First, in the trout, we chose to assess the impact of those congeners previously shown to enhance the RyR1 in mammals (Pessah et al., 2006). Second, to better represent realistic exposure scenarios, congeners that are found at high concentrations in fish tissue in US lakes (Stahl et al., 2009; USEPA, 2009) were evaluated. The congeners assessed included the non-coplanar PCB 77 as a reference for DL PCB effects. We did not assess the non-ortho PCB 126 because it has previously been shown to be inactive toward the RyR1 in mammals (Pessah et al., 2006) and its frequency of detection was only about 11% of the predatory fish analyzed (Table 1). Also, due to the fact that PCBs are often present in the environment with other halogenated compounds we began addressing the potential impacts of PCBs in the presence of polybrominated diphenyl ethers (PBDE). We chose to assess the activity of PBDE 49, alone and in the presence of a NDL PCB, because it is a non-coplanar compound commonly detected in fish (Hites et al., 2004b) that is highly efficacious toward the RyR1 in mammals (Kim et al., 2010).
2. Materials and Methods
2.1 Chemicals
The PCB and PBDEs utilized in this study were purchased from AccuStandard (New Haven, CT) as neat preparations and upon receipt solubilized in anhydrous dimethyl-sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). Chemical purity for all PCB congeners purchased was greater than 99.7%. All other chemicals utilized for the creation of buffers were purchased from Sigma-Aldrich unless otherwise stated.
2.2 Crude microsomal protein preparations
Rainbow trout were obtained from Mt. Lassen Trout Farms (Paynes Creek, CA) at approximately 9 months post hatch. Following an overdose of tricaine methanesulfonate the left skeletal muscle fillet of trout were excised, immediately flash frozen in liquid nitrogen and stored at −80ºC until prepared for biochemical assays.
To obtain crude microsomal protein samples enriched in RyR1, approximately 5 g of skeletal muscle tissue, which represented the partial filets combined from 4–5 fish, was minced with scissors in 5 volumes ice cold homogenization buffer consisting of 300 mM sucrose, 20 mM HEPES and the following protease and phosphatase inhibitors: 2μg ml−1 leupeptin, 1mM PMSF, 10mM NaF, 2mM β-Glycerol, 5mM Na4P2O7 and 0.5mM Na3VO4 (pH 7.2). Tissue was further homogenized with a Polytron E2000 with 2 bursts of 20s at half speed, with 2 min on ice between bursts. Homogenates were centrifuged at 8,000 RPM (4ºC for 15min) and the supernatants passed through two layers of cheesecloth. The pellet was subsequently re-homogenized as above in 5 ml homogenization buffer and the centrifugation steps repeated. Resulting supernatants were combined and underwent ultracentrifugation at 32,500 RPM (4ºC for 1h). The supernatant was discarded and the pellet re-suspended in 300 mM sucrose, 20 mM HEPES using a glass Dounce homogenizer. Protein concentrations were determined in triplicate using a BCA Protein Assay (Fisher Scientific, Pittsburgh, PA) and the re-suspension placed in 100 μl aliquots and stored at −80ºC.
2.3 Chemical-enhanced ryanodine receptor activity
The plant alkaloid ryanodine (Ry), for which the RyR is named, binds preferentially to the open channel state of the receptor, where increased Ry binding signifies increased receptor activity (Pessah et al., 1987). We utilized [3H]ryanodine ([3H]Ry; 59.4 Ci mmol−1; Perkin Elmer) to assess the open channel state of the receptor in the presence or absence of PCBs, PCB mixtures, or PCB/PBDE mixtures. Radioligand binding assays were performed following slightly modified protocols as previously described (Franck et al., 1998; Pessah et al., 2006). Here, 40 μg ml−1 of the crude protein homogenate from trout muscle was incubated in the presence of a DMSO solvent control, which never exceeded 1%, or individual chemicals or chemical mixtures. All tests were run in triplicates of 300 μL in a buffer containing 8 nM [3H]Ry, 250 mM KCl, 20 mM HEPES, 15 mM NaCl, and 50 μM CaCl2, pH 7.1. Non-specific binding in the presence of the DMSO control or 10 μM of a given compound was run alongside each binding experiment. Here, crude microsomal preparations were incubated under the same assay conditions as outlined above but these tubes also contained 10 μM unlabeled ryanodine (1250-fold in excess of [3H]Ry) and 200 μM EGTA. Assays were all incubated for 16h at 25ºC and then terminated by rapid filtration through Whatman GF/B filters (retention 1 μm) and washed with 15 ml of ice cold buffer consisting of 140 mM KCl, 10 mM HEPES, and 0.1 mM CaCl2, pH 7.4. Total radioligand bound was determined by liquid scintillation counting (model LS6500; Beckman) and specific binding determined by subtracting non-specific values from total binding for each assay. Non-specific binding never exceeded 20% of the total binding and neither PCBs nor PBDEs altered non-specific binding as compared to the DMSO control.
Concentration-response relationships, as measured by increased specific [3H]Ry bound, for individual chemicals were determined in the presence of 0.05–50 μM PCB or PBDEs. The possible interaction of non-coplanar compounds towards RyR activation was assessed utilizing mixtures of either PCB 95 and PCB 136, which have similar receptor activities (Pessah et al., 2006), or two distinct chemotypes PCB 95 and PBDE 49 known to have variable potencies toward the RyR1 (Kim et al., 2010). Concentration-response relationships for each chemical individually were run alongside binary mixtures, where the total amount of halogenated compound present was created using equal potent concentrations of each chemical in the respective mixture. To assess the impact of a DL PCB on the activity of a NDL PCB, binding experiments were conducted in the presence of 0.05–10 μM PCB 95, alone or in combination with 0.1, 1 or 10 μM PCB 77, a DL PCB that did not enhance RyR activity alone (see results). Environmentally relevant mixture exposures were recreated by combining all 20 PCB congeners assessed in the current study at levels proportional to the estimated maximum concentration found in predatory fish in US lakes (Table 1; without PCB 126 as noted in the introduction). For each congener, tissue levels originally reported in μg kg−1 were converted to μM concentrations based on that congener’s molecular weight. Subsequently 100X total PCB stock solutions were created to contain levels of each congener proportional to that found in fish. The crude protein homogenate were incubated with total PCB concentrations ranging from 0.05–40 μM dissolved in 1% DMSO.
2.4 Statistical analysis
To assess concentration-response variability most chemicals were assessed in repeat radioligand binding assays, as described above, where assays utilized different protein preparations created from the filets of different fish. For some congeners there were slight variations in the maximum response achieved even though the overall responses observed between different protein preparations did not vary significantly (data not shown). For statistical purposes the repeated tests were combined in order to provide a more accurate concentration-response curve for a given chemical or chemical mixture (see Table 2; n=3–12). The concentration-response relationships were all determined utilizing Prism 5.0 (Graphpad Software) where the responses for individual chemicals were fit to a sigmoidal curve, and used to determine the maximum percent response, as compared to the DMSO control, and the effect concentration that would cause half of the maximum response (EC50). For each congener a slope was obtained through linear regression of data fit to a log-probit plot and the maximum binding at saturation (Bmax) was determined utilizing non-linear regression.
Table 2.
Potency and efficacy of PCB congeners towards activation of the ryanodine receptor, isoform 1, in rainbow trout skeletal muscle.
| Congener | Maximum Response (% of Control) | EC50 (μM) | EC50 95%CI (μM) | Log-Probit Slope | Bmax (pmol/mg) | (n) |
|---|---|---|---|---|---|---|
| 20 | 116.40 ± 3.13 | 1.80 | 0.75–4.36 | 2.83 ± 0.10 | 0.34 ± 0.02 | 3 |
| 28 | - | - | - | - | - | 3 |
| 44 | 169.90 ± 3.50 | 4.5 | 3.82–5.29 | 4.20 ± 0.10 | 0.50 ± 0.03 | 3 |
| 52 | 250.10 ± 7.88 | 2.94 | 2.37–3.64 | 2.66 ± 0.09 | 0.77 ± 0.05 | 6 |
| 66 | 130.20 ± 4.34 | 1.16 | 0.60–2.25 | 2.93 ± 0.00 | 0.42 ± 0.02 | 6 |
| 77 | - | - | - | - | - | 6 |
| 95 | 237.40 ± 5.69 | 1.06 | 0.89–1.27 | 2.15 ± 0.05 | 0.95 ± 0.04 | 12 |
| 99 | 136.50 ± 5.66 | 3.59 | 1.88–6.88 | 2.30 ± 0.05 | 0.61 ± 0.03 | 6 |
| 101 | 194.30 ± 7.99 | 1.98 | 1.34–2.94 | 1.83 ± 0.04 | 0.64 ± 0.04 | 6 |
| 110 | 120.00 ± 4.44 | 1.08 | 0.5–2.39 | 2.60 ± 0.01 | 0.48 ± 0.03 | 9 |
| 132 | 121.80 ± 9.49 | 1.32 | 0.28–6.24 | 1.70 ± 0.04a | 0.46 ± 0.02 | 9 |
| 136 | 269.10 ± 7.03 | 1.30 | 1.09–1.56 | 3.16 ± 0.14 | 0.96 ± 0.04 | 6 |
| 149 | 257.90 ± 4.45 | 0.81 | 0.69–0.94 | 3.40 ± 0.08 | 0.76 ± 0.04 | 6 |
| 151 | 177.30 ± 8.38 | 2.76 | 1.73–4.40 | 1.91 ± 0.04 | 0.77 ± 0.04 | 6 |
| 153 | 167.10 ± 14.57 | 5.75 | 2.7–12.25 | 2.25 ± 0.05 | 0.54 ± 0.02 | 6 |
| 170 | - | - | - | - | - | 6 |
| 180 | 131.80 ± 9.87 | 1.53 | 0.39–5.91 | 1.84 ± 0.06 | 0.40 ± 0.03 | 6 |
| 183 | 143.70 ± 9.55 | 1.82 | 0.63–5.28 | 1.58 ± 0.09 | 0.42 ± 0.03 | 6 |
| 187 | 134.60 ± 3.27 | 1.30 | 0.84–1.96 | 3.05 ± 0.07 | 0.45 ± 0.02 | 9 |
| 194 | 130.00 ± 2.99 | 1.27 | 0.80–2.01 | 8.94 ± 0.34 | 0.36 ± 0.02 | 3 |
Notes: Percent responses were determined relative to the [3H]Ry binding in the presence of a DMSO control; binding parameters represent Mean ± SEM; (−) inactive;
Abbreviations: half maximum effective concentration (EC50); 95% confidence interval (95% CI); maximal level of binding at saturation (Bmax).
parameter determined using activating concentrations only
Best fit parameters for binary or environmental mixtures were compared using an extra sum of squares F-test. Additivity between binary mixtures of active congeners or of the environmentally relevant mixture was assessed using the concentration addition (CA) model as previously described (Faust et al., 2000; Jonker et al., 2005; Belden and Lydy, 2006; Boik et al., 2009). The CA model assumes that all compounds in a mixture have a similar mode of toxic action and thus can be summed once corrected for differences in potency. Therefore, the total concentration that should elicit an expected response was calculated using the following equation as previously described:
where ECXmix is the total PCB concentration in the mixture that is expected to cause x effect, piis the proportion of chemical i present in the mixture and ECXi is the concentration of chemical i shown to cause x effect. This equation was calculated from multiple expected effect concentrations for individual congeners and fit to a sigmoidal curve. The expected sigmoidal dose response curve was compared to that calculated from the observed responses normalized to a baseline (control) response of 0 and the maximum achieved response set at 100%. When the expected curve fell out-side of the determined 95% Confidence Interval for the observed response it was determined to deviate from additivity.
The EC50 of an individual congener was deemed inappropriate for the evaluation of individual congener’s potency because it is dependent on the maximum response observed for that congener. For example, the EC50 for PCB 20 and PCB 101 was similar (see Table 2) but the congers led to significantly different maximal responses. Therefore, for determination of relative potency parameters percent responses were first normalized to the presumed overall maximum response (Q) observed for any congener, 253.83% (average PCB 52, 95, 136, and 149; see results), placing all congeners on the same scale from 0 to 100. Therefore, the only congeners to reach the maximum response (Q100) were 52, 95, 136 and 149. Once normalized, the concentration expected to lead to 20% (Q20) of the normalized maximum was calculated and compared to the predicted Q20 for the highly efficacious PCB 95.
3. RESULTS
3.1 Individual PCB congeners enhance RyR1 activity
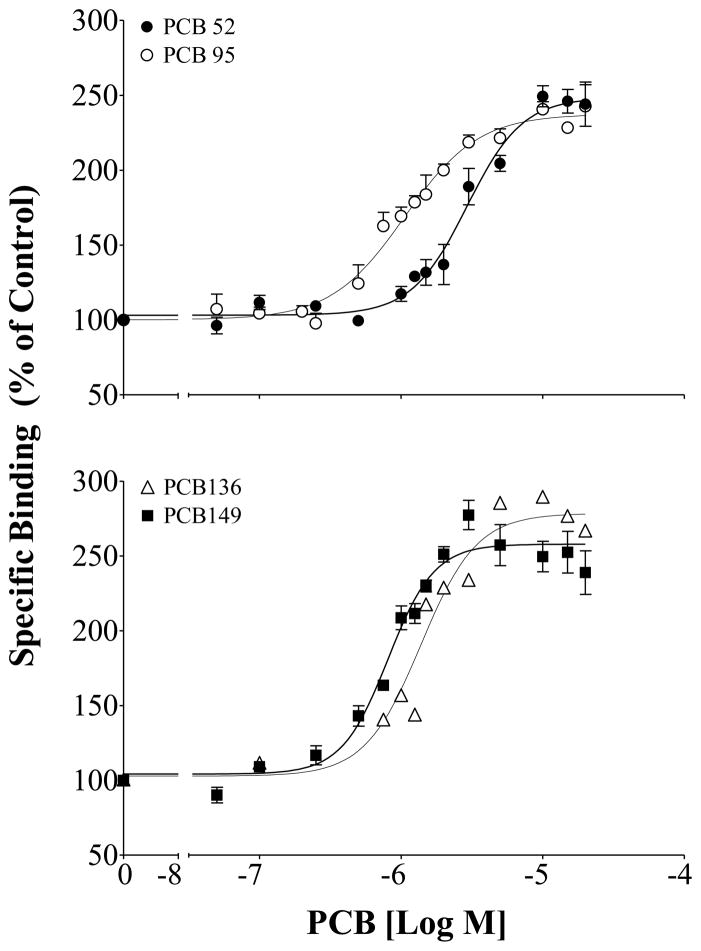
The ligand binding parameters for individual congeners are summarized in Table 2, where concentration-responses were determined with PCB concentrations ranging from 0–20 μM as levels exceeding 20 μM often led to a decrease in the maximum [3H]Ry binding, or plateau for a given congener. These levels are well above what is found in fish and may have exceeded the solubility limit, thus assay availability, for given congeners and therefore were excluded from the calculations for all congeners. Of the congeners assessed, the most efficacious at the receptor included PCB 52, 95, 136, and 149 (Figure 1; Table 2). These congeners caused an approximately 250% increase in [3H]Ry binding compared to the DMSO control, with EC50 values of 2.94, 1.06, 1.30 and 0.81 μM for PCB 52, 95, 136, and 149 respectively. Other efficacious congeners included PCB 44, 101 151 and 153, which all caused greater than a 160% increase in receptor activity. These congeners all have at least two chlorines in the ortho position (Table 1), whereas the non-ortho or mono-ortho PCBs assessed had no or negligible activity toward RyR1. This included PCB 77, a coplanar congener lacking ortho substitution, and the mono-ortho PCB 28, which were inactive toward RyR1 in the rainbow trout preparation. Mono-ortho PCB 20 and 66 did enhance receptor activity but in general were less efficacious than di- and tri-ortho substituted congeners.
Figure 1.
[3H]Ry binding in rainbow trout skeletal muscle homogenates in the presence of PCB 52, 95, 136, or 149. Specific binding shown as a percentage of the DMSO solvent control; Mean ± SEM. PCB 136,149 and 52 (n=6); PCB 95 (n=12).
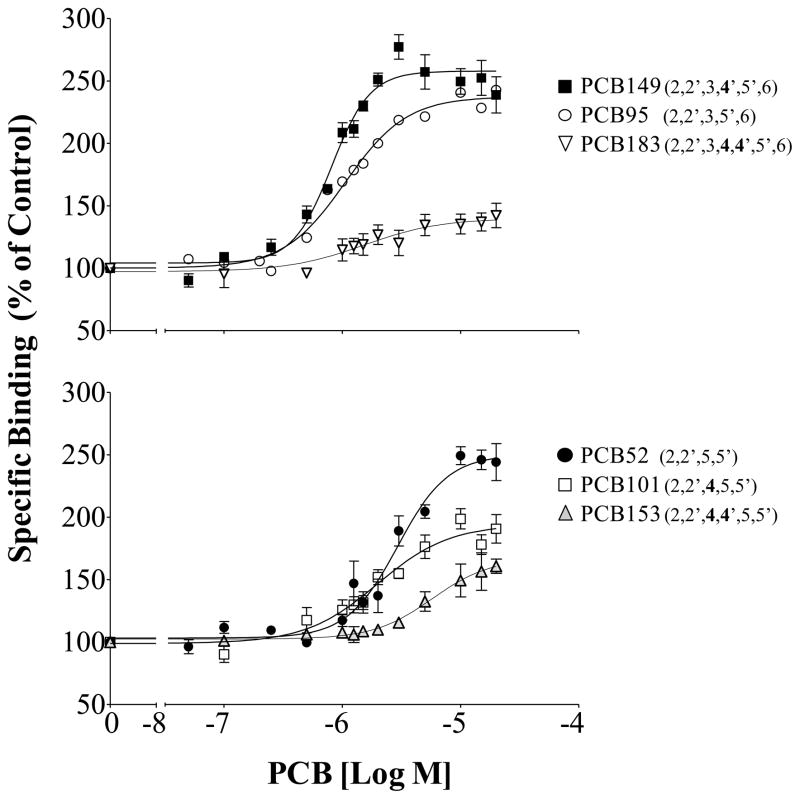
In addition to enhanced receptor activity for those congeners containing two or more chlorines in the ortho position, other patterns in chlorine substitution appeared to influence the ability of PCB congeners to activate RyR1 (Figure 2). When tri-ortho substituted PCB 95 was compared to PCB 149, which has the same chlorine substitution pattern but an additional chlorine in the para position, there was no difference in efficacy. However, PCB 183 that has two para chlorine substitutions was far less efficacious with a maximum activation of 143%. The effect of para substitution on decreased receptor efficacy appeared to be consistent for the 20 congeners assessed and was especially true for the di-ortho PCBs. For example the di-ortho substituted PCB 52, 101 and 153 all share a 2,2′,5,5′ substitution pattern but showed decreasing activity at the receptor with one or both of the para positions occupied, respectively (Figure 2). Interestingly, PCB 170 was inactive at the trout RyR1 despite the required di-ortho chlorine substitution and PCB 132 enhanced receptor activity at concentrations up to 10 μM followed by a consistent decrease in activity that reached 88% of baseline at 20 μM (data not shown).
Figure 2.
Impact of para chlorine substitution on PCB efficacy at RyR1. Specific binding shown as a percentage of the DMSO solvent control; Mean ± SEM; PCB 149, 183, 52, 101, 153 (n=6); PCB 95 (n=12).
3.2 Binary and complex PCB mixtures display additivity at the RyR1
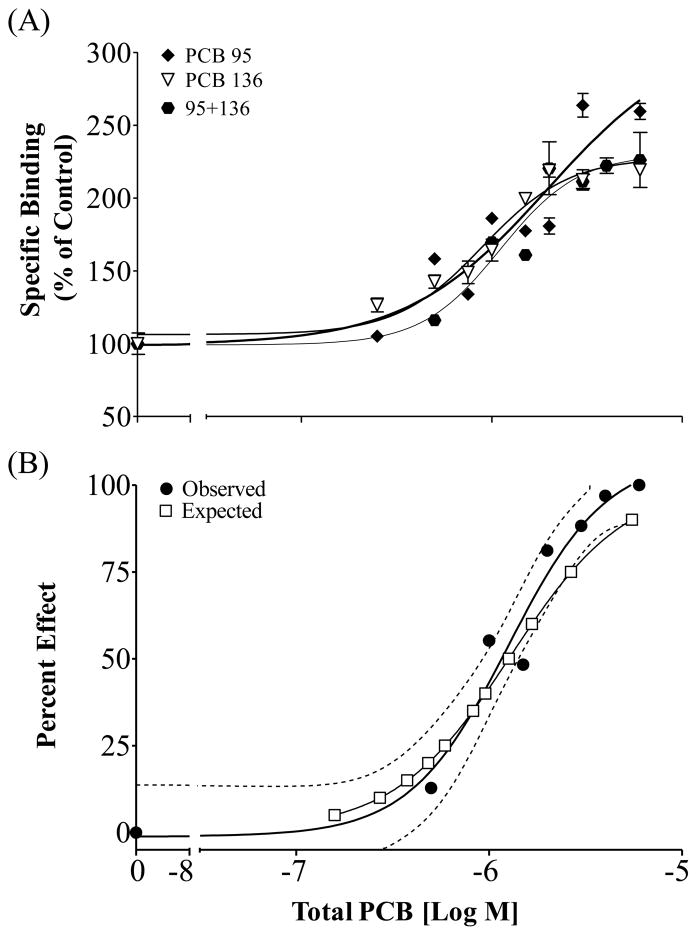
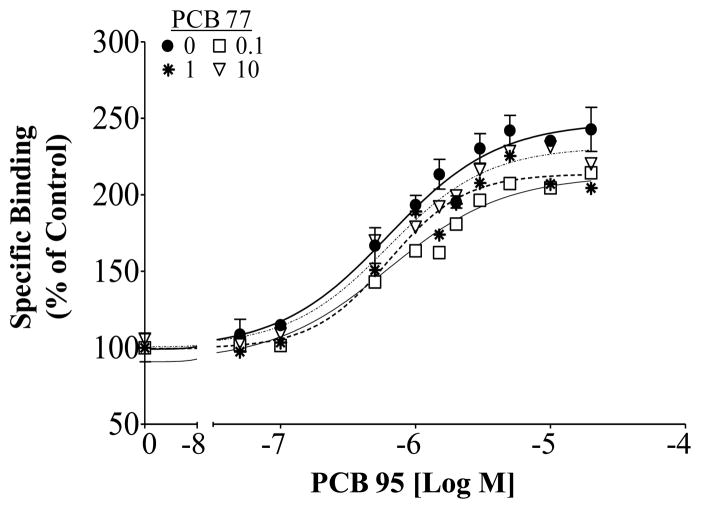
When the two efficacious NDL PCB congeners PCB 95 and PCB 136 were combined, the binary mixture followed the assumptions described under the CA model (Figure 3). Here, the mixture led to a 230% maximum response with an EC50 of 1.25 μM total PCB (95% CI 0.84–1.87 μM). The expected concentration-response curve, calculated from the individual congener responses, predicted that the mixture would result in an EC50 of 1.29 μM and the calculated expected curve fell within the 95% confidence interval of that observed for the mixture, suggesting that active NDL PCB congeners display additivity toward RyR1. Combining the active congener PCB 95 with the coplanar congener PCB 77, which lacked activity at the receptor, resulted in significantly different dose-response curves as compared to PCB 95 alone (Figure 4; p<0.0001; F=6.988). Even though the curves differed significantly, as a result of slightly decreased maximal response, the calculated EC50 for PCB 95 alone was not significantly different from that observed in the presence of PCB 77 (p=0.27; F=1.32).
Figure 3.
[3H]Ry binding in rainbow trout skeletal muscle in the presence of PCB 95 and PCB 136 individually or in a mixture. (A) Enhanced activity compared to a DMSO control; (B) Observed Effect is the specific binding of the mixture normalized with baseline set at 0 and the maximal response set at 100%; Mean ± SEM, n=3; (----) 95% Confidence Interval for the Observed response. Expected values calculated following the Concentration Addition Model.
Figure 4.
[3H]Ry binding in rainbow trout skeletal muscle due to PCB 95 alone or in the presence of PCB 77. Specific binding shown as a percentage of the DMSO solvent control; Mean ± SEM, n=3.
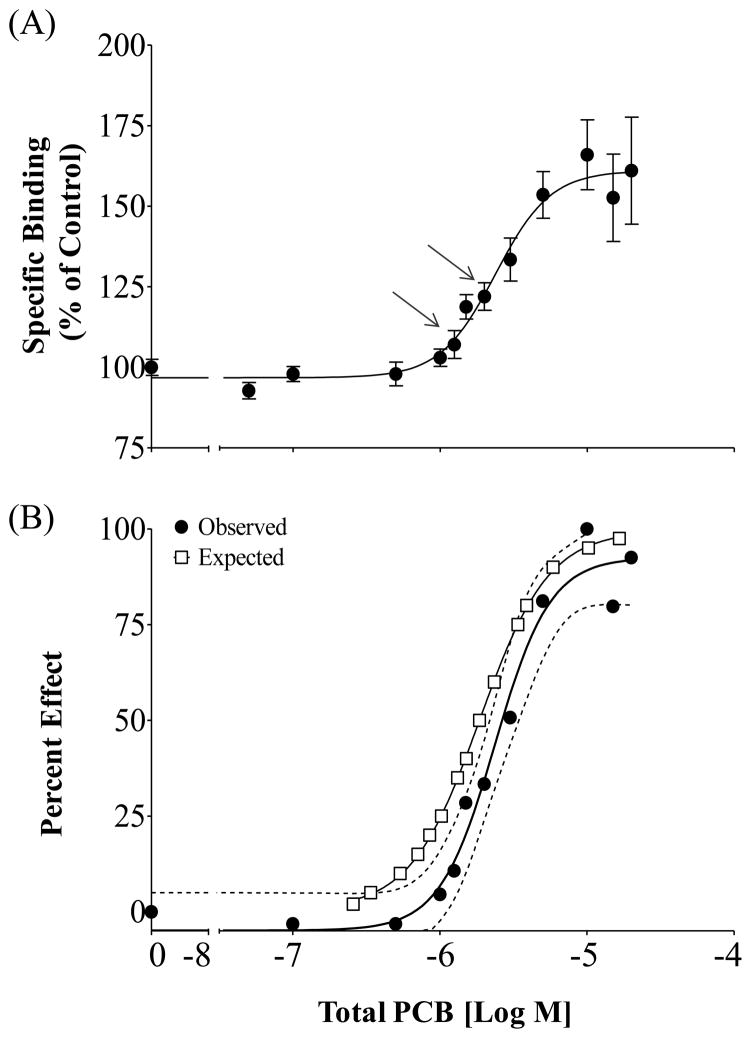
When all 20 congener, addressed in the current study, were combined at levels proportional to the maximal estimated concentration found in predatory fish across the US they led to an enhanced RyR1 response equal to 161.00% of the DMSO control with an EC50 of 2.37 μM (SEM ± 5.19%; 95% CI 1.71–3.29 μM; Figure 5A). The observed percent effect of the mixture again appeared to follow the predictions of the CA model (Figure 5B) further supporting congener additivity at the RyR1. Here, the expected dose-response curve, calculated from the percent effects for all 20 individual congeners, feel within the 95% confidence interval of that observed for the mixture. There were slight variations at the lower end of the expected dose-response curve; however, the predicted EC50 (1.87 μM) did not differ from that observed for the mixture (p=0.68; F=0.17) and one curve was found to adequately describe both observed and expected data sets (p=0.64; F=0.63).
Figure 5.
Environmentally relevant PCB mixtures alter the activity of RyR1 in rainbow trout skeletal muscle. (A) Specific Binding relative to the DMSO control; Mean ± SEM n=12. RESPONSE: Arrows represent the estimated concentrations of PCBs or mixtures currently detected in fish tissue; bottom arrow is the sum concentration of the 20 congeners addressed in this study converted to μM based on individual congener MW; top arrow is the total PCB concentrations of all 209 congener (704 μg kg−1) converted to μM based on average PCB molecular weight. (B) Percent effect of Observed response compared to the Expected dose response as predicted by the Concentration Addition Model. Observed responses based on the specific binding, found in graph (A), with control set at 0 and the maximum mixture response set at 100%; (- - -) 95% Confidence Interval for the Observed response.
3.3 Other halogenated compounds enhance RyR1 activity and display additivity when present with PCBs
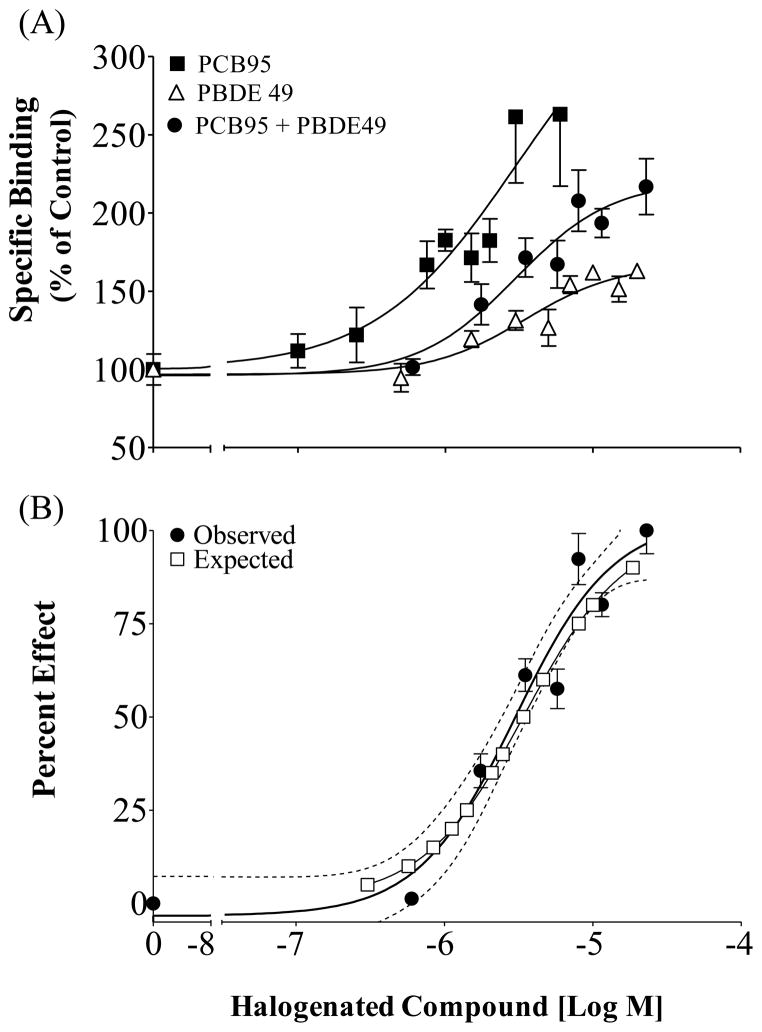
Alone, PBDE49 lead to enhanced receptor activity with a maximal response of 167% and an EC50 of 3.48 μM (SEM ± 13.15%; 95% CI 1.65–7.35 μM; Figure 6A). When PBDE 49 was placed in a binary mixture with PCB 95 the mixture lead to a maximal response of 220% with an EC50 of 3.0 μM (SEM ± 12.38%; 95% CI 1.37–6.59 μM; Figure 6A) The mixture of the two halogenated chemicals appeared to follow the assumptions of the CA model (Figure 6B) supporting additivity of active compounds at the RyR1.
Figure 6.
[3H]Ry binding in rainbow trout skeletal muscle in the presence of PCB 95 and PBDE 49 individually or in a mixture. (A) Enhanced activity compared to a DMSO control; (B) Observed Effect is the specific binding of the mixture normalized with baseline set at 0 and the maximal response set at100 %; Mean ± SEM, n=3; (----) 95% Confidence Interval for the Observed response. Expected values calculated following the Concentration Addition Model.
3.4 Relative potency scheme
The TEQ developed for DL chemicals, including DL PCBs, is based on the potency of a given chemical relative to the most potent AhR agonist, TCDD (Van den Berg et al., 2006). In an effort to establish a similar relative potency scheme for NDL PCBs, and other halogenated congeners, in fish a similar approach was taken. The EC50 of an individual congener was deemed inappropriate for such measures because it is dependent on the maximum response observed for a that congener. For example, the EC50 for PCB 20 and PCB 101 was similar (see Table 2) but the congers led to significantly different maximal responses. Therefore, the concentration needed to elicit 20% of the maximal possible response (Q20) observed for any congener (~ 253%) was determined. The Q20 for individual congeners can be found in Table 3 along with the relative potency as compared to that of PCB 95.
Table 3.
Non-dioxin like PCB relative potency factors (RPFa) in fish and mammals.
| Congener | Fish Normalized Q20 (μM) | Fish RPF | Mammal EC2X (μM)b | Mammal RPF | Comparison (Ratio) |
|---|---|---|---|---|---|
| 20 | 79.33 | 0.01 | 2c | 0.11 | 0.05 |
| 28 | - | - | 5c | 0.04 | - |
| 44 | 5.35 | 0.08 | 0.8c | 0.28 | 0.29 |
| 52 | 1.40 | 0.31 | 0.49 | 0.45 | 0.69 |
| 66 | 10.66 | 0.04 | 2.38 | 0.09 | 0.44 |
| 77 | - | - | 5.00 | 0.04 | - |
| 95 | 0.43 | 1.00 | 0.22 | 1.00 | 1.00 |
| 99 | 15.53 | 0.03 | 2.80 | 0.08 | 0.35 |
| 101 | 1.03 | 0.42 | 0.86 | 0.26 | 1.64 |
| 110 | NC | - | 1.13 | 0.19 | - |
| 132 | 18.57 | 0.02 | 0.91 | 0.24 | 0.10 |
| 136 | 0.72 | 0.60 | 0.23 | 0.96 | 0.63 |
| 149 | 0.45 | 0.96 | 0.33 | 0.67 | 1.45 |
| 151 | 2.21 | 0.20 | 0.50 | 0.44 | 0.45 |
| 153 | 5.81 | 0.07 | 1.21 | 0.18 | 0.41 |
| 170 | - | - | 0.73 | 0.30 | - |
| 180 | 11.16 | 0.04 | 0.96 | 0.23 | 0.17 |
| 183 | 6.76 | 0.06 | 0.55 | 0.40 | 0.16 |
| 187 | 7.11 | 0.06 | 0.58 | 0.38 | 0.16 |
| 194 | 11.36 | 0.04 | 0.5c | 0.44 | 0.09 |
| Average | 0.50 |
RPFs shown relative to PCB 95
Values from Pessah et al. 2006
Estimated from Rayne and Forest 2010
notes: Inactive (−) or Not converged (NC)
4.0 DISCUSSION
The current study demonstrated that, similar to the mode of toxic action in mammalian species, NDL PCBs enhance the activity of the RyR1 found in skeletal muscle of the rainbow trout. The RyR1 is essential to countless physiological processes (Berridge, 2006; Pessah et al., 2010) and disruption due to either genetic or environmental factors has been correlated to impacts on muscle contraction and swimming performance in mammals (Bellinger et al., 2008; Betzenhauser and Marks, 2010; Cherednichenko et al., 2012) and fish respectively (Hirata et al., 2007; Seebacher et al., 2012; Fritsch et al., 2013). Disruption by NDL PCBs, specifically, is known to increase the open probability of the RyR1 resulting in a leaky channel and ultimately depleted SR Ca2+ stores (Pessah et al., 2010). While the direct impact of NDL PCB mediated depletion on muscle contraction requires further investigation depleted SR stores is a known mechanism of muscle fatigue and reduced force production (Allen et al., 2008) and is associated with decreased fatigue resistance in carp (Seebacher et al., 2012). In the current study, we demonstrated individual congener effects and we showed that binary and complex mixtures display additivity at the receptor, which is supported by trends published elsewhere (Kostyniak et al., 2005; Pessah et al., 2006). The impact on the receptor occurred with congeners prevalent in fish and at the levels currently detected in their tissue suggesting that further studies regarding the effect of NDL PCBs on the muscle contraction and swimming performance of fish are needed to fully address the risks posed by PCBs in aquatic ecosystems.
In this study we addressed the effect of PCBs spanning a wide array of chlorine substitution patterns and while this was not a complete profile the congeners used here are similar to that utilized in rabbit studies (Pessah et al., 2006), which were shown to adequately explain the potential range of RyR1 toxicity induced by all 209 PCBs (QSAR; Rayne and Forest, 2010). From these studies several assumptions can be made regarding PCB mediated toxicity at the RyR1: 1) activity likely requires a minimum of mono-ortho substitution; 2) congeners with two or more chlorines in the ortho-position are highly efficacious; and 3) congeners with increased para substitution are likely less efficacious at the RyR1 when compared to those with similar chlorine patterns but lacking chlorines in the para position. Here, the QSAR developed by Rayne and Forrest (2010) suggested that para-substitution on both biphenyl rings may not describe less efficacious congeners; however, thus far in vitro experiments conducted on non-coplanar halogenated compounds support this conclusion (current study; Pessah et al., 2006; Kim et al., 2010).
In addition to the similarities between the vertebrate taxa there were also several notable differences including lower maximal responses achieved for any congener and higher effective concentrations in the trout. These differences may be due to slight variations in assay conditions including the use of trout crude microsomal protein preparations versus more purified rabbit preparations of junctional SR (Pessah et al., 2006). Preparation could have accounted for differences in relative lipid concentrations contributing to altered concentration-responses. Additionally, variable affinity for the radioligand to the RyR1 could lead to an overall response difference as the maximal achieved binding of the ligand varies between mammals and teleost species (Thomas et al., 1996; Qi et al., 1998).
Species sensitivity to NDL PCBs might also be described by variable characteristics of skeletal muscle physiology. Fillets utilized in this study consisted primarily of white skeletal muscle, where RyR1 and RyR, isoform 3 (RyR3), are known to be co-expressed in several fish species (O’Brien et al., 1995; Franck et al., 1998). In contrast adult mammalian muscle, as used in previous studies (Pessah et al., 2006), do not express RyR3 (Legrand et al., 2008). Sensitivity to NDL PCBs may vary between RyR isoforms explaining the lower efficacy seen in fish, which is supported by studies showing distinct pharmacology and gating behavior between RyR1 and RyR3 (Fessenden et al., 2000). Also, in mammalian systems NDL PCB congeners cause an over-activation of the RyR through a mechanism involving the immunophilin FKBP12/12.6, which are accessory proteins that stabilize the conformational state of the channels (Wong and Pessah, 1997; Qi et al., 1998; Pessah et al., 2006; Pessah et al., 2010). There is limited research regarding FKBP12 binding to the RyR in fish; however, what has been done suggests that the stoichiometry of the complex varies between fish and mammals (Qi et al., 1998). Differential regulation of the conformational state of the channel by FKBP12 may explain the observed differences in NDL PCB sensitivity.
Regardless of differences in overall sensitivity and efficacy we found that NDL PCBs enhance RyR1 activity in the trout at levels found in the environment. Namely, when the estimated maximum concentrations of the 20 PCBs utilized in this study were summed it represented levels equal to 1.24 μM (based on congener MW), which is above the calculated 10% of maximal effect concentration (EC10 = 0.92 μM) observed for the congener mixture (Figure 5A, bottom arrow). The ability of this mixture to enhance the activity of the receptor is likely conservative because many of the other 209 congeners, which were not tested here, are known or predicted to enhance RyR activity (Pessah et al., 2006; Rayne and Forest, 2010). The maximum concentration of all 209 PCBs detected by Stahl et al. 2009 was estimated at 2.16 μM (704 μg kg−1; based on average PCB MW). This is relative to the observed EC50 of the 20 congener mixture at 2.37 μM, shown to enhance receptor activity at an approximate 130% (Figure 5A, top arrow). Making the assumption that all active congeners display additivity at the RyR1 and that non-active congeners only slightly decrease the impact of PCBs on the receptor we hypothesize that many of the other congeners in the total PCB burden in fish would contribute to the observed toxicity at the RyR1. This is supported by findings that PCB extracts from (Pessah et al., 2006) or representative of congener profiles (Kostyniak et al., 2005) found in various forms of environmental media are capable of activating the RyR1in mammals at total PCB levels as low as 1 μM.
The relevance of a 10%, or greater, over-activation of fish RyR1 at environmental PCB concentrations has not been established but chemical perturbation by NDL PCBs correlates with enhanced Ca2+ efflux from the SR in mammalian cellular assays, SR Ca2+ depletion, and is linked to weak muscle contraction and developmental neurotoxicity (Pessah et al., 2010). Additionally, chemicals known to act on the RyR, or related proteins, alter muscle contraction and swimming performance in exposed teleost species (Seebacher et al., 2012; Fritsch et al., 2013). The whole animal and ecological relevance of chronic over-activation of the RyR1 by NDL PCBs needs to be further addressed to fully understand the risks posed by complex PCB mixtures.
Current regulatory practices assess the relative toxicity of a given PCB congener or group of congeners compared to that of the potent AhR activator TCDD to create what is known as TCDD Equivalency Factors (TEF) or TCDD Equivalents (TEQ) respectively. Utilizing this scheme the threat of individual PCB congeners, or environmentally relevant mixtures, can be evaluated (Van den Berg et al., 1998; Van den Berg et al., 2006) providing a useful regulatory tool (USEPA, 2000; Stahl et al., 2009). However, TCDD based toxicity has only been demonstrated for a select number of PCBs and thus the sole use of the TEQ scheme may inaccurately represent the total impact of PCB contamination (Giesy and Kannan, 1998; Pessah et al., 2010).
Several groups have suggested the development of a similar relative toxicity scheme for NDL PCBs focusing on inducible neurotoxicity in in vitro or in vivo assays (Simon et al., 2007; Rayne and Forest, 2010). This has led to so called Neurotoxic Equivalence Factors (NEF) or Neurotoxic Equivalents (NEQ) for individual congeners or mixtures of PCB classes, respectively (Simon et al., 2007; Rayne and Forest, 2010), and includes the neurologic or neurodevelopment toxicity mediated by the RyR1 or RyR2 (Kim et al., 2010; Pessah et al., 2010; Wayman et al., 2012a; Wayman et al., 2012b). However, to date NEF/NEQ parameters have yet to be utilized to evaluate the neurotoxic risk associated with exposure to or body burdens of PCBs. Here, the calculated relative potency values in trout were generally lower than those previously determined for the rabbit (Table 3) suggesting that the application of developed NEQs for mammals would also be protective in fish. Therefore, using the NEQs developed by Ranye and Forest (2010), together with documented PCB concentrations, future studies could evaluate the neurotoxic risk associated with a given waterbody or exposure scenario.
It should also be noted that PCBs are often present in the environment together with other halogenated compounds. Particularly, PBDEs share many structural similarities with the non-coplanar PCBs and our study shows that the commonly detected PBDE 49 (Hites et al., 2004b), does in fact enhance RyR1 activity in the rainbow trout (Figure 6A), which is consistent with its effects seen in mammals (Kim et al., 2010). Given the additivity of PBDE 49 and PCB 95 at the receptor (Figure 6B) it is likely that PBDEs contribute to altered RyR1 function when present together with PCBs. A full quantitative structure activity relationship for PBDE congeners has not been established, but work to date suggests that similar characteristics as that seen for PCBs may mediate toxicity at the receptor (Kim et al., 2010). Taken together this suggests that a NEQ scheme, evaluating environmentally relevant impacts of non-coplanar compounds on the RyR, would be a useful tool that when utilized with the currently established TEQ would provide a more inclusive measure of the risks that legacy and current use persistent organic pollutants pose to aquatic environments.
HIGHLIGHTS.
Non-dioxin like PCBs enhance ryanodine receptor activity
Ortho and para chlorine substitution define efficacy at the ryanodine receptor
Active congeners display additivity toward ryanodine receptor activation
PCB concentrations that occur in fish tissue enhance ryanodine receptor activity
PBDEs may contribute to PCB induced toxicity at the ryanodine receptor
Acknowledgments
The UC Davis NIEHS Superfund Research Program P42 ES004699 supported this project (to INP and EBF). Additional Support came from NIEHS 1R01-ES014901 and 1R01-ES017425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. We appreciate Matthias Hasenbein who assisted with sample collection. Appreciation is also extended to Dr. Inge Werner (Swiss Centre for Applied Ecotoxicolgy) whose review and advice helped strengthen the final version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal Muscle Fatigue: Cellular Mechanisms. Physiological Reviews. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Belden JB, Lydy MJ. Joint toxicity of chlorpyrifos and esfenvalerate to fathead minnows and midge larvae. Environmental Toxicology and Chemistry. 2006;25:623–629. doi: 10.1897/05-370r.1. [DOI] [PubMed] [Google Scholar]
- Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. The Journal of Clinical Investigation. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: Organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Cell SIgnaling Pathways. Cell Signaling Biology Module. 2012;2 [Google Scholar]
- Betzenhauser MJ, Marks AR. Ryanodine receptor channelopathies. Pflügers Archiv-European Journal of Physiology. 2010;460:467–480. doi: 10.1007/s00424-010-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Reviews of Environmental Contamination and Toxicology Vol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Boik J, Kirakosyan A, Kaufman PB, Seymour EM, Spelman K. Recent Advances in Plant Biotechnology. 2009. Interactions of Bioactive Plant Metabolites: Synergism, Antagonism, and Additivity; pp. 213–230. [Google Scholar]
- Brar NK, Waggoner C, Reyes JA, Fairey R, Kelley KM. Evidence for thyroid endocrine disruption in wild fish in San Francisco Bay, California, USA. Relationships to contaminant exposures. Aquatic Toxicology. 2010;96:203–215. doi: 10.1016/j.aquatox.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Cherednichenko G, Zhang R, Bannister RA, Timofeyev V, Li N, Fritsch EB, Feng W, Barrientos GC, Schebb NH, Hammock BD, Beam KG, Chiamvimonvat N, Pessah IN. Triclosan impairs excitation–contraction coupling and Ca2+ dynamics in striated muscle. Proceedings of the National Academy of Sciences. 2012;109:14158–14163. doi: 10.1073/pnas.1211314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbandi S, Franck JPC. A comparative study of ryanodine receptor (RyR) gene expression levels in a basal ray-finned fish, bichir (Polypterus ornatipinnis) and the derived euteleost zebrafish (Danio rerio) Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2009;154:443–448. doi: 10.1016/j.cbpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Dellinger JA, Moths MD, Dellinger M, Ripley MP. Contaminant Trends in Freshwater Fish from the Laurentian Great Lakes: A 20-Year Analysis. Human and Ecological Risk Assessment: An International Journal 2013 [Google Scholar]
- Denison MS, Nagy SR. Activation of the Aryl Hydrocarbon Receptor by Structurally Diverse Exogenous and Endogenous Chemicals*. Annual review of pharmacology and toxicology. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environment International. 2007;33:397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith LS, Smith-Simon C, De Rosa CT. Concise International Chemical Assessment Document 55. World Health Organization; Geneva: 2003. Polychlorinated Biphenyls: Human Health Aspects. [Google Scholar]
- Faust M, Altenburger R, Backhaus T, Boedeker W, Scholze M, Grimme L. Predictive assessment of the aquatic toxicity of multiple chemical mixtures. Journal of environmental quality. 2000;29:1063–1068. [Google Scholar]
- Fernandes ECA, Hendriks HS, van Kleef RG, Reniers A, Andersson PL, van den Berg M, Westerink RH. Activation and Potentiation of Human GABAA Receptors by Non-Dioxin–Like PCBs Depends on Chlorination Pattern. Toxicological Sciences. 2010;118:183–190. doi: 10.1093/toxsci/kfq257. [DOI] [PubMed] [Google Scholar]
- Fessenden JD, Wang Y, Moore RA, Chen SRW, Allen PD, Pessah IN. Divergent Functional Properties of Ryanodine Receptor Types 1 and 3 Expressed in a Myogenic Cell Line. Biophysical Journal. 2000;79:2509–2525. doi: 10.1016/S0006-3495(00)76492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JPC, Morrissette J, Keen JE, Londraville RL, Beamsley M, Block BA. Cloning and characterization of fiber type-specific ryanodine receptor isoforms in skeletal muscles of fish. American Journal of Physiology - Cell Physiology. 1998;275:C401–C415. doi: 10.1152/ajpcell.1998.275.2.C401. [DOI] [PubMed] [Google Scholar]
- Fritsch EB, Connon RE, Werner I, Davies RE, Beggel S, Feng W, Pessah IN. Triclosan Impairs Swimming Behavior and Alters Expression of Excitation-Contraction Coupling Proteins in Fathead Minnow (Pimephales promelas) Environmental Science & Technology. 2013;47:2008–2017. doi: 10.1021/es303790b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue-and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Dioxin-Like and Non-Dioxin-Like Toxic Effects of Polychlorinated Biphenyls (PCBs): Implications For Risk Assessment. Critical Reviews in Toxicology. 1998;28:511–569. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- Hayward D, Wong J, Krynitsky AJ. Polybrominated diphenyl ethers and polychlorinated biphenyls in commercially wild caught and farm-raised fish fillets in the United States. Environmental Research. 2007;103:46–54. doi: 10.1016/j.envres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Hirata H, Watanabe T, Hatakeyama J, Sprague SM, Saint-Amant L, Nagashima A, Cui WW, Zhou W, Kuwada JY. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development. 2007;134:2771–2781. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global Assessment of Organic Contaminants in Farmed Salmon. Science. 2004a;303:226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. Global Assessment of Polybrominated Diphenyl Ethers in Farmed and Wild Salmon. Environmental Science & Technology. 2004b;38:4945–4949. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- Howell NL, Suarez MP, Rifai HS, Koenig L. Concentrations of polychlorinated biphenyls (PCBs) in water, sediment, and aquatic biota in the Houston Ship Channel, Texas. Chemosphere. 2008;70:593–606. doi: 10.1016/j.chemosphere.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Hwang HM, Green PG, Young TM. Tidal salt marsh sediment in California, USA. Part 1: Occurrence and sources of organic contaminants. Chemosphere. 2006;64:1383–1392. doi: 10.1016/j.chemosphere.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Jonker MJ, Svendsen C, Bedaux JJM, Bongers M, Kammenga JE. Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Environmental Toxicology and Chemistry. 2005;24:2701–2713. doi: 10.1897/04-431r.1. [DOI] [PubMed] [Google Scholar]
- Khan MA, Hansen LG. ortho-Substituted polychlorinated biphenyl (PCB) congeners (95 or 101) decrease pituitary response to thyrotropin releasing hormone. Toxicology Letters. 2003;144:173–182. doi: 10.1016/s0378-4274(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, Lein PJ, Pessah IN. Para- and Ortho-Substitutions Are Key Determinants of Polybrominated Diphenyl Ether Activity toward Ryanodine Receptors and Neurotoxicity. Environ Health Perspect. 2010;119(4):519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, Heideman W, Peterson RE. Reproductive and developmental toxicity of dioxin in fish. Molecular and Cellular Endocrinology. 2012;354:121–138. doi: 10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochemical Pharmacology. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL. Formulation and Characterization of an Experimental PCB Mixture Designed to Mimic Human Exposure from Contaminated Fish. Toxicological Sciences. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lanner J. In: Ryanodine Receptor Physiology and Its Role in Disease. Islam MS, editor. Calcium Signaling; Springer Netherlands: 2012. pp. 217–234. [DOI] [PubMed] [Google Scholar]
- Legrand C, Giacomello E, Berthier C, Allard B, Sorrentino V, Jacquemond V. Spontaneous and voltage-activated Ca2+ release in adult mouse skeletal muscle fibres expressing the type 3 ryanodine receptor. The Journal of Physiology. 2008;586:441–457. doi: 10.1113/jphysiol.2007.145862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Valdivia HH, Block BA. Physiological differences between the alpha and beta ryanodine receptors of fish skeletal muscle. Biophysical Journal. 1995;68:471–482. doi: 10.1016/S0006-3495(95)80208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacology & Therapeutics. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure–Activity Relationship for Noncoplanar Polychlorinated Biphenyl Congeners toward the Ryanodine Receptor-Ca2+ Channel Complex Type 1 (RyR1) Chemical Research in Toxicology. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Stambuk RA, Casida JE. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Molecular Pharmacology. 1987;31:232–238. [PubMed] [Google Scholar]
- Qi Y, Ogunbunmi EM, Freund EA, Timerman AP, Fleischer S. FK-binding Protein Is Associated with the Ryanodine Receptor of Skeletal Muscle in Vertebrate Animals. Journal of Biological Chemistry. 1998;273:34813–34819. doi: 10.1074/jbc.273.52.34813. [DOI] [PubMed] [Google Scholar]
- Rayne S, Forest K. Quantitative structure-activity relationship (QSAR) studies for predicting activation of the ryanodine receptor type 1 channel complex (RyR1) by polychlorinated biphenyl (PCB) congeners. Journal of Environmental Science and Health, Part A. 2010;45:355–362. doi: 10.1080/10934520903467980. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Pollard SR, James RS. How well do muscle biomechanics predict whole-animal locomotor performance? The role of Ca2+ handling. The Journal of Experimental Biology. 2012;215:1847–1853. doi: 10.1242/jeb.067918. [DOI] [PubMed] [Google Scholar]
- Simon T, Britt JK, James RC. Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regulatory Toxicology and Pharmacology. 2007;48:148–170. doi: 10.1016/j.yrtph.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Stahl L, Snyder B, Olsen A, Pitt J. Contaminants in fish tissue from US lakes and reservoirs: a national probabilistic study. Environmental Monitoring and Assessment. 2009;150:3–19. doi: 10.1007/s10661-008-0669-8. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Ramu K. Monitoring temporal and spatial trends of legacy and emerging contaminants in marine environment: Results from the environmental specimen bank (es-BANK) of Ehime University, Japan. Marine Pollution Bulletin. 2012;64:1459–1474. doi: 10.1016/j.marpolbul.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Hamman BN, Tibbits GF. Dihydropyridine and ryanodine binding in ventricles from rat, trout, dogfish and hagfish. Journal of Experimental Biology. 1996;199:1999–2009. doi: 10.1242/jeb.199.9.1999. [DOI] [PubMed] [Google Scholar]
- OoSaT, editor. USEPA. Guidance for assessing chemical contaminant data for use in fish adivisories, volume 2: Risk assessement and fish consumption limits. 3. USEPA Office of Water; Washington DC: 2000. [Google Scholar]
- USEPA. The National Study of Chemical Residues in Lake Fish Tissue. In: UOoWOoSa, editor. Technology. Washington DC: 2009. [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld A, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environmental Health Perspectives. 1998;106:775. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DM, Mills MA, Cade BS, Burkard LP. Trophic Magnification of PCBs and Its Relationship to the Octanol–Water Partition Coefficient. Environmental Science & Technology. 2011;45:3917–3924. doi: 10.1021/es103158s. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ. PCB-95 Modulates the Calcium-Dependent Signaling Pathway Responsible for Activity-Dependent Dendritic Growth. Environ Health Perspect. 2012a;120(7):1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ. PCB-95 Promotes Dendritic Growth via Ryanodine Receptor–Dependent Mechanisms. Environ Health Perspect. 2012b;120(7):997–1002. doi: 10.1289/ehp.1104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PW, Pessah IN. Noncoplanar PCB 95 Alters Microsomal Calcium Transport by an Immunophilin FKBP12-Dependent Mechanism. Molecular Pharmacology. 1997;51:693–702. doi: 10.1124/mol.51.5.693. [DOI] [PubMed] [Google Scholar]