Abstract
Epidemiologic and case control population based studies over the past few decades have identified diet as an important determinant of cancer risk. This evidence has kindled an interest into research on bioactive food components and has till date resulted in the identification of many compounds with cancer preventive and therapeutic potential. Among such compounds has been fisetin (3,7,3’,4’-tetrahydroxyflavone), a flavonol and a member of the flavonoid polyphenols that also include quercetin, myricetin and kaempferol. Fisetin is commonly found in many fruits and vegetables such as apples, persimmons, grapes, kiwis, strawberries, onions and cucumbers. We evaluated the effects of fisetin against melanoma and cancers of the prostate, pancreas and the lungs. Using prostate and lung adenocarcinoma cells, we observed that fisetin acts as a dual inhibitor of the PI3K/Akt and the mTOR pathways. This is a significant finding considering the fact that mTOR is phosphorylated and its activation is more frequent in tumors with overexpression of PI3K/Akt. Dual inhibitors of PI3K/Akt and mTOR signaling have been suggested as valuable agents for treating such cancers. Here, we summarize our findings on the dietary flavonoid fisetin and its effects on cancer with particular focus on prostate cancer. Our observations and findings from other laboratories suggest that fisetin could be a useful chemotherapeutic agent that could be used either alone or as an adjuvant with conventional chemotherapeutic drugs for the management of prostate and other cancers.
Fisetin: An overview
There is an increased interest in the scientific community on the use of plant based polyphenols based on their varied biological properties including antioxidative, antimicrobial, anticarcinogenic as well as cardioprotective activity [1, 2]. An important advantage with plant based polyphenols, especially those from dietary sources, is that they are perceived as non-toxic and have wide human acceptance [3]. Several non-nutritive, macronutrient phytochemicals are being evaluated for the management of cancer and other diseases [3]. Flavonoids form a large family of polyphenolic macronutrients that are abundant in plants. In laboratory studies flavonoids have been shown to affect cellular signaling pathways thus influencing cell survival and proliferation [2].
Fisetin (3,7,3’,4’-tetrahydroxyflavone) belongs to the flavonol subgroup of flavonoids along with quercetin, myricetin and kaempferol. It is present in many fruits and vegetables most notably strawberries, apples, persimmons, kiwis, cucumbers and onions [4]. The bioavailability of fisetin has been studied following intravenous and oral administration [5]. Serum levels of free fisetin decline rapidly within the first few hours while the levels of sulfated/glucuronidated fisetin increase. Following oral administration at 50 mg/kg, the serum concentration of fisetin sulfates/glucuronides was maintained at ~10 μM for >24 h. Following a single intraperitoneal injection, fisetin was detected in the brains of rats and this correlated with a significant reduction in cerebral damage in a stroke model [6].
Fisetin has broad biological properties ranging from antibacterial to antioxidative to cancer therapeutic effects (Table 1). In earlier studies, fisetin was identified as an antimicrobial agent and later shown to prevent oxidative stress-induced nerve cell death [7, 8]. Fisetin was also found to possess neurotrophic activity, promoting nerve cell differentiation via activation of extracellular signal-regulated kinase (Erk) [9]. Oral administration of fisetin to mice promoted ERK-dependent long-term potentiation and enhanced memory [10]. In addition, fisetin reduced cytotoxicity of lipopolysaccharide-stimulated microglia toward B35 neuroblastoma cells in a co-culture system indicating that fisetin has a strong anti-inflammatory activity in brain microglia, and could be a potential therapeutic agent for the treatment of neuroinflammatory diseases [11]. Sung et al [12] showed that fisetin mediates its anti-proliferative and anti-inflammatory effects through modulation of NF-κB. Fisetin suppressed NF-κB activation induced by various inflammatory agents and carcinogens and blocked the phosphorylation and degradation of IκBα, which in turn led to suppression of the phosphorylation and nuclear translocation of NF-κB/p65 [12].
Table 1.
Summary of biological effects associated with fisetin.
| Biological Effects | Reference No. |
|---|---|
| • Antibacterial and antimicrobial | 7 |
| • Prevents oxidative-stress induced nerve cell death | 8 |
| • Promotes nerve cell differentiation via Erk activation | 9 |
| • Enhances memory via ERK-dependent long-term potentiation | 10 |
| • Microtubule stabilizer and antimitotic: Reduces aurora B kinase | 13, 14 |
| • Induces apoptosis in promyeloleukemic and hepatocellular carcinoma cells through activation of the caspase 3 cascade | 15, 16 |
| • Inhibits adhesion, migration and invasion of human A549 lung cancer cells via inhibition of ERK, MMP-2 and u-PA | 17 |
| • Confers protection against benzo(a)pyrene [B(a)P] induced lung Carcinogenesis | 18 |
| • Inhibits activities of cdks leading to cell cycle arrest in HT-29 human colon cancer cells | 19 |
| • Enhances the radio-sensitivity of p53-mutant HT-29 human colon cancer Cells | 20 |
| • Activates death receptors to induce apoptosis in human HCT-116 human colon cancer cells | |
| • Induces apoptosis and inhibits invasion of AsPC-1 pancreatic cancer cells through suppression of DR3-mediated NFκB activation | 23 |
| • Potent inhibitor of the type 1 5 alpha-reductase activity | 24 |
| • Decreases viability of prostate cancer cells through physical interaction with the ligand binding domain of the androgen receptor | 26, 27 |
| • Inhibits tumor growth and reduces serum PSA levels | 28 |
| • Exerts inhibitory effects on the adhesion, migration, and invasion of prostate cancer cell via inactivation of the PI3K/Akt and JNK signaling Pathways | 29 |
| • Induces autophagic-programmed cell death through inhibition of mTOR kinase signaling pathway | 30 |
| • Physically interacts with the mTOR molecule | 40 |
| • Inhibits mTOR signaling in prostate cancer cells | 45 |
| • Inhibits mTOR signaling in human lung cancer cells | 40 |
| • Fisetin enhances cytotoxic effects when com |
Touil et al [13] compared 24 flavonoids for their cytotoxicity on B16 and Lewis lung cancer cells and their morphological effect on endothelial cells (EC) that could predict antiangiogenic activity. Ten flavonoids including fisetin inhibited cell proliferation at concentrations below 50 μM. Fisetin, among other flavonoids was also found to induce the formation of cell extensions and filopodias at noncytotoxic concentrations in EC cells. Increase in acetylated alpha-tubulin demonstrated that fisetin was a microtubule stabilizer [13]. Using a cell-based high-throughput screen for small molecules, fisetin was identified as an antimitotic compound that caused premature initiation of chromosome segregation and exit from mitosis without normal cytokinesis [14]. These effects of fisetin were observed to be linked to the significantly reduced activity of Aurora B kinase that was identified as a direct target of fisetin [14].
Fisetin and cancer
Effects of fisetin against cancer have been investigated both in vitro and in vivo. Treatment with fisetin induced apoptosis in promyeloleukemic and hepatocellular carcinoma cells through activation of the caspase 3 cascade [15, 16]. Fisetin exerted an inhibitory effect on adhesion, migration and invasion of human A549 lung cancer cells via inhibiting the phosphorylation of ERK1/2 and downregulation of the expressions of matrix metalloproteinase (MMP)-2 and u-PA at both the protein and mRNA levels [17]. An in vivo study showed that fisetin confers protection against benzo(a)pyrene [B(a)P] induced lung carcinogenesis. Treatment with fisetin significantly reduced the degree of histological lesions, restored the levels of lipid peroxidation, enzymic and non-enzymic anti-oxidants in B(a)P-induced mice [18]. Studies on the effect of fisein on colon cancer cells have been conducted employing two cell lines; the human HT29 and HCT-116 colon cancer cells. Fisetin inhibited the activities of cdks leading to cell cycle arrest in HT-29 human colon cancer cells. Treatment of COX2-overexpressing HT-29 cells with fisetin resulted in induction of apoptosis, downregulation of COX2 protein expression with no effect on COX1 and inhibition of secretion of prostaglandin E2. In addition, fisetin inhibited Wnt signaling activity through downregulation of β-catenin and T cell factor 4 and decreased the expression of target genes such as cyclin D1 and MMP-7. Fisetin treatment of colon cancer cells inhibited the activation of epidermal growth factor receptor and NFκB pathways [19]. In another study it was shown that fisetin pretreatment enhanced the radiosensitivity of p53-mutant HT-29 cancer cells, prolonged radiation-induced G(2)/M arrest, and enhanced radiation-induced caspase-dependent apoptosis [20].
Fisetin-induced apoptosis in HCT-116 colon cancer cells occurred via the activation of the death receptor and mitochondrial-dependent pathways. Induction of p53 resulted in the translocation of Bax to the mitochondria, and subsequent activation of the caspase cascade [21]. Securin is highly-expressed in various tumors including those of the colon. It was shown that fisetin-induced apoptosis in HCT-116 cells was enhanced in HCT-116 securin-null cells or in wild-type cells in which securin was knockdown by siRNA, but attenuated when wild-type or non-degradable securin was reconstituted. Moreover, fisetin did not induce apoptosis in HCT-116 p53-null and HT-29 p53-mutant cells. Knockdown of securin in HCT-116 p53-null cells potentiated fisetin-induced cytotoxicity by induction of apoptosis suggesting that securin depletion sensitizes human colon cancer cells to fisetin-induced apoptosis [22].
Fisetin treatment was found to negatively regulate the growth of chemoresistant pancreatic cancer cells [23]. Fisetin induced apoptosis and inhibited invasion of AsPC-1 pancreatic cancer cells through suppression of DR3-mediated NFκB activation. A cDNA array analysis revealed that fisetin modulated expression of more than twenty genes with maximum decrease observed in DR3 expression and a parallel increase observed in the expression levels of IκBα, the NFκB inhibitor [23]. Down-regulation of DR3 was found to down regulate activated NFκB/p65, MMP-9 and XIAP associated with chemoresistance in pancreatic cancer cells. Transient down-regulation of DR3 and by RNA interference and blocking of DR3 receptor with an extra cellular domain blocking antibody significantly augmented fisetin induced changes in cell proliferation, cell invasion and apoptosis paralleled with decrease in MMP9, XIAP and NFκB DNA binding activity [23]. These data provide evidence that fisetin has potential activity against several forms of cancer.
Fisetin and prostate cancer
We and other have made important observations on the effects of fisetin against prostate cancer. In humans the enzyme 5 alpha-reductase catalyzes the conversion of testosterone to 5 alpha-dihydrotestosterone and its activity is critical for male sexual differentiation and also in the development of benign prostatic hyperplasia and prostate cancer [24]. Several flavonoids including fisetin were found to be potent inhibitors of the type 1 5alpha-reductase activity. Since some of these compounds are consumed as part of the normal diet or in supplements, they have the potential to inhibit 5 alpha-reductase activity, which may be useful for the prevention or treatment of androgen-dependent disorders [24]. Haddad et al [25] compared the effect of two flavonoids, 2,2’-dihydroxychalcone (DHC) and fisetin in prostate cancer cells and observed concomitant induction of apoptosis and a decrease in clonogenic survival. Gene expression studies showed that amongst the most highly represented functional categories of genes altered by both compounds was the cell cycle category [25]. In total, hundred cell cycle genes were altered by DHC and fisetin including twenty seven genes with key functions in G2/M phase that were downregulated by both compounds. Other functional categories altered included chromosome organization, apoptosis, and stress response [25].
Studies from our laboratory also suggest that fisetin possesses potent antiproliferative activity against prostate cancer cells. We showed that fisetin decreased viability of prostate cancer cells LNCaP, CWR22Rν1 and PC-3 cells but had only minimal effects on normal prostate epithelial cells [26, 27]. Fisetin through physical interaction with the ligand binding domain of the androgen receptor interfered with the amino-/carboxyl-terminal interaction resulting in blunting of androgen receptor (AR)-mediated transactivation of target genes including prostate specific antigen [28]. Furthermore, treatment with fisetin in athymic nude mice implanted with AR-positive CWR22ν1 prostate cancer cells resulted in inhibition of tumor growth and reduction in serum PSA levels [28]. Chen et al [29] observed that fisetin exerts inhibitory effects on the adhesion, migration, and invasion of prostate cancer cells. They showed that the antimetastatic potential of fisetin is linked to the inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2 and 9 expressions in these cells [29].
We recently showed that fisetin treatment of androgen-independent and phosphate and tensin homolog (PTEN)-negative human PC3 prostate cancer cells leads to induction of autophagic-programmed cell death with inhibition of mammalian target of rapamycin (mTOR) kinase signaling pathway [30]. This was an exciting observation since AKT/mTOR and MAP kinase pathways are among the major signaling networks that have been implicated in advanced prostate cancer. Deregulated expression of the PTEN occur with high frequency in prostate cancer, leading to aberrant activation of AKT kinase activity as well as its downstream effectors, including the mTOR signaling pathway [31-33]. Further, many prostate tumors exhibit deregulated growth factor signaling resulting in activation of the ERK MAP kinase signaling [34, 35]. Notably, previous studies have demonstrated that the AKT/mTOR and MAPK signaling pathways are alternatively and/or coordinately expressed in advanced prostate cancer and function cooperatively to promote tumor growth and the emergence of hormone-refractory disease [35-39].
Treatment of cells with fisetin inhibited mTOR activity and downregulated components of the mTOR complexes Raptor, Rictor, PRAS40 and GβL that resulted in loss of formation of mTOR complexes 1 and 2 [30]. Using in silico molecular modeling studies, we found that fisetin physically interacts with the mTOR molecule and binds at two distinct sites with binding energies in the range of -7 to -8 kcal/mol [40]. These interactions could interfere with the formation of the mTOR complexes. The mTOR complex 1 promotes mRNA translation and inhibits autophagy by integrating nutrient signals that are generated by amino acids, growth factors, various stressors including hypoxia and DNA damage [41]. The mTOR complex 2 is activated by growth factor receptors and forms a core component of the phosphoinositide 3-kinase (PI3K)/Akt pathway [41]. The two mTOR complexes are linked through Akt since mTORC2-activated Akt activates mTORC1 through phosphorylation of TSC2 and PRAS40. Clinical trials with mTOR inhibitors such as rapamycin have proved disappointing since chronic administration results in activation of the Akt [42]. It is recommended that dual inhibition of mTOR and other signaling pathways such as PTEN/PI3K/Akt would be an effective strategy in targeting cancer. As prostate cancer advances mutations in the tumor suppressor PTEN result in hyperactivation of Akt that subsequently leads to activation of mTOR signaling. Our observations that fisetin targets not only the mTOR signaling pathway but also the phosphorylation of AKT are novel and could have clinical potential.
Activated mTOR transmits its signal to p70-S6 kinase to activate it and conducts negative signals to eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), which binds and inhibits eukaryotic translation initiation factor (eIF) 4E [41]. Downstream of the mTOR complex, 4E-BP1 plays a key role in cell proliferation, and its inactivation is suggested to directly contribute to the growth of cancers [43]. It is suggested that mTORC1-mediated inhibition of 4E-BP1, activates eIF4E which preferentially drives the translation of mRNAs for pro-tumorigenic genes, including cell cycle regulators [41]. Interestingly, we observed inhibition of downstream targets of mTOR including p70-S6 kinase by fisetin. Fisetin-mediated inhibition of mTOR resulted in hypophosphorylation of 4E-BP1 and suppression of Cap-dependent translation and induction of autophagy [30]. We also observed that fisetin induces autophagy in prostate cancer PC3 cells leading to autophagic cell death rather than cytoprotective autophagy. Autophagy is a catabolic process involving physiological turnover of long-lived proteins and damaged organelles thereby promoting cell survival. It is being increasingly investigated as a new target for cancer therapy. Our data that fisetin suppresses Cap-dependent translation and induces autophagic cell death through inhibition of both mTORC1 and mTORC2 complexes provides a good rationale for further research and development of fisetin as a dual mTORC1/2 inhibitor against prostate cancer. Fisetin may be beneficial to patients with mixed population of prostate cancer cells. Therefore, the observed effects of fisetin in prostate cancer cells are quite interesting and could have clinical relevance.
Using a unique family of prostate epithelial cells (RWPE-1, WPE1-NA22, WPE1-NB14, WPE1-NB11 and WPE1-NB26) which mimic steps in prostate carcinogenesis and progression [44] we observed that mTOR signaling increased in cells with increasing tumorigenic potential [45]. Further, we also observed that cells with higher mTOR signaling were more prone to cell growth inhibition by fisetin. Cells expressing higher mTOR signaling formed cell number of colonies in presence of fisetin. Greater efficacy of fisetin in the inhibition of PI3K, Akt, mTOR and p-S6K70 in cells expressing higher mTOR signaling was also observed [45]. These results suggested that the mTOR signaling plays an essential role in the tumorigenesis of prostate cancer and mTOR specific inhibitors may be promising anticancer therapeutics.
Fisetin effects on the mTOR pathway were similarly observed using human non-small cell lung cancer A549 cells [40]. Fisetin treatment reduced formation of A549 cell colonies that was associated with decrease in the protein expression of PI3K, inhibition of phosphorylation of Akt, mTOR, p70S6K1, eIF-4E and 4E-BP1. Fisetin-treated cells also exhibited dose-dependent inhibition of the constituents of mTOR signaling complex Rictor, Raptor, GβL and PRAS40.
CONCLUSIONS
The mTOR pathway drives a feedback loop and keeps the PI3K/Akt activity under tight control. One consequence of mTOR inhibition is alleviation of this negative feedback loop resulting in activation of PI3K and subsequent activation of Akt. Therefore, simultaneously targeting of both PI3K/Akt and mTOR has the potential to inhibit both upstream and downstream signaling in the pathway. By physically interacting with the mTOR molecule and inhibiting its activity, fisetin treatment of human prostate cancer cells inhibits mTOR signaling both upstream and downstream. Additionally, fisetin also inhibits the PI3K/Akt pathway which ensures that the feedback loop remains in check. Our findings suggest that fisetin inhibits the mTOR pathway in addition to inhibition of the PI3K/Akt pathway. In addition fisetin has been shown to enhance cytotoxic effects when combined with other known chemotherapeutic drugs [46-48]. Fisetin also sensitized TRAIL-resistant androgen-dependent LNCaP and the androgen-independent DU145 and PC3 prostate cancer cells to TRAIL-induced death [49]. These observation sunderscore the importance of testing fisetin in combination with known and conventional chemotherapeutic drugs. Therefore, based on these observations fisetin could be developed as a novel agent for the management of prostate cancer.
Figure 1. Graphic representation of the effect of fisetin on PI3K/Akt/mTOR signaling.
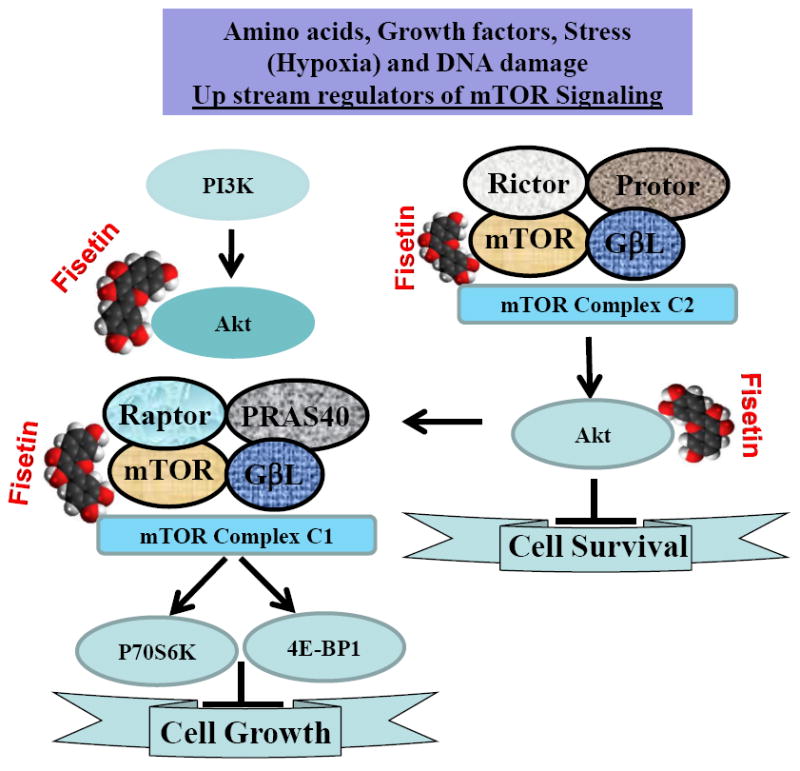
Simultaneously targeting both PI3K/Akt and mTOR has the potential to inhibit both upstream and downstream signaling in the pathway. Fisetin inhibits the mTOR pathway and keeps the feedback loop in check by also inhibiting the PI3K/Akt pathway and inhibits cell survival and growth.
Acknowledgments
The original work from the author’s (H Mukhtar) laboratory outlined in this paper was supported by United States Public Health Service Grant RO1 CA 160867.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 2.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8:168–75. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 5.Shia CS, Tsai SY, Kuo SC, Hou YC, Chao PD. Metabolism and pharmacokinetics of 3,3’,4’,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J Agric Food Chem. 2009;57:83–9. doi: 10.1021/jf802378q. [DOI] [PubMed] [Google Scholar]
- 6.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–25. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 8.Gabor M, Eperjessy E. Antibacterial effect of fisetin and fisetinidin. Nature. 1966;212:1273. doi: 10.1038/2121273a0. [DOI] [PubMed] [Google Scholar]
- 9.Sagara Y, Vanhnasy J, Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem. 2004;90:1144–55. doi: 10.1111/j.1471-4159.2004.02563.x. [DOI] [PubMed] [Google Scholar]
- 10.Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103:16568–73. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng LT, Ock J, Kwon BM, Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int Immunopharmacol. 2008;8:484–94. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 13.Touil YS, Fellous A, Scherman D, Chabot GG. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr Cancer. 2009;61:310–21. doi: 10.1080/01635580802521346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmela AL, Pouwels J, Varis A, Kukkonen AM, Toivonen P, Halonen PK, et al. Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint. Carcinogenesis. 2009;30:1032–40. doi: 10.1093/carcin/bgp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Shih CM, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–9. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol. 2002;63:225–36. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 17.Liao YC, Shih YW, Chao CH, Lee XY, Chiang TA. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57:8933–41. doi: 10.1021/jf902630w. [DOI] [PubMed] [Google Scholar]
- 18.Ravichandran N, Suresh G, Ramesh B, Siva GV. Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem Toxicol. 2011;49:1141–7. doi: 10.1016/j.fct.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–7. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen WS, Lee YJ, Yu YC, Hsaio CH, Yen JH, Yu SH, et al. Enhancement of p53-mutant human colorectal cancer cells radiosensitivity by flavonoid fisetin. Int J Radiat Oncol Biol Phys. 2010;77:1527–35. doi: 10.1016/j.ijrobp.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Jung J, Cho HJ, Lim DY, Lee HS, Chun HS, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–90. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 22.Yu SH, Yang PM, Peng CW, Yu YC, Chiu SJ. Securin depletion sensitizes human colon cancer cells to fisetin-induced apoptosis. Cancer Lett. 2011;300:96–104. doi: 10.1016/j.canlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002;63:1165–76. doi: 10.1016/s0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 25.Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, et al. Antiproliferative mechanisms of the flavonoids 2,2’-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr Cancer. 2010;62:668–81. doi: 10.1080/01635581003605524. [DOI] [PubMed] [Google Scholar]
- 26.Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz S, Zaid MA, et al. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin in pigmented fruits and vegetables. Int J Cancer. 2008;123:1508–15. doi: 10.1002/ijc.23675. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan N, Asim M, Afaq FM, Abu Zaid M, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–63. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–80. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 30.Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–33. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–71. [PubMed] [Google Scholar]
- 32.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–6. [PubMed] [Google Scholar]
- 33.Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, et al. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–12. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 34.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 35.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–84. [PubMed] [Google Scholar]
- 36.Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–54. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci USA. 2006;103:14477–82. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64:6190–9. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- 39.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130:1695–705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–6. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webber MM, Quader ST, Kleinman HK, Bello-DeOcampo D, Storto PD, Bice G, et al. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. Prostate. 2001;47:1–13. doi: 10.1002/pros.1041. [DOI] [PubMed] [Google Scholar]
- 45.Adhami V, Lall RK, Mukhtar H. Fisetin, a dietary flavonoid and a novel mTOR inhibitor, for treatment and prevention of prostate cancer. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31- Apr 4; Chicago, IL Philadelphia (PA). AACR; 2012. Abstract. [Google Scholar]
- 46.Tripathi R, Samadder T, Gupta S, Surolia A, Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol Cancer Ther. 2011;10:255–68. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- 47.Kandaswami C, Perkins E, Soloniuk DS, Drzewiecki G, Middleton E., Jr Ascorbic acid-enhanced antiproliferative effect of flavonoids on squamous cell carcinoma in vitro. Anticancer Drugs. 1993;4:91–6. doi: 10.1097/00001813-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Touil YS, Seguin J, Scherman D, Chabot GG. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445–55. doi: 10.1007/s00280-010-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szliszka E, Helewski KJ, Mizgala E, Krol W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int J Oncol. 2011;39:771–9. doi: 10.3892/ijo.2011.1116. [DOI] [PubMed] [Google Scholar]


