Abstract
Background
Ability of the heart to undergo pathological or physiological hypertrophy upon increased wall stress is critical for long-term compensatory function in response to increased workload demand. While substantial information has been published on the nature of the fundamental molecular signaling involved in hypertrophy, the role of extracellular matrix (ECM) protein Fibronectin (Fn) in hypertrophic signaling is unclear.
Objective
Delineate the role of Fn during pressure overload-induced pathological cardiac hypertrophy and physiological growth prompted by exercise.
Methods and Results
Genetic conditional ablation of Fn in adulthood blunts cardiomyocyte hypertrophy upon pressure overload via attenuated activation of Nuclear Factor of Activated T cells (NFAT). Loss of Fn delays development of heart failure and improves survival. In contrast, genetic deletion of Fn has no impact on physiological cardiac growth induced by voluntary wheel running. Down regulation of the transcription factor c/EBPβ (Ccaat-enhanced binding protein β), which is essential for induction of the physiological growth program, is unaffected by Fn deletion. Nuclear NFAT translocation is triggered by Fn in conjunction with up-regulation of the fetal gene program and hypertrophy of cardiomyocytes in vitro. Furthermore, activation of the physiological gene program induced by Insulin stimulation in vitro is attenuated by Fn, whereas Insulin had no impact on Fn-induced pathological growth program.
Conclusion
Fn contributes to pathological cardiomyocyte hypertrophy in vitro and in vivo via NFAT activation. Fn is dispensable for physiological growth in vivo, and Fn attenuates the activation of the physiological growth program in vitro.
Keywords: cardiomyocytes, pathological and physiological hypertrophy, fibronectin, heart failure
Introduction
Hypertrophy is the primary phenotypic characteristic of cardiomyocytes in response to increased functional demand that reduces wall stress and maintains cardiac output. Physiological hypertrophy is induced by endurance exercise, resulting in enhanced cardiac function with increased output under stress. In contrast, pathological hypertrophy due to pressure or volume overload ultimately leads to decompensated heart failure (HF) associated with reduced survival. Priming cardiac tissue with physiological hypertrophy decreases susceptibility to failure if the heart is secondarily stressed with a pathological stimulus in rodents [23]. Supervised exercise training in HF patients improves clinical presentation, hospitalization rates and most likely survival [30]. Therefore, striking a balance between physiological versus pathological hypertrophic growth programs is critical to maximize successful long-term outcome.
Pathological hypertrophic growth is characterized by Induction of natriuretic peptides (Atrial Natriuretic Peptide (ANP), Brain Natriuretic Peptide (BNP)) and reactivation of the fetal gene program, namely α – skeletal actin and β – myosin heavy chain (αSA, βMHC). The calcineurin - Nuclear Factor of Activated T cell (Cn-NFAT) cascade is a major signaling pathway essential and sufficient for pathological hypertrophy, but seemingly irrelevant for physiological growth [43]. Activated Cn dephosphorylates NFAT resulting in nuclear translocation and activation of the pro-hypertrophic fetal gene program [12, 41]. Regulator of CAlcineuriN 1.4 (RCAN1.4) expression is induced due to several NFAT binding motifs in its promoter region and correlates with NFAT cascade activation [28]. In contrast, physiological growth significantly increases heart weight without reactivation of the fetal gene program or induction of natriuretic peptides [22]. Down-regulation of the transcription factor c/EBPβ (Ccaat-enhanced binding protein β plays a decisive role in physiological hypertrophy through induction of a distinct transcriptional program involving increased expression of αMHC, CITED4 (cbp/p300 interacting transactivator 4) and PGC1α (Peroxisome proliferator-activated receptor Gamma Coactivator 1α) [4]. Although induction of PGC1α expression might me controversial in the literature [19].
Extracellular matrix (ECM) is a pivotal component of myocardial remodeling responses during homeostasis and pathological injury, providing both structural support as well as essential signaling cues. Expression of the ECM protein Fibronectin (Fn) is elevated during early postnatal development and declines with progression to adulthood [8]. After pathological injury such as myocardial infarction or pressure overload, Fn expression is re-induced as a characteristic part of pathologic remodeling [14, 18]. Short term induction of Fn following pathological injury may be tied to a beneficial role in myocardial reparative response by promoting cardiac resident stem cell proliferation [13, 16]. Determination of the role for Fn in the context of adult myocardial remodeling has proven challenging since genetic deletion of Fn results in embryonic lethality [16]. As such, involvement of Fn for physiological as well as pathological cardiomyocyte growth in the adult heart has not been determined. Published studies indicate that hypertrophy of neonatal cardiomyocytes in vitro is induced by Fn [6, 27, 39], but no further assessment of Fn participation in hypertrophic growth of the intact heart has been described. Therefore, this study details the role of Fn in pathological growth induced by transaortic constriction (TAC) or alternatively voluntary wheel running for induction of physiological growth. An inducible genetic deletion of Fn in transgenic mice was established and tested in both models to elucidate the role of Fn on the balance of both described growth programs.
Methods
Animals
To create inducible Fn knockdown animals, mice expressing Tamoxifen-sensitive Cre under CMV-enhanced global actin promoter (Cre+/−, Jackson, #004682) were crossed for two generations with mice homozygous floxed for fibronectin (Fnfl/fl, generously provided by Prof. Dr. Reinhard Fässler) [35]. The obtained mice (Cre+/− Fnfl/fl) were breed with Fnfl/fl animals for multiple generations to build up the colony. Both mice strains have a C57BL/6 background. At the age of 8 weeks all mice received Tamoxifen intra-peritoneally for 10 days 1mg/day resuspended in a 1:10 mixture of 100% ethanol in sunflower oil to induce recombination. Cre+/− Fnfl/fl mice were considered knockdown animals, whereas Cre negative littermates represent controls. The experiments were started at the age of 12 weeks. The 4 week window allowed successful knockdown of Fn since the half-life time for Fn has been reported to be between 13 hours and 3.5 days [32] [29]. The 4-week voluntary running experiment has been performed as described recently [5]. The Institutional Animal Care and Use Committee of San Diego State University approved all animal procedures and treatments.
Cardiomyocyte isolation, treatment and culture
Neonatal rat cardiomyocytes (NRCMs) were isolated from hearts of 1- to 2-day-old neonatal rats by trypsin digestion using standard procedures. After enzymatic digestion, cell preparations were preplated for 2hours in M199 medium (Cell-Gro) with 15% fetal bovine serum (FBS) to reduce non-myocyte contamination. NRCMs were plated in dishes pretreated with gelatin (0.1%, Sigma), or Fn (20 μg/mL) together with gelatin for 2 hours at 37°C. For cellular hypertrophy experiments in vitro, NRCMs were cultured with low serum media (0.5% FCS) for 48 hours.
Surgical procedures and echocardiographic assessment
Twelve week old male animals were anesthetized under isoflurane 2%, intubated, and ventilated. A thoracotomy was performed and the aorta has been ligated using a 7-0 suture between the innominate and left common carotid artery with an overlying 26-gauge needle. After ligation the needle was withdrawn. Consecutive non-invasive assessment of cardiac function in the parasternal short axis view in M-mode has been performed after 1, 2, 4, 6 and 8 weeks post surgery. Echocardiographic assessment of function as well as surgery and data analysis were performed in a blinded fashion. Echocardiography was performed under mild isoflurane sedation (.5 –1.5%) using a Vevo 770 High resolution system. For histochemistry hearts were arrested in diastole using high cadmium solution and perfused with phosphate-buffered formalin for 15 minutes (Sigma-Aldrich) via retrograde canulation of the abdominal aorta. Retroperfused hearts were removed from the chest cavity and placed in formalin for at least another 24 hours. Alternatively for whole heart lysates, hearts were removed directly and frozen down in liquid nitrogen until further processing.
(Immuno-)histochemistry
Heart sections were deparaffinized and rehydrated using consecutive incubations in alcohol with decreasing concentrations and finally incubated in destilled water. For antigen retrieval specimen were boiled for 15 minutes in citrate (1 mM, pH6.0) using a standard microwave oven followed by a 1-hour block in TNB buffer from Perkin Elmer, Woodbridge, Ontario. If horseradish peroxidase amplification based detection was needed endogenous peroxidase activity was blocked by an additional incubation with 3% hydrogen peroxide for 20 minutes at room temperature. Primary antibodies were incubated overnight at 4°C in TNB. After 3 wash-steps in Tris/NaCl secondary antibodies were incubated for 1.5 hours at room temperature. For amplification Tritc or Fitc based tyramide reagent pack from Perkin Elmer were used according manufacturers instructions. Specimens were mounted with Vectashield (Vector laboratories, Burlingame). Sytox blue was given 1:2000 into the mounting solution to stain for nuclei or topro dye was given in the last washing step after incubation with secondary antibodies at 1:5000. Masson Trichrome stainings were performed as per manufacturer instructions (Trichomes Accustain (Masson), Sigma-Aldrich). Micrographs were acquired using a Leica DMRE confocal microscope. For cardiomyocyte cell size measurements, only strictly cross-sectional cells were analyzed in micrographs taken at the anterior, posterior, septal and lateral wall at mid-ventricular position. At least 10 cells were analyzed at each location per field in every heart resulting in >40 cells per heart. Observers were blinded to the experimental group when scanning the slides as well as analyzing the data.
Antibodies
A complete list of applied antibodies is provided in the supplemental files.
NFAT translocation assay
NRCMs were transduced with adenovirus harboring eGFP-NFAT in low serum medium overnight. The next day cells were refed with fresh medium and after another 24 hours cells were fixed with 4% paraformaldehyde and stained with antibodies to detect desmin and nuclear dyes. Total number of GFP+ cells and number of GFP+ nuclei were counted in a blinded fashion. Percentage of bright GFP+ nuclei relative to all GFP+ cells was calculated. For in vivo analysis, twelve-week-old mice underwent intubation anesthesia as described above, and at 6 positions along the long axis of the left ventricle 5μL of NFAT-GFP adenovirus with a concentration of 109 pfu were injected per animal. Two days following viral delivery, animals underwent TAC or sham surgery as described earlier. Another 2 days later hearts were harvested for retroperfusion. Percentage of bright GFP+ nuclei relative to all GFP+ cardiomyocytes was calculated.
Sample preparation, Immunoblotting, qRT-PCR
Whole heart and isolated cell lysates were prepared as described [25]. Immunoblotting was performed using standard procedures. Protein samples were loaded onto a 4–12% NuPAGE Novex Bis-Tris Gel (Invitrogen) for electrophoresis. Separated proteins were then transferred onto a polyvinylidene fluoride (PVDF) membrane, blocked with 7% skim milk in Tris-Buffered Saline Tween-20 (TBST) for 1 h at room temperature, and exposed to primary antibodies over night. Alkaline phosphatase (AP), horseradish peroxidase (HRP), Fitc- or Cy5-conjugated IgGs (Jackson ImmunoResearch, West Grove, PA) were used as secondary antibodies. Fluorescence signal was detected and quantified by using a Typhoon 9400 fluorescence scanner together with ImageQuant 5.0 software (Amersham Biosciences). mRNA was enriched using the Quick RNA Mini Prep kit from ZymoResearch according to the manufacturers instructions. cDNA was transcribed using the cDNA preparation kit from Biorad. For RT-PCR Sybr green (Biorad) was used following the manufacturer protocol. Data were analyzed with the ΔΔC(t) method. A complete list of the used primers is provided in the supplemental files.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0 (Graphpad Software Inc; www.graphpad.com). P values <0.05 were considered significant. To compare two groups with normal distribution Student’s t-test was applied, otherwise a non-parametric test was used. For comparision of more than two groups 1-way ANOVA was applied, for the echocardiographic time course analysis 2-way ANOVA was used, in both cases inclusive Bonferroni post hoc tests.
Results
Loss of Fn does not interfere with physiological growth due to voluntary exercise
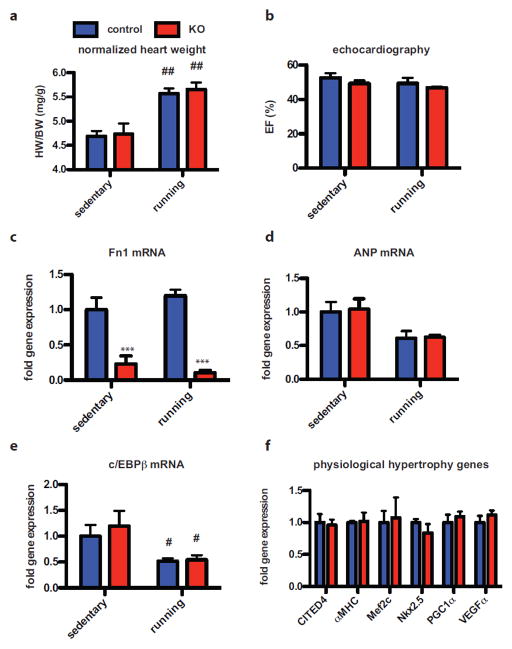
Germline deletion of Fn is embryonic lethal with cardiovascular malformation, so studies in adult mice involving Fn deletion required establishment of a conditional global Fn knock out strain [16]. Mice possessing heterozygous expression of Cre-ERT under a CMV-enhanced β-actin promoter were crossed with mice carrying floxed Fn gene leading to loss of Fn expression after tamoxifen (Tx)-induced recombination (knockout animals, KO). A globally expressed promoter was chosen to eliminate Fn rather than a cardiomyocyte-specific deletion since Fn is secreted by heterogeneous cell types within the heart and unlikely to be efficiently removed by excision from only myocytes within the myocardium. After mice had completed physiological cardiac growth at 8 weeks of age, Tx was injected for 10 days in KO and controls. One month later at 12 weeks of age both control and Tx-treated groups were subjected to voluntary wheel running. The intervening 4-week window allowed recombination and consecutive loss of Fn as well as recovery from transient cardio-depression due to Cre activation by Tx in the heart (suppl. Fig. 1a–b) [2, 15]. Heart weight to body weight ratio (HW/BW) increased similarly about 20% four weeks after training in control and KO animals (Fig. 1a). Cardiac function was stable and indistinguishable between both groups assessed by echocardiography (Fig. 1b). Successful Fn knock out (Fig. 1c) and lack of ANP induction upon exercise were confirmed with RT-PCR (Fig. 1d) also without any difference between the groups. Recently, down-regulation of c/EBPβ has been identified as central signal in physiological hypertrophy [4]. c/EBPβ expression decreased similarly after exercise by 48% in controls and 46% in KO mice, respectively (Fig. 1e). Also, the c/EBPβ dependent gene program was not significant different between the two groups after training as expression of CITED4, αMHC, Mef2c, Nkx2.5, PGC1α and VEGFα were comparable (Fig. 1f). Knockdown efficiency for other organs is provided as suppl. Fig. 1d. In summary, loss of Fn has no impact on physiological hypertrophy with regard to cardiac function and activation of underlying c/EBPβ-dependent transcriptional program due to voluntary wheel running.
Figure 1. Loss of Fn does not impact physiological growth after voluntary wheel running.
(a) Heart weight to body weight ratio (HW/BW) after voluntary wheel running in control and KO mice. (b) Cardiac function assessed as ejection fraction (EF) is stable in both groups after exercise. (c) RT-PCR confirming successful knock out of Fn without induction of ANP (d) after exercise. (e) Exercise induced decrease in c/EBPβ is similar in control and KO mice. (f) There is no significant difference in the c/EBPβ dependent physiological growth program between controls and KO mice after exercise. ***: p<0.001 compared to control. #: p<0.05; ##: p<0.01 compared to sedentary.
Loss of Fn attenuates ventricular hypertrophy upon pressure overload and delays development of heart failure
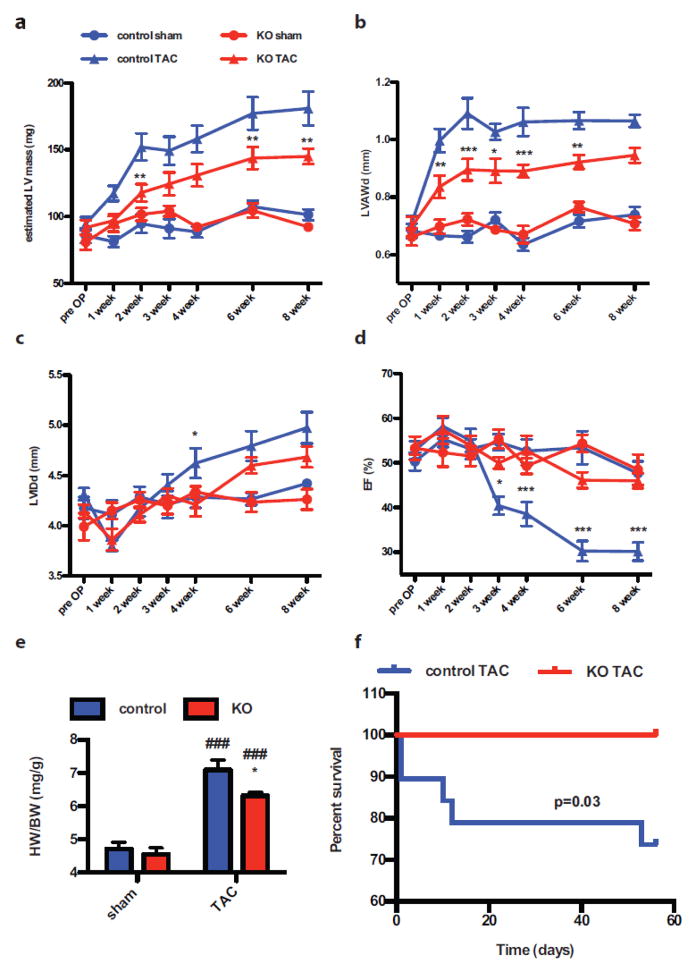
Pathological hypertrophy was induced by transaortic constriction (TAC) in parallel experiments using the same time course for Tx-treatment and recovery (suppl. Fig. 1b) as described for exercise-induced hypertrophic studies (Fig. 1). Left ventricular (LV) mass estimated by echocardiography increased by 78% starting as early as one week after TAC (Fig. 2a) in controls. Increase in LV anterior wall thickness in diastole (LVAWd) was detectable at one week post-TAC and reached a plateau for the remaining seven weeks (44% increase) (Fig. 2b). Cardiac ejection fraction (EF) was stable for the first 2 weeks post surgery and heart failure became overt at 3 weeks in parallel with a continuous dilatation of the left ventricle measured as LV internal diameter in diastole (LVIDd, Fig. 2c–d) in the control group. EF decreased to 30% and LVIDd reached 4.98mm in control mice at the end of the observation period. Hypertrophic response was attenuated in KO mice as the estimated LV mass increased later than in controls and was significantly reduced at any given time point reaching 57% increase at the end of the observation (Fig. 2a). Also LVAWd increase was attenuated in KO mice (33% increase) (Fig. 2b). Most importantly, cardiac function was preserved during the entire 8 week-study, however, left ventricular dilatation began at 6 weeks post TAC, reaching 4.7mm at the 8 week time point (Fig. 2c–d). Heart rate during echocardiographic analysis was similar between groups (suppl. Fig. 1c). HW/BW ratio was increased in the controls after 8 weeks of TAC by 56% and significantly lower in KO animals (41% gain) (Fig. 2e), supporting the echocardiographic findings. Finally, a clear survival benefit in the Kaplan-Meyer mortality analysis was observed for KO animals (Fig. 2f) as 5 out of 19 control animals died after TAC and all 15 KO mice survived the follow-up period. None of the sham treated animals died in either group. Taken together, loss of Fn attenuates cardiac hypertrophy upon TAC, delaying development of heart failure and ultimately improving short term survival.
Figure 2. Loss of Fn attenuates ventricular hypertrophy upon pressure overload and delays development of heart failure.
Knockdown of Fn attenuates increase in echocardiographic estimated left ventricular mass (LV mass (a)), LV anterior wall thickness in diastole (LVAWd (b)) and LV internal diameter in diastole (LVIDd (c)). Cardiac function presented as ejection fraction (EF (d)) is preserved in KO mice after TAC. HW/BW ratio is depicted in (e). Kaplan-Meier curve showing significant survival benefit of KO animals after TAC. None of the sham animals died in both groups. (f). *: p<0.05; **:p<0.01; ***: p<0.001 compared to control. ###: p<0.001 compared to sham.
Loss of Fn blunts cardiomyocyte hypertrophy and NFAT activation upon pressure overload
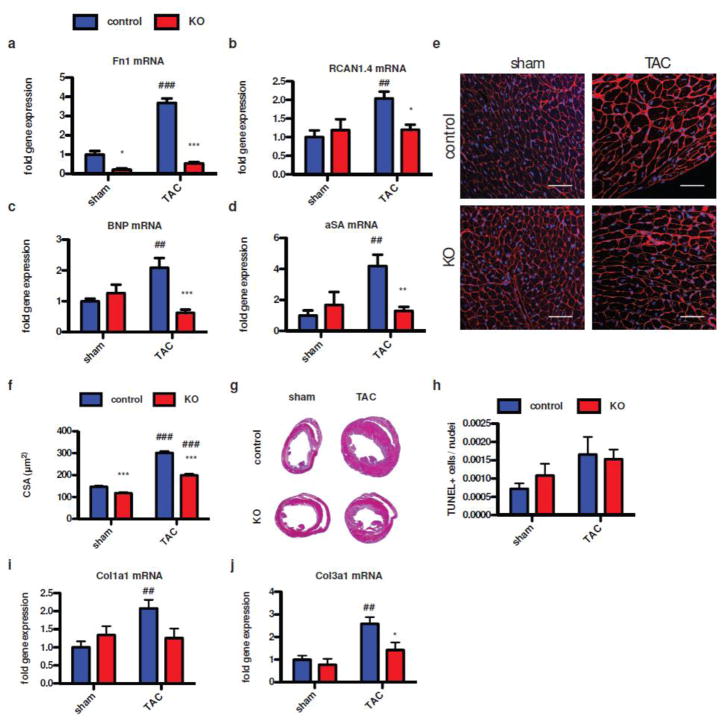
NFAT activation is a major contributor for pathological hypertrophy in response to pathological TAC challenge. Therefore, whole heart lysates 2 days after sham or TAC procedure were prepared from control and KO animals to assess NFAT-related signaling in response to TAC. Under sham condition Fn expression was significantly reduced in KO mice by 79%, confirming efficient down-regulation of Fn in our mouse model. Fn expression was significantly induced after TAC in control animals by 268%, which was markedly reduced in the KO animals after TAC by 46% compared to sham controls (Fig. 3a). Strikingly, RCAN1.4 induction by 100% in controls after TAC was obviated in the KO animals, which showed only 20% induction upon pressure overload (Fig. 3b). Consistent with functional data, expression levels of natriuretic peptides and the pro-hypertrophic fetal gene program were significantly blunted in the KO animals (Fig. 3c–d). Analysis of cross sectional area of left ventricular cardiomyocytes confirmed attenuated hypertrophic response in KO mice after TAC compared to controls at the single cell level (Fig. 3e–f). In controls, cross sectional area increased from 147mm2 to 302 mm2, whereas KO animals showed 118mm2 under sham condition, increasing to 199mm2 only after TAC. Representative midventricular sections after Masson-Trichrome staining are depicted in Figure 3g for control and KO mice after sham and TAC procedure. TAC procedure induced increase in number of TUNEL positive cells, however, no significant difference was seen between control and KO animals (Fig. 3h). In contrast, analysis of fibrosis using RT-PCR for collagen 3α1 (Col3a1) and 1α1 (Col1a1) expression revealed reduction of both isoforms in the KO animals upon TAC procedure compared to control (Fig. 3i and j). Furthermore, representative immunohistochemistry specimen and immunoblots depicting Fn expression on protein level are provided as suppl. Figure 2. In summary, loss of Fn attenuates NFAT-dependent gene expression and cardiomyocyte hypertrophy in vivo.
Figure 3. Loss of Fn blunts cardiomyocyte hypertrophy and NFAT activation upon pressure overload.
Gene expression analysis 2 days post TAC confirming successful Fn knock out (a). Loss of Fn prevents NFAT activation measured as RCAN1.4 induction (b) and attenuation of NFAT regulated genes as BNP (c) and alpha-skeleton actin (d). Immunohistochemistry of wheat-germ-agglutinin (WGA) staining showing representative images of the hypertrophic response on the single cell level for sham (left) and after TAC (right) in control (top) and KO (bottom) animals 3 weeks after TAC (scale: 50μm) (e). (f) Quantitative analysis of the cross-sectional area under the conditions as depicted in e. (g) Masson-Trichrome stainings of mid-ventricular sections in the short axis for the same conditions as depicted in e and f. (h) TUNEL analysis for apoptotic cells is depicted. Expression of collagen isoforms 1α1 (i) and 3α1 (j) were quantified using RT-PCR. *: p<0.05; **:p<0.01; ***: p<0.001 compared to control. ##: p<0.01; ###: p<0.001 compared to sham.
Fn induces hypertrophy and NFAT activation in cardiomyocytes
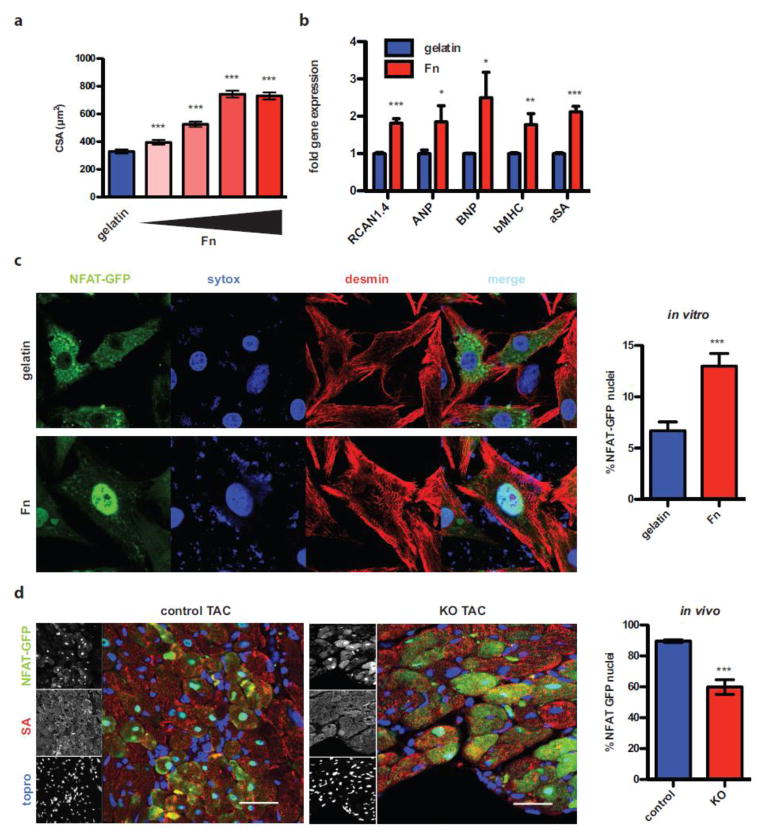
To test direct functional impact of Fn on cardiomyocyte biology, NRCMs were plated on Fn or gelatin coated dishes in vitro and cell area was quantified. Fn induced dose-dependent hypertrophy of cardiomyocytes (Fig. 4a). Upon dephosphorylation, NFAT translocates into the nucleus, so tagging NFAT with GFP allows direct subcellular visualization of NFAT nuclear translocation. NRCMs were infected with adenovirus encoding NFAT-GFP fusion protein (AdNFAT-GFP) [21]. Percentages of clearly fluorescent NFAT+ nuclei relative to all infected GFP+ cardiomyocytes were determined in a blinded fashion. Fn induced significant increases in the percentage of cells with GFP+ nuclei consistent with cell size data (Fig. 4c). NFAT-dependent transcriptional activation was analyzed by RT-PCR to assess functional relevance of NFAT translocation. Fn induced significant up-regulation of RCAN1.4, natriuretic peptides (ANP and BNP) as well as fetal genes (αSA, βMHC) (Fig. 4b). The significance of Fn for nuclear translocation of NFAT in vivo was assessed in mice, which had received intra-myocardial injection of AdNFAT-GFP two days before TAC surgery. At day two after TAC, GFP+ cardiomyocyte nuclei were counted relative to all GFP+ cardiomyocytes similar to the in vitro protocol. KO hearts showed significantly reduced GFP+ nuclei compared to controls (Fig. 4d and suppl. Figure 3 showing low magnification images for Fig. 4 c and d). To test whether NFAT activation involves calcineurin, NRCMs were treated with cyclosporine A (CsA) and the pathological gene program was analyzed upon Fn stimulation. As depicted in suppl. Figure 3c, CsA attenuated the induction of the fetal genes. Collectively, these results support the postulate that Fn activates NFAT via calcineurin leading to cardiomyocyte hypertrophy in vitro and in vivo.
Figure 4. Fn induces hyertrophy and NFAT activation in cardiomyocytes.
(a) Cross-sectional analysis of cardiomyocytes plated on gelatin or increasing concentrations of Fn (0.3125μg/mL, 1.25μg/mL, 5μg/mL, 20μg/mL). (b) RT-PCR assessment of NFAT regulated genes after stimulation with Fn (20μg/mL). (c) NRCM plated on gelatin (top) or Fn (20μg/mL, bottom) were infected with AdNFAT-GFP. Quantification of GFP positive nuclei relative to all GFP positive cardiomyocytes (right). (d) Mice received AdNFAT-GFP via intra-myocardial injection 2 days before surgery. Hearts were harvested 2 days after TAC. Control mouse (left) and KO animal (right) after TAC are depicted (scale: 40μm). Quantification thereof as described in c. *: p<0.05; **:p<0.01; ***: p<0.001 compared to control.
Fn attenuates activation of physiologic growth reprogramming in vitro
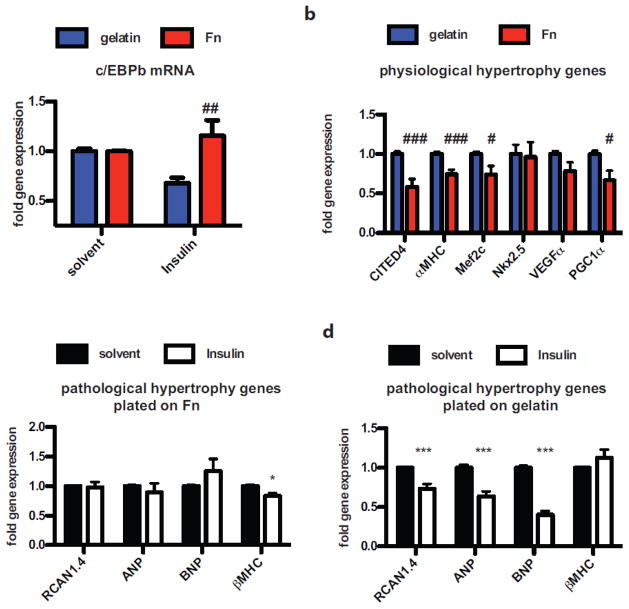
Fn gene expression was unaltered in healthy adult hearts before and after exercise (Fig 1c), but Fn is sufficient for induction of pathological growth in vitro (Fig. 3 and 4). Therefore, the impact of Fn upon physiological hypertrophy signaling was assessed by mimicking induction of physiological growth in vitro by insulin stimulation in the presence of gelatin or Fn. Insulin stimulation reduced c/EBPβ expression in cardiomyocytes plated on gelatin (Fig. 5a), but cells plated on Fn were insensitive for insulin-induced c/EBPβ reduction. Induction of c/EBPβ dependent transcriptional reprogramming was attenuated in Fn-treated cardiomyocytes as expression of CITED4, αMHC, MEF2c and PGC1α were significantly reduced after insulin treatment compared to gelatin-plated cells (Fig. 5b). Induction of physiological growth is beneficial, so experiments were performed to determine if insulin treatment would attenuate Fn-induced hypertrophic reprogramming in cultured cells. Interestingly, Fn-induced activation of natriuretic peptides (ANP, BNP) and the fetal genes (βMHC) was not blunted by concurrent insulin stimulation (Fig. 5c). These same hypertrophy-associated genes were markedly reduced by insulin stimulation when cells were plated on gelatin (Fig. 5d). In summary, Fn promotes pathological hypertrophic reprogramming in vitro and in vivo and presence of Fn attenuates activation of the physiological growth program in vitro.
Figure 5. Fn attenuates activation of the physiological gene program in vitro.
(a) c/EBPβ expression quantified by RT-PCR in cardiomyocytes plated on gelatin or Fn stimulated with solvent or Insulin. Data are normalized to respective solvent control. (b) Physiological gene program in cardiomyocytes plated on gelatin or Fn after Insulin treatment. (c) Pathological gene program in cardiomyocytes plated on Fn after solvent or Insulin stimulation. (d) Pathological gene program in cardiomyocytes plated on gelatin after stimulation with solvent control or Insulin. *: p<0.05; ***<0.001 compared to solvent; #: p<0.05; ##: p<0.01 compared to gelatin.
Discussion
Cellular hypertrophy is an essential growth response of post-mitotic myocytes challenged by increased contractile demand to improve hemodynamic output and decrease wall stress. The overall balance of multiple concurrent and sequential stimuli dictates whether the heart as an organ rises to challenge with physiological beneficial growth leading to increased cardiac output or pathological remodeling ultimately leading to HF and increased mortality. Molecular signaling literature is rife with examples of proteins that exacerbate or mitigate myocardial responses to hypertrophic challenge [11, 40] [20] [34]. Results presented here establish Fn up-regulation after TAC challenge as a contributory factor in pathological cardiomyocyte hypertrophy in vivo. Adult-onset conditional genetic deletion of Fn delays myocardial hypertrophic remodeling upon pressure overload and thereby improves short term survival. The hypertrophic reprogramming induced by Fn appears to be mediated through NFAT signaling in vivo and in vitro. In contrast, physiological growth, known to be NFAT-independent, is unaffected by Fn deletion as evidenced by induction of the established c/EBPβ-mediated transcriptional program and functional outcome comparable to wild-type mice. Lack of Fn expression is permissive for physiological growth in cultured cardiomyocytes, whereas presence of Fn attenuates activation of the physiological transcriptional program. Therefore, Fn mediates cardiac hypertrophy allowing physiological growth when absent or contributing to pathological growth upon induction.
Changes in the ECM and matricellular proteins after pressure overload are well described in literature[10, 38]. Also induction of Fn in the heart upon pressure overload is well documented leading to speculation about promotion of myocyte growth by Fn in vivo[36] and in vitro. Similarly, Fn expression corrrelates with remodeling secondary to the underlying pathology of the stressed heart such as TAC[36], volume overload[31], myocardial infarction[1], hypertension[7, 36], rejection after transplantation[3] or myocarditis[17]. Furthermore, typical growth inducing factors as isoproterenol or angiotensin II do induce Fn expression, which might contribute to the pro-hypertrophic response of cardiomyocytes [42] [26]. Up to this point the determination of Fn functional role has not been assessed by genetic deletion in vivo, but results presented here show Fn contributes to myocyte growth upon pressure overload and therefore represents a potential target for therapeutic interference. Fn expression decreases during postnatal development and shows only low level expression in adult organs; therefore, side effects of a putative therapeutic intervention are expected to be minimal in other healthy organs as supported by our finding that loss of Fn under basal conditions, as well as during physiological growth induced by exercise, had no impact on cardiac function. However, Fn is re-expressed upon most types of injury in many organs, therefore, under these conditions systemic therapeutic inhibition of Fn signaling might be critical and the consequences of interference with induction would need to be assessed in tissue-specific fashion.
A necessary methodological limitation in our study is the global knock out model, which was essential since Fn is synthesized in many different cell types upon injury such as smooth muscle cells, endothelial cells, cardiomyocytes or fibroblasts. As such, we can not exclude potential systemic, indirect, non-cardiac effects upon hypertrophy, but in vitro experiments reducing the variables to a minimum recapitulate direct effects of Fn upon cardiomyocyte biology irrespective of the cellular source of Fn in vivo and potential systemic effects. In general, Fn shows low expression in healthy adult tissue and reactivates specifically in damaged organs upon local injury. Four weeks after Tx-treatment to induce Fn gene excision, no cardiac phenotype in echocardiographic assessments or other non-cardiac macroscopic abnormalities were evident. Use of AdNFAT-GFP as well as analysis of cross sectional area of myocytes in vitro and in vivo provides clear evidence of Fn upon NFAT-mediated cardiomyocyte hypertrophy (Fig. 3 and 4). In addition to direct effects of Fn upon cardiomyocytes, Fn as also impacts non-cardiac cell types during cardiac remodeling such as smooth muscle cells or fibroblasts. Potential additional effects of Fn loss upon other cell types within the heart will be the subject of future studies.
Pre-treatment of NRCMs with the calcineurin inhibitor CyclosporinA completely blocked Fn-induced activation of the fetal gene program; therefore, we think calcineurin is involved in the activation of NFAT upon Fn stimulation. In general, calcineurin is activated upon calcium induced calmodulin activation, and there are three potential pathways possible for calcium release: 1) Fn might activate membrane bound calcium channels, which release calcium stored in the sarcoplasmatic reticulum[37], 2) Fn has been shown to co-signal together with Wnt [24]. The non-canonical Wnt/calcium pathway also activates calmodulin upstream of calcineurin, or 3) syndecan-mediated calcineurin activation as proposed recently[9]. This pathway is of particular interest since it has been shown that Fn co-signals with Wnt via syndecan, which would integrate two of these pathways into one signal[24]. Furthermore, knockout of syndecan attenuates pressure overload induced hypertrophy[9]. These proposed signaling events upstream of calcineurin are speculations and require further study.
As shown in our recent publication[16], loss of Fn led to further deterioration of cardiac function in a mouse model of myocardial infarction due to impaired wound healing and reduced progenitor cell recruitment. In the present study, loss of Fn was beneficial for the stressed heart upon pressure overload. Beside the quality of the applied stress, induction of Fn expression upon myocardial infarction was a very quick, high, increase returning to almost the levels before injury within 4 weeks. In contrast, upon pressure overload Fn continuously raised over time at a lower level of expression. Therefore, we speculate that the duration and intensity might impact the ultimate outcome of Fn stimulation. After loss of Fn hearts are still capable to respond with physiological hypertrophy and hearts are protected from pathological remodeling upon pressure overload. Therefore, Fn KO hearts seem to represent an advantageous phenotype regarding hypertrophy. However, in case of ischemic injury loss of Fn is disadvantageous correlating with further reduction of function and impairment of repair. Also in the context of atherosclerosis, blockade of Fn might be disadvantageous because formation of the protective fibrotic cap on the plaque is attenuated and therefore, prone for rupture potentially leading to myocardial infarction[33].
In summary, Fn contributes to the acceleration of pathological hypertrophy and concomitant failure of the heart after pressure overload. Findings presented in this study link Fn signaling to NFAT activation. Taken in the context of pro-regenerative beneficial signals mediated by Fn for stem cells [16], maladaptive effects of Fn in the case of cardiomyocytes highlight the need for further research to tailor induction or inhibition of Fn signals to maximize therapeutic benefit while avoiding loss of beneficial signaling upon selected cell types.
Supplementary Material
Acknowledgments
This study was supported by grants of the National Institute of Health to M. Sussman (R01HL067245, R01HL105759, R01HL113656, R21HL102613, R21HL104544, R21HL102714, R37HL091102, RC1HL100891), and to M. Quintana (SDSU MARC 5T34GM008303-23), by a pre-doctoral fellowship of the American Heart Association to S. Din (12PRE12060248) and the Deutsche Forschungsgemeinschaft DFG (1659/1-1 to M. Völkers and 3900/1-1 to M. Konstandin). We would like to thank Prof. Dr. Reinhard Fässler for generously providing Fnfl/fl mice.
Footnotes
Author Contributions
M.H.K. planned and performed experiments and wrote the manuscript; H.T., G.G., P.Q., A.DTL. M.Q., B.C., S.D., D.A., N.G. performed experiments; M.A.S. supervised all procedures and edited the manuscript.
Disclosures: None. There is no conflict of interest.
References
- 1.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 2.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth AJ, Wood SC, Cornett AM, Dreffs AA, Lu G, Muro AF, White ES, Bishop DK. Recipient-derived EDA fibronectin promotes cardiac allograft fibrosis. J Pathol. 2012;226:609–618. doi: 10.1002/path.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carraway MS, Suliman HB, Jones WS, Chen CW, Babiker A, Piantadosi CA. Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart. Circ Res. 2010;106:1722–1730. doi: 10.1161/CIRCRESAHA.109.214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Huang XN, Yan W, Chen K, Guo L, Tummalapali L, Dedhar S, St-Arnaud R, Wu C, Sepulveda JL. Role of the integrin-linked kinase/PINCH1/alpha-parvin complex in cardiac myocyte hypertrophy. Lab Invest. 2005;85:1342–1356. doi: 10.1038/labinvest.3700345. [DOI] [PubMed] [Google Scholar]
- 7.Crawford DC, Chobanian AV, Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ Res. 1994;74:727–739. doi: 10.1161/01RES.74.4.727. [DOI] [PubMed] [Google Scholar]
- 8.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finsen AV, Lunde IG, Sjaastad I, Ostli EK, Lyngra M, Jarstadmarken HO, Hasic A, Nygard S, Wilcox-Adelman SA, Goetinck PF, Lyberg T, Skrbic B, Florholmen G, Tonnessen T, Louch WE, Djurovic S, Carlson CR, Christensen G. Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway. PloS one. 2011;6:e28302. doi: 10.1371/journal.pone.0028302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2011;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 12.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 13.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992;89:1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstandin MH, Toko H, Gastelum GM, Quijada PJ, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Volkers MJ, Gude NA, Fassler R, Sussman MA. Fibronectin is Essential for Reparative Cardiac Progenitor Cell Response Following Myocardial Infarction. Circ Res. 2013;113:115–125. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leipner C, Grun K, Muller A, Buchdunger E, Borsi L, Kosmehl H, Berndt A, Janik T, Uecker A, Kiehntopf M, Bohmer FD. Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced chronic myocarditis. Cardiovasc Res. 2008;79:118–126. doi: 10.1093/cvr/cvn063. [DOI] [PubMed] [Google Scholar]
- 18.Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20:502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Muhlfeld C, Niemann B, Pan R, Li R, Hilfiker-Kleiner D, Chen Y, Rohrbach S. Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol. 2011;106:1221–1234. doi: 10.1007/s00395-011-0213-9. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Bian ZY, Zhang R, Zhang Y, Liu C, Yan L, Zhang SM, Jiang DS, Wei X, Zhu XH, Chen M, Wang AB, Chen Y, Yang Q, Liu PP, Li H. Interferon regulatory factor 3 is a negative regulator of pathological cardiac hypertrophy. Basic Res Cardiol. 2013;108:326. doi: 10.1007/s00395-012-0326-9. [DOI] [PubMed] [Google Scholar]
- 21.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 25.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 26.Nadu AP, Ferreira AJ, Reudelhuber TL, Bader M, Santos RA. Reduced isoproterenol-induced renin-angiotensin changes and extracellular matrix deposition in hearts of TGR(A1–7)3292 rats. J Am Soc Hypertens. 2008;2:341–348. doi: 10.1016/j.jash.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa E, Saito Y, Harada M, Kamitani S, Kuwahara K, Miyamoto Y, Ishikawa M, Hamanaka I, Kajiyama N, Takahashi N, Nakagawa O, Masuda I, Kishimoto I, Nakao K. Outside-in signalling of fibronectin stimulates cardiomyocyte hypertrophy in cultured neonatal rat ventricular myocytes. J Mol Cell Cardiol. 2000;32:765–776. doi: 10.1006/jmcc.2000.1119. [DOI] [PubMed] [Google Scholar]
- 28.Oh M, Dey A, Gerard RD, Hill JA, Rothermel BA. The CCAAT/enhancer binding protein beta (C/EBPbeta) cooperates with NFAT to control expression of the calcineurin regulatory protein RCAN1–4. J Biol Chem. 2010;285:16623–16631. doi: 10.1074/jbc.M109.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver N, Babu M, Diegelmann R. Fibronectin gene transcription is enhanced in abnormal wound healing. J Invest Dermatol. 1992;99:579–586. doi: 10.1111/1523-1747.ep12667776. [DOI] [PubMed] [Google Scholar]
- 30.Piepoli MF, Conraads V, Corra U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13:347–357. doi: 10.1093/eurjhf/hfr017. [DOI] [PubMed] [Google Scholar]
- 31.Plante E, Lachance D, Gaudreau M, Drolet MC, Roussel E, Arsenault M, Couet J. Effectiveness of beta-blockade in experimental chronic aortic regurgitation. Circulation. 2004;110:1477–1483. doi: 10.1161/01.CIR.0000141733.55236.9D. [DOI] [PubMed] [Google Scholar]
- 32.Reilly JT, McVerry BA, Mackie MJ. Fibronectin in blood products--an in vitro and in vivo study. J Clin Pathol. 1983;36:1377–1381. doi: 10.1136/jcp.36.12.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Duner P, Nilsson J, Soehnlein O, Fassler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med. 2012;4:564–576. doi: 10.1002/emmm.201200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roof SR, Tang L, Ostler JE, Periasamy M, Gyorke S, Billman GE, Ziolo MT. Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise. Basic Res Cardiol. 2013;108:332. doi: 10.1007/s00395-013-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 36.Samuel JL, Barrieux A, Dufour S, Dubus I, Contard F, Koteliansky V, Farhadian F, Marotte F, Thiery JP, Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest. 1991;88:1737–1746. doi: 10.1172/JCI115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 39.Taylor JM, Rovin JD, Parsons JT. A role for focal adhesion kinase in phenylephrine-induced hypertrophy of rat ventricular cardiomyocytes. J Biol Chem. 2000;275:19250–19257. doi: 10.1074/jbc.M909099199. [DOI] [PubMed] [Google Scholar]
- 40.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, de Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem. 2002;277:48617–48626. doi: 10.1074/jbc.M206532200. [DOI] [PubMed] [Google Scholar]
- 42.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation. 1993;88:2849–2861. doi: 10.1161/01.CIR.88.6.2849. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.