Abstract
At present, several eukaryotic expression systems including yeast, insect and mammalian cells and plants are used for the production of recombinant proteins. Proteins with potential N-glycosylation sites are efficiently glycosylated when expressed in these systems. However, the ability of the eukaryotic expression systems to glycosylate may be not desirable for some proteins. If target proteins that do not carry N-linked glycans in the native host contain potential N-linked glycosylation sites, they can be aberrantly glycosylated in the eukaryotic expression systems, thus, potentially impairing biological activity. Recently, we have developed a strategy of enzymatic deglycosylation of proteins in vivo by co-introducing bacterial PNGase F via agroinfiltration followed by transient expression in plants.1 Here, we summarize our work on this topic and its potential implications.
Keywords: N-linked glycosylation, PNGase F, deglycosylation, plant transient expression, recombinant proteins, malaria vaccine candidate Pfs48/45
The recent biotechnology boom has triggered an interest in using plants as an alternative platform for production of recombinant proteins. Plants offer several advantages compared with other recombinant protein expression systems, including simple, highly scalable, cost-effective manufacturing, relative product safety due to the lack of any harbored mammalian pathogens, and the presence of the eukaryotic post-translational modification (PTM) machinery.2,3 N-linked glycosylation is a PTM that is critical for correct folding, stability and biological activity of many proteins including recombinant subunit vaccines and therapeutic proteins produced in heterologous expression systems.4 However, some eukaryotic as well as bacterial proteins contain no N-glycans in the native host, but may contain multiple potential glycosylation sites which are aberrantly glycosylated when these proteins are expressed in heterologous eukaryotic expression systems, potentially leading to reduced functionality and immunogenicity due to incorrect or altered folding or masking of epitopes. For example, Pfs48/45 protein of Plasmodium falciparum5 or A chain of human factor XIII6,7 do not carry N-linked glycans and protective antigen (PA) of Bacillus anthracis is not a glycoprotein; however, these proteins contain potential N-linked glycosylation sites which can be aberrantly glycosylated during expression in yeast, mammalian or plant systems. It has been shown that aberrant N-glycosylation poses problems for many therapeutic applications. For example, aberrantly increased N-glycosylation is often observed in proteins of cancer cells.8 In addition, aberrant N-glycosylation of cell surface receptors, including integrins and cadherins, appears to be associated with changes in carcinoma progression and metastasis,9-11 indicating significant changes in these proteins’ behavior. In fact, the attachment of carbohydrates strongly affects physico-chemical properties of a protein, and therefore can alter its essential biological properties such as the specific activity, ligand-receptor interactions and immunogenicity, which may pose a safety risk when the protein is used in vivo. At this point, the ability of the eukaryotic expression systems to glycosylate may be not desirable for those targets that do not require N-linked glycosylation. Therefore, it is important to develop strategies for producing non-glycosylated forms of target proteins to preserve their native conformation and biological activity. One of such strategies is to use tunicamycin, a specific inhibitor of the enzyme that transfers acetylglucosaminephosphate (GlcNAc-1-P) onto dolichol phosphate (Dol-P) to block N-glycosylation. However, this approach has been previously demonstrated to result in a non-uniform expression of proteins in plants.12,13 Moreover, tunicamycin is very toxic, and even a short-term treatment of plants significantly affects protein folding,14,15 inhibits extracellular secretion of proteins,16 whereas a long-term treatment has a lethal effect on plants.4 Therefore, this strategy is not practical for production of recombinant proteins in a non-glycosylated form. Thus, we sought to develop a robust strategy to produce non-glycosylated protein targets that do not require N-glycosylation, similar to native Pfs48/45.
PNGase F is a 34.8 kDa enzyme secreted by gram-negative bacterium Flavobacterium meningosepticum17 which cleaves a bond between the innermost GlcNAc and asparagine residues of high-mannose, hybrid and complex oligosaccharides in N-linked glycoproteins, except when the α(1–3) core is fucosylated. We hypothesized that co-expression of target protein with bacterial PNGase F in the endoplasmic reticulum may lead to its deglycosylation and allow for production of non-glycosylated forms of the protein in plants. Recombinant PNGase F has not been previously expressed in plants. Therefore, we first aimed to achieve the expression of active bacterial PNGase F in plants. The bacterial PNGase F sequence encompassing 314 amino acids (the full length of the catalytically active protein without a signal sequence) was optimized for the expression in Nicotiana benthamiana plants, cloned into the plant expression vector pGRD418 and expressed in N. benthamiana plants with a FLAG tag.1 The expression of ~36 kDa PNGase F was confirmed by Western blot analysis using an anti-FLAG monoclonal antibody (mAb) (Fig. 1A). The average expression level of PNGase F was approximately 150 mg/kg of fresh leaf biomass. In plants, most proteins of the extracellular compartment and the endomembrane system are glycosylated and N-linked glycosylation of proteins has a great impact on their biological functions.19 In this regard, N. benthamiana plants expressing PNGase F remained healthy at 7, 8 and 9 d post infiltration (dpi) with no visible symptom development or change in growth when co-expression of target reached the highest level, suggesting that due to the transient nature of expression and brief time span, the effect of PNGase F on the endogenous protein folding and extracellular secretion is not significant. PNGase F was then purified from N. benthamiana leaves using an anti-FLAG agarose column, and its enzymatic deglycosylation activity was confirmed in vitro. Thus, since recombinant PNGase F has not been previously expressed in plants, our results support the utility of plants as an expression system for production of an active, endotoxin-free PNGase F at reduced costs.
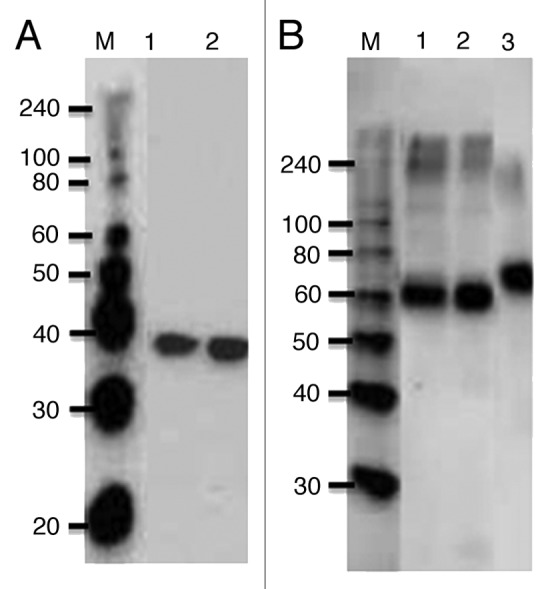
Figure 1. western blot analysis of Pfs48F1 (B) co-expressed with bacterial PNGase F (A) in N. benthamiana plants. N. benthamiana plants were infiltrated with pGRD4-PNGase F/pBI-Pfs48F1 constructs, for the production of deglycosylated Pfs48F1. Leaf samples were taken at 7 dpi and homogenized in 3 volumes of extraction buffer. After centrifugation at 13,000 g for 20 min, 10 μL of 10-fold diluted samples were run on SDS-PAGE prior to Western blotting. PNGase F and deglycosylated Pfs48F1 protein bands were detected in the same samples. PNGase F and Pfs48F1 bands were probed using an anti-FLAG monoclonal antibody (Sigma) and anti-4xHis tag mAb, respectively. Lanes: 1 - TP, total protein; 2- TSP, total soluble protein; 3- glycosylated Pfs48F1, expressed in N. benthamiana alone without PNGase F.
To evaluate an in vivo activity of plant-produced PNGase F, the enzyme was transiently co-expressed in N. benthamiana with several recombinant proteins, including malaria vaccine candidate Pfs48F1, PA of B. anthracis and an antibody against PA of B. anthracis.1 Co-expression with PNGase F led to the accumulation of ~60 kDa Pfs48F1 protein in soluble fraction (Fig. 1B). The expression level of deglycosylated Pfs48F1 co-expressed with PNGase F was about 50 mg/kg of fresh leaf biomass and solubility was about 95%. Deglycosylated Pfs48F1 had a similar size as the in vitro deglycosylated Pfs48F1,1 suggesting that Pfs48F1 was enzymatically deglycosylated by PNGase F in vivo. The efficiency of Pfs48F1 in vivo deglycosylation was confirmed by the glycan detection and mass spectrometry analyses.1
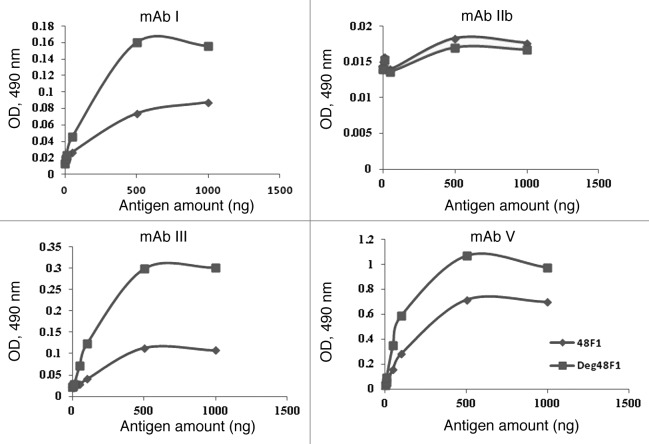
Malaria is a vector-borne infectious disease caused by protozoan parasites. It is widespread in tropical and subtropical regions, including parts of the Americas, Asia, and Africa with over 300–500 million cases and more 1 million deaths each year from around the world. Although some vaccines are under development, no vaccine is currently available for this infectious disease that provides a high level of protection. Pfs48/45, a member of a Plasmodium-specific protein family, displays conformation-dependent epitopes and is one of the leading candidates for transmission-blocking (TB) vaccines. As described above, the native Pfs48/45 protein does not carry N-linked glycans5 but contains seven potential N-linked glycosylation sites which can be aberrantly glycosylated during during expression in yeast, mammalian or plant systems. In previously published studies, several recombinant protein expression systems have been employed to express the full-length form of this protein, but the resulting protein did not exhibit TB activity and did not induce TB antibodies in mice.20,21 Pfs48/45 was also expressed in N. benthamiana plant at Fraunhofer USA Center for Molecular Biotechnology (FhCMB) using a transient expression system, but the TB activity of this plant-derived vaccine candidate was low. We hypothesized that the low TB activity of Pfs48/45 may be associated with an incorrect or altered folding or masking of important epitopes of the protein due to glycosylation. For example, a C-terminal fragment (containing 10 cysteine residues) of the Pfs48/45 protein of P. falciparum expressed in Escherichia coli as a maltose-binding protein fusion has been shown to be correctly folded (when co-expressed with 4 E. coli chaperones) and to elicit functional TB antibodies in mice.21 In contrast, the full-length form of Pfs48/45 (M-Pfs16C, 16 cysteine residues and 7 putative glycosylated sites) did not properly fold, was rapidly and extensively degraded by host cell proteases in the E. coli periplasm, and had poor solubility and much weaker epitope recognition. Plants, as mentioned above, offer several advantages over other recombinant protein expression systems, including an eukaryotic PTM machinery. Plant viral vector-based transient expression in N. benthamiana plants has been successfully used for time- and cost-effective production of recombinant vaccine candidates against various infectious diseases.22,23 Thus, the PNGase F co-expression strategy may be the preferred approach to transiently express functionally active Pfs48F1protein in N. benthamiana plants. Using this strategy, we produced non-glycan forms of Pfs48F1 in plant cells, purified them and tested for binding affinity to antibodies raised against epitopes I, III and V of native Pfs48/45 of P. falciparum.24 Epitope-specific mAbs I, III and V recognized the deglycosylated form of Pfs48F1 2–6-fold better than the glycosylated form of the same protein (Fig. 2).1 The binding affinity of mAb V for glycosylated Pfs48F1 and in vitro and in vivo deglycosylated Pfs48F1 was further evaluated using the KinExA 3200 instrument (Sapidyne) and was determined to be 9.7 nM, 3.8 nM and 2.7 nM, respectively.1 In addition, qualitative results of the signal inhibition analysis suggested that mAb III had the highest affinity to in vivo deglycosylated Pfs48F1 compared with both in vitro deglycosylated and glycosylated Pfs48F1.1 Taken together, these data suggest that aberrant glycosylation might have led to masking of important epitopes or caused incorrect or altered folding of Pfs48F1 and that deglycosylated Pfs48F1 may have a high TB activity and therefore, be a good candidate for malaria vaccine development.
Figure 2. Comparative ELISA analysis of glycosylated (♦) and deglycosylated (■) forms of Pfs48F1. Recognition of glycosylated and deglycosylated forms of Pfs48F1 by various rat mAbs that detect epitopes I, IIb, III and V of Pfs45/48 was measured by ELISA.
Since the biological activity of many recombinant proteins depends on their glycosylation status, efforts are under way to humanize N-linked glycosylation and N-glycans of biopharmaceuticals produced in heterologous expression systems. Enzymatic deglycosylation of proteins in vivo has not been achieved previously in any eukaryotic system, including plants. Our studies demonstrated that enzymatic deglycosylation of target proteins can be achieved in vivo by introducing a bacterial deglycosylation enzyme, PNGase F, into a plant expression system and that it has potential to become a robust strategy for production of therapeutic target proteins in a non-glycan form. This strategy is expected to have many potential applications in molecular farming and to be used to produce subunit vaccines, therapeutic proteins and antibodies in deglycosylated forms. This can be particularly important for the proteins that are not glycosylated in their native host but may be aberrantly glycosylated when expressed in other eukaryotic hosts, potentially leading to reduced functionality and immunogenicity. Plant-produced PA of B. anthracis has a great potential as a target for a safe, effective and low-cost vaccine against anthrax, which would help counteracting the threat of bioterrorism. PA of B. anthracis is not a glycoprotein, but has six potential glycosylation sites and is glycosylated when expressed in N. benthamiana. The PNGase F-based in vivo deglycosylation approach has been also applied toward producing non-glycosylated forms of PA of B. anthracis and a mAb against PA in the plant transient expression system. Western blot analysis demonstrated a shift in the mobility of PA co-expressed with PNGase F.1 When PNGase F was co-expressed with an anti-PA mAb, the protein mobility shift on SDS-PAGE was observed with the heavy chain that has one glycosylation site, but not with the light chain that lacks glycosylation sites.1 Collectively, these results, along with the observation of in vivo deglycosylation of Pfs48F1 following co-expression with PNGase F, demonstrate that PNGase F successfully cleaved N-linked glycans from all tested glycoproteins and suggest that the PNGase F co-expression strategy can be used to produce non-glycosylated therapeutic proteins in the N. benthamiana-based transient expression system. Plant complex N-glycans contain β1, 2-xylose and α1, 3-fucose residues that are not present in human complex glycans, and therefore, these findings can be also important for producing fucose- and xylose-free recombinant proteins, which would reduce a potential risk of immunogenic and allergenic reactions to these epitopes in humans. The methods developed and used in this study may have broad applications in modifying many targets (e.g., therapeutic proteins, antibodies and vaccine candidates) in plants such as N. benthamiana by co-expressing these proteins with modifying enzymes such as PNGase F and. In addition, using this strategy, non-N-glycosylated forms of recombinant complex proteins can be also produced in other eukaryotic expressions systems, such as mammalian cells and yeast. This strategy may allow for better understanding of the importance and roles of glycans in plant glycoproteins. Finally, this approach can be applied to different PTMs for producing proteins with desired activity in heterologous systems.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr Stephen J. Streatfield for useful discussion and Dr Natasha Kushnir for editorial assistance. This study was supported by Fraunhofer USA Center for Molecular Biotechnology.
Submitted
12/03/12
Revised
12/25/12
Accepted
12/30/12
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/23449
References
- Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, et al. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F. Plant Biotechnol J. 2012;10:773–82. doi: 10.1111/j.1467-7652.2012.00694.x. [DOI] [PubMed] [Google Scholar]
References
- 1.Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, et al. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F. Plant Biotechnol J. 2012;10:773–82. doi: 10.1111/j.1467-7652.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- 2.Mett V, Farrance CE, Green BJ, Yusibov V. Plants as biofactories. Biologicals. 2008;36:354–8. doi: 10.1016/j.biologicals.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Yusibov V, Mamedov TG. Plants as an Alternative System for Expression of Vaccine Antigens. Proc ANAS (Biol Sci) 2010; 65:195–200. [Google Scholar]
- 4.Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, et al. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J. 2010;8:564–87. doi: 10.1111/j.1467-7652.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 5.Milek RL, DeVries AA, Roeffen WF, Stunnenberg H, Rottier PJ, Konings RN. Plasmodium falciparum: heterologous synthesis of the transmission-blocking vaccine candidate Pfs48/45 in recombinant vaccinia virus-infected cells. Exp Parasitol. 1998;90:165–74. doi: 10.1006/expr.1998.4315. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft AE, Grant PJ, Ariëns RA. A study of human coagulation factor XIII A-subunit by electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:1607–11. doi: 10.1002/1097-0231(20000915)14:17<1607::AID-RCM69>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz ML, Pizzo SV, Hill RL, McKee PA. The subunit structures of human plasma and platelet factor XIII (fibrin-stabilizing factor) J Biol Chem. 1971;246:5851–4. [PubMed] [Google Scholar]
- 8.Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009;69:5673–80. doi: 10.1158/0008-5472.CAN-08-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–45. [PubMed] [Google Scholar]
- 10.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–4. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 11.Isaji T, Gu J, Nishiuchi R, Zhao Y, Takahashi M, Miyoshi E, et al. Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. J Biol Chem. 2004;279:19747–54. doi: 10.1074/jbc.M311627200. [DOI] [PubMed] [Google Scholar]
- 12.Frank J, Kaulfürst-Soboll H, Rips S, Koiwa H, von Schaewen A. Comparative analyses of Arabidopsis complex glycan1 mutants and genetic interaction with staurosporin and temperature sensitive3a. Plant Physiol. 2008;148:1354–67. doi: 10.1104/pp.108.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hori H, Elbein AD. Tunicamycin inhibits protein glycosylation in suspension cultured soybean cells. Plant Physiol. 1981;67:882–6. doi: 10.1104/pp.67.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico L, Valsasina B, Daminati MG, Fabbrini MS, Nitti G, Bollini R, et al. Bean homologs of the mammalian glucose-regulated proteins: induction by tunicamycin and interaction with newly synthesized seed storage proteins in the endoplasmic reticulum. Plant J. 1992;2:443–55. doi: 10.1111/j.1365-313x.1992.00443.x. [DOI] [PubMed] [Google Scholar]
- 15.Sparvoli F, Faoro F, Daminati MG, Ceriotti A, Bollini R. Misfolding and aggregation of vacuolar glycoproteins in plant cells. Plant J. 2000;24:825–36. doi: 10.1046/j.1365-313x.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 16.Faye L, Chrispeels MJ. Apparent Inhibition of beta-Fructosidase Secretion by Tunicamycin May Be Explained by Breakdown of the Unglycosylated Protein during Secretion. Plant Physiol. 1989;89:845–51. doi: 10.1104/pp.89.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plummer TH, Jr., Elder JH, Alexander S, Phelan AW, Tarentino AL. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984;259:10700–4. [PubMed] [Google Scholar]
- 18.Shoji Y, Bi H, Musiychuk K, Rhee A, Horsey A, Roy G, et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine. 2009;27:1087–92. doi: 10.1016/j.vaccine.2008.11.108. [DOI] [PubMed] [Google Scholar]
- 19.Rayon C, Lerouge P, Faye L. The protein N-glycosylation in plants. J Exp Bot. 1998;49:1463–72. [Google Scholar]
- 20.Milek RL, Stunnenberg HG, Konings RN. Assembly and expression of a synthetic gene encoding the antigen Pfs48/45 of the human malaria parasite Plasmodium falciparum in yeast. Vaccine. 2000;18:1402–11. doi: 10.1016/S0264-410X(99)00392-8. [DOI] [PubMed] [Google Scholar]
- 21.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci U S A. 2008;105:4301–5. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, et al. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin. 2011;7(Suppl):191–8. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]
- 23.Mett V, Chichester JA, Stewart ML, Musiychuk K, Bi H, Reifsnyder CJ, et al. A non-glycosylated, plant-produced human mAb against anthrax protective antigen protects mice and non-human primates from B. anthracis spore challenge. Hum Vaccin. 2011;7(Suppl):183–90. doi: 10.4161/hv.7.0.14586. [DOI] [PubMed] [Google Scholar]
- 24.Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K, et al. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. 2007;282:17148–56. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]