Abstract
The yes-associated protein (YAP) transcription co-activator has been reported either as an oncogene candidate or a tumor suppressor. Liver tissue chips revealed that about 51.4% human hepatocellular carcinoma (HCC) samples express YAP and 32.9% HCC samples express phosphorylated YAP. In this study, we found that chemotherapy increased YAP protein expression and nuclear translocation in HepG2 cells, as well as p53 protein expression and nuclear translocation. However, little is known about YAP functions during chemotherapy. Our results show that overexpression of YAP increases chemosensitivity of HepG2 cells during chemotherapy. Dominant negative transfection of Flag-S94A (TEAD binding domain mutant) or Flag-W1W2 (WW domain mutant) to HepG2 cells decreases p53 expression/ nuclear translocation and chemosensitivity when compared with control HepG2 cells. Furthermore, rescue transfection of Flag-5SA-S94A or Flag-5SA-W1W2, respectively to HepG2 cells regains p53 expression/nuclear translocation and chemosensitivity. These results indicate that YAP promotes chemosensitivity by modulating p53 during chemotherapy and both TEAD and WW binding domains are required for YAP-mediated p53 function. ChIP assay results also indicated that YAP binds directly to the p53 promoter to improve its expression. In addition, p53 could positively feedback YAP expression through binding to the YAP promoter. Taken together, our current data indicate that YAP functions as a tumor suppressor that enhances apoptosis by modulating p53 during chemotherapy.
Keywords: YAP, p53, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), is one of the most common malignancies and the leading cause of cancer-related deaths worldwide, whose global burden is significant.1 Most patients present at an advanced stage when operation is no longer feasible. Chemotherapy is a common treatment modality for inoperable HCC; however, the intrinsic and acquired resistance of cancer cells to drug treatment represents a critical problem. Under these circumstances, it is most critical to establish the mechanisms of chemoresistance or to find suitable sensitizers.
The transcriptional coactivator Yes-associated protein (YAP), one of the members of hippo signaling pathway,2-4 was initially isolated through its binding to the Src family member non-receptor tyrosine kinase YES (Yes kinase-associated protein).5,6 It was reported to play contradictory roles in the development of cancer. Thus, YAP could interact with and enhance p73-dependent apoptosis,7-9 but it was also shown to act as a transcriptional activator when binding to transcription factors TEAD (TEA domain/transcription enhancer factor family members),10-12 Runx2 (Runt-related transcription factor 2),13,14 ErbB4 (epidermal growth factor receptor family 4)15,16 and et al. Therefore, the roles of YAP in chemosensitivity of hepatocellular carcinoma remain still to be clearly defined.
Here, we report that YAP is widely expressed in HCC cases and cell lines. YAP can be activated and upregulated when treated with chemotherapeutics. Overexpression of YAP in HepG2 cells resulted in high expression of p53, and both TEAD binding and WW domains were found important for YAP to carry out those functions. In addition, YAP regulated p53 through binding to the p53 promoter, whereas p53 could bind to the YAP promoter to induce feedback of YAP regulation. Collectively, these findings suggest that YAP contributes significantly to chemosensitivity of hepatocelluar carcinoma.
Results
Evaluation of YAP expression in liver cancer and normal liver
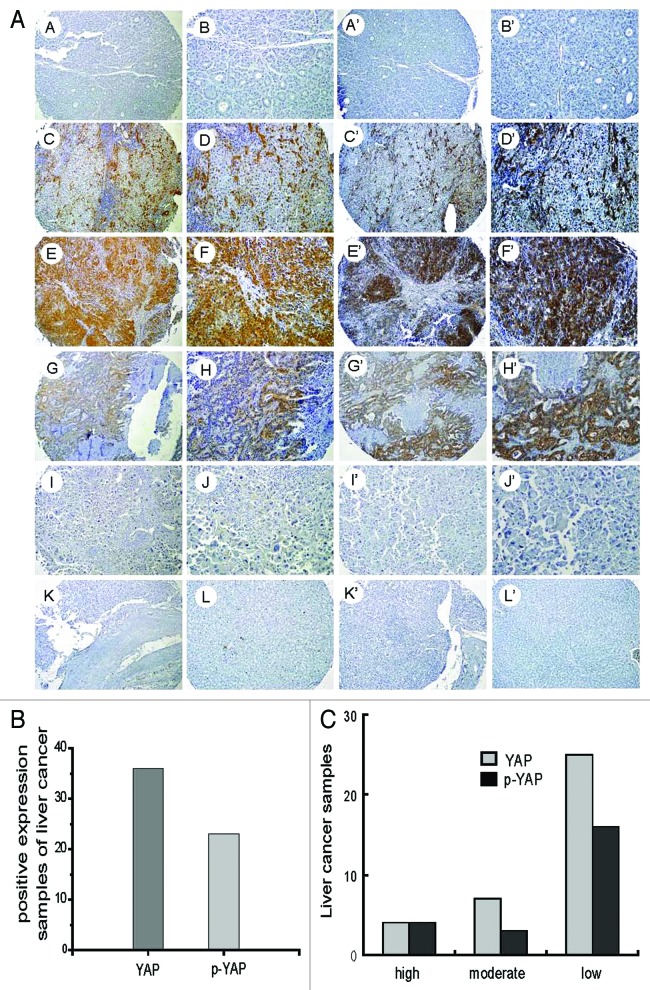
In the assessment of nuclear and cytoplasm YAP expression intensity, samples from tissue chips with 10 normal liver specimens and 70 HCC hepatocelluar carcinoma specimens were analyzed. In the normal liver, we found very low level of YAP. In those HCC specimens, 36 of 70 (51.4%) cases had some level of YAP, whereas 4/70 (5.7%) had a high level, 7/70 (10%) had moderate, 25/70 (35.7%) had low (Fig. 1). At the same time, p-YAP was also tested. No significant expression of p-YAP was found in normal liver specimens, whereas 23/70 (32.9%) HCC specimens expressed p-YAP. Of these specimens 4/70 (5.7%) expressed high, 3/70 (4.3%) moderate and 16/70 (22.9%) low p-YAP (Fig. 1). The results showed that no matter how strongly YAP are expressed, most of them were phosphorylated, indicating that YAP was precisely regulated. This is coincidence with a previous report of YAP in head and neck cancer.17
Figure 1. Evaluation of YAP expression in hepatocellular carcinoma and normal liver samples. (A) A–L show YAP expression in HCC and normal liver samples. A’–L’ reveal p-YAP expression in HCC and normal liver samples. A, A’, B and B’ represent Grade I HCC; C, C’, D and D’ represent Grade II HCC; E, E’, F and F’ represent Grade III HCC; G, G’, H and H’ represent Grade II cholangiohepatoma; I, I’, J and J’ represent Grade IV HCC; K and K’ represent sarcomas hepatocellular carcinoma; L,L; represent normal liver samples. (B) Statistics of positive expression of YAP and p-YAP in liver cancer samples. (C) Statistics of YAP and p-YAP expression levels in liver cancer samples.
YAP expression in normal liver cell lines and hepatocellular carcinoma cell lines
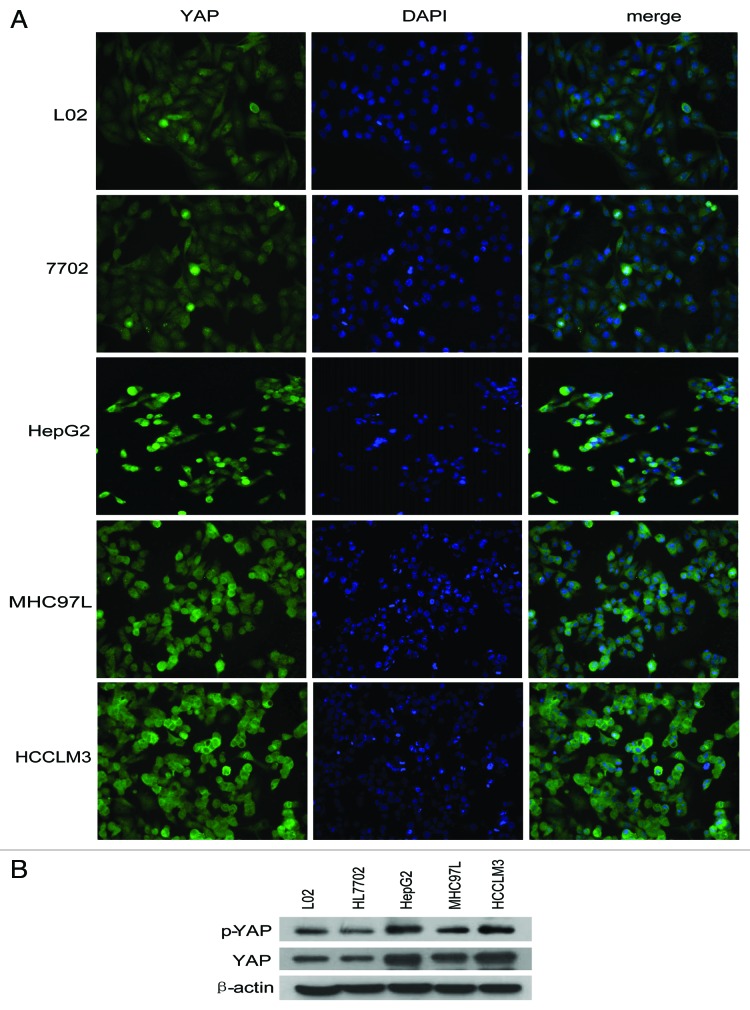
Based on previous observations of YAP expression in tissue microarray, we selected two normal liver cell lines (L02 and HL7702) and three HCC cell lines (HepG2, MHC97L and HCCLM3) to analyze YAP and p-YAP expression and distribution by western blot and YAP distribution by immunofluorescence methods (Fig. 2A and B). The results indicated that YAP localized both in the nucleus and cytoplasm, a result which coincided with tissue microarray data (Fig. 1).
Figure 2. YAP is expressed in both HCC and normal liver cell lines. (A) YAP proteins are widely expressed in both cytoplasm and nucleus. (B) Test of YAP expression by western blots.
YAP is activated when treated with doxorubicin
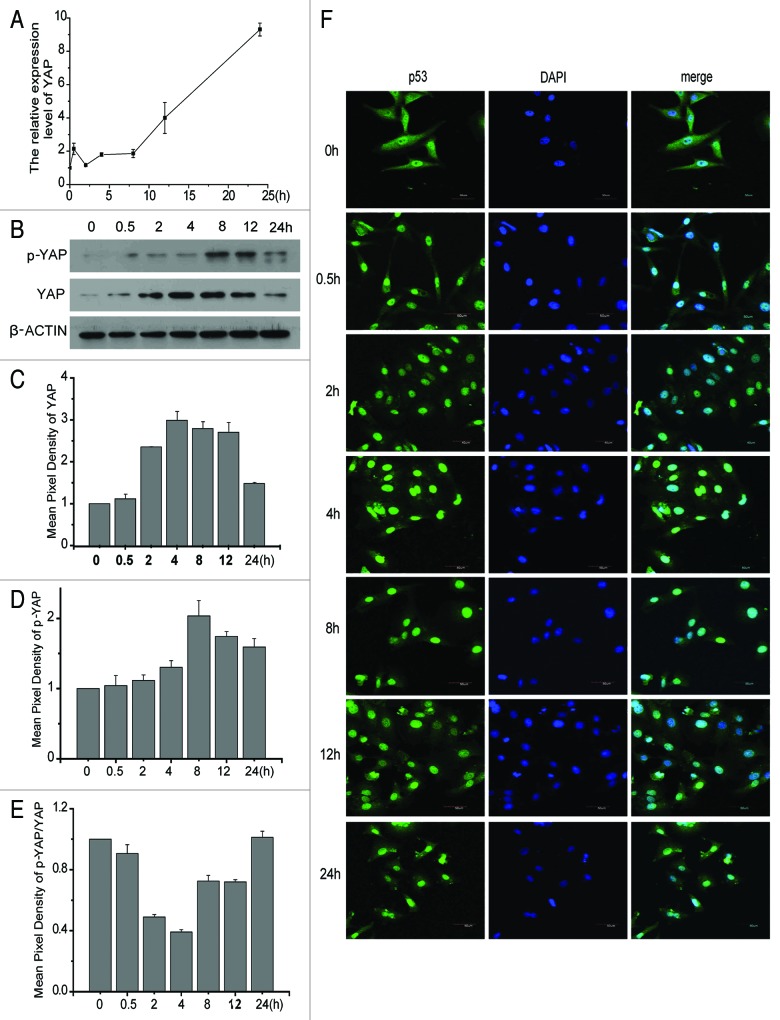
To investigate the changes of YAP when treated with doxorubicin, the HCC cell line HepG2 was treated with 0.7 μg/ml doxorubicin, and then analyzed for YAP at the protein or RNA levels at several time points. It was shown that increasing yap mRNA are time dependent (Fig. 3A), while YAP proteins gradually increased at first and then decreased and became time-dependent (Fig. 3B). This same phenomenon also occurred with p-YAP (Fig. 3C–E). These results indicate that YAP protein was degradated at a later stage. Then the question arose us to whether stimulation with doxorubin affected YAP distribution. Next, when YAP distribution was tested by immnofluorescence, (Fig. 3F) most of YAP translocated first to the nuclus and then was gradually released. Such phenomena suggest that YAP, as a transcription activator, is activated when stimulated by doxorubicin. This finding is in coincidence with a previous report indicating that YAP translocated into the nucleus once treated with DDP (cisplatin).18
Figure 3. YAP is activated when treated with doxorubicin. HepG2 cells were treated with 0.7 μg/ml doxorubicin from 0 to 24 h; then cells were harvested to test mRNA (A) and protein expression (B) levels of YAP at different time point. The pixel density from the western blot results of Yap (C) and p-YAP (D) were analyzed and plotted as Means + SD (n = 3). (E) The ratio of protein expression level of YAP/ p-YAP were plotted as Means+SD (n = 3). (F) The same as for (B) except that analysis was done by immunofluorescence.
YAP enhances chemosensitivity of HCC
Although prior data had shown that YAP was activated when stimulated with doxorubicin, the kind of function of YAP in this case is yet to be established. In other words, would cells be rescued or killed? In order to investigate this, we transiently transfected WT-YAP and mutant-YAP into HepG2 cells, then stimulated these cells with doxorubicin, to them test cell viabilities by WST-1 after 72 h (Fig. 4A). This experiment showed that the chemosensitivity of HepG2 was enhanced when transfected with WT-YAP relative to empty vector control. Furthermore, after HepG2 cells transfected with Flag-S94A (TEAD binding domain mutant) or Flag-W1W2 (WW domain mutant), their chemosensitivities decreased relative to WT-YAP, but still remained stronger than that of the empty vector control. Once cells were transfected with Flag-5SA-S94A and Flag-5SA-W1W2, their chemosensitivities decreased and they could rescue functions of S94A and W1W2 mutants (Table 1). In addition, when another chemotherapeutic drug DDP(cisplatin) was used to treat HepG2 cells (Fig. 4B), results indicated that YAP enhanced chemosensitivity as well (Table 2). At the same time, IC50 of Dox in the cells with suppressed YAP expression was also examined, compared with the scramble control, knockdown of YAP enhanced the chemoresistance of HepG2 cells (Fig. 4C, Table 3).Taken together, our results suggested that YAP might function as an apoptotic enhancer and that both TEAD binding domain and WW domain were required for YAP to fulfill these functions.
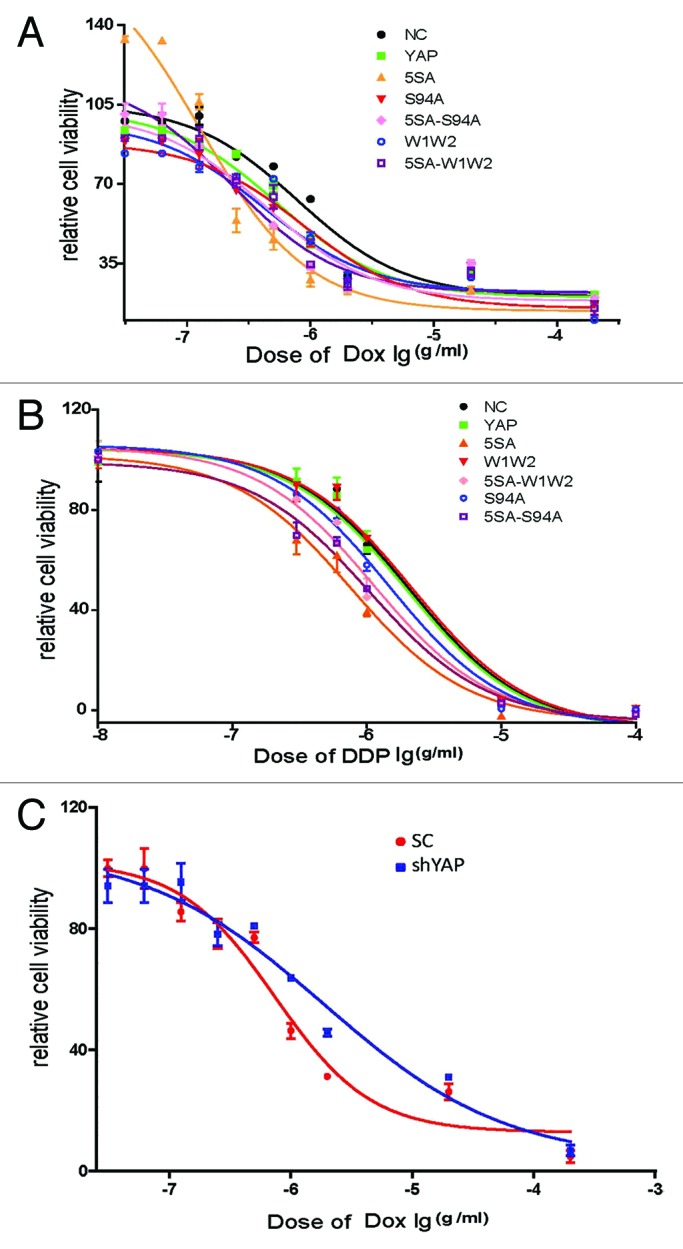
Figure 4. YAP2 improves the apoptosis of HepG2 cells after treatment with DDP and doxorubicin. HepG2 cells were transiently transfected with WT-YAP or YAP-mutants, after 48 h, cells were harvested and seeded into 96-well plate. Once cells attached to the plates, they were treated with different concentration of (A) doxorubicin and (B) DDP for 72 h and then subjected to WST-1 analysis. (C) HepG2 cells were transiently transfected with sh-YAP or scramble control; after 48 h, cells were harvested and seeded into 96-well plate. Once cells attached to the plates, they were treated with different concentration of doxorubicin.
Table 1. IC50 values of YAP or YAP mutants treated by doxorubicin.
| Cell lines | IC50 value (× 10−7 g/ml) | p value |
|---|---|---|
| NC |
7.817 ± 0.145 |
|
| YAP |
5.430 ± 0.183 |
< 0.001 |
| 5SA |
1.268 ± 0.021 |
< 0.001 |
| W1W2 |
8.679 ± 0.101 |
0.01 |
| 5SA-W1W2 |
4.369 ± 0.075 |
0.001 |
| S94A |
4.140 ± 0.289 |
< 0.001 |
| 5SA -S94A | 2.299 ± 0.304 | < 0.001 |
Table 2. IC50 values of YAP or YAP mutants treated by DDP.
| Cell lines | IC50 value (× 10−6 g/ml) | p value |
|---|---|---|
| NC |
2.982 ± 0.147 |
|
| YAP |
1.975 ± 0.054 |
0.01 |
| 5SA |
0.760 ± 0.018 |
< 0.001 |
| W1W2 |
2.173 ± 0.159 |
0.5 |
| 5SA-W1W2 |
1.116 ± 0.100 |
< 0.001 |
| S94A |
1.457 ± 0.073 |
0.002 |
| 5SA-S94A | 1.040 ± 0.012 | < 0.001 |
Table 3. IC50 values of shYAP treated by doxorubicin.
| Cell lines | IC50 value (× 10−7 g/ml) | p value |
|---|---|---|
| NC |
7.444 ± 0.0526 |
|
| shYAP | 18.870 ± 0.0497 | < 0.01 |
p53 is activated when treated with doxorubicin
It is known that p53 became activated in cells exposed to damaged DNA as well as to other type of stress.19-21 To investigate the changes induced by p53 when treated with doxorubicin, the HCC cell line HepG2 was treated with 0.7 μg/ml of this drug and then analyzed for p53 and p-p53 at the protein level at several time points by western blots and p53 localization by immnofluorescence. The results showed that the levels of p53 and p-p53 protein increased and that most of p53 translocated into the nucleus (Fig. 5A and B).
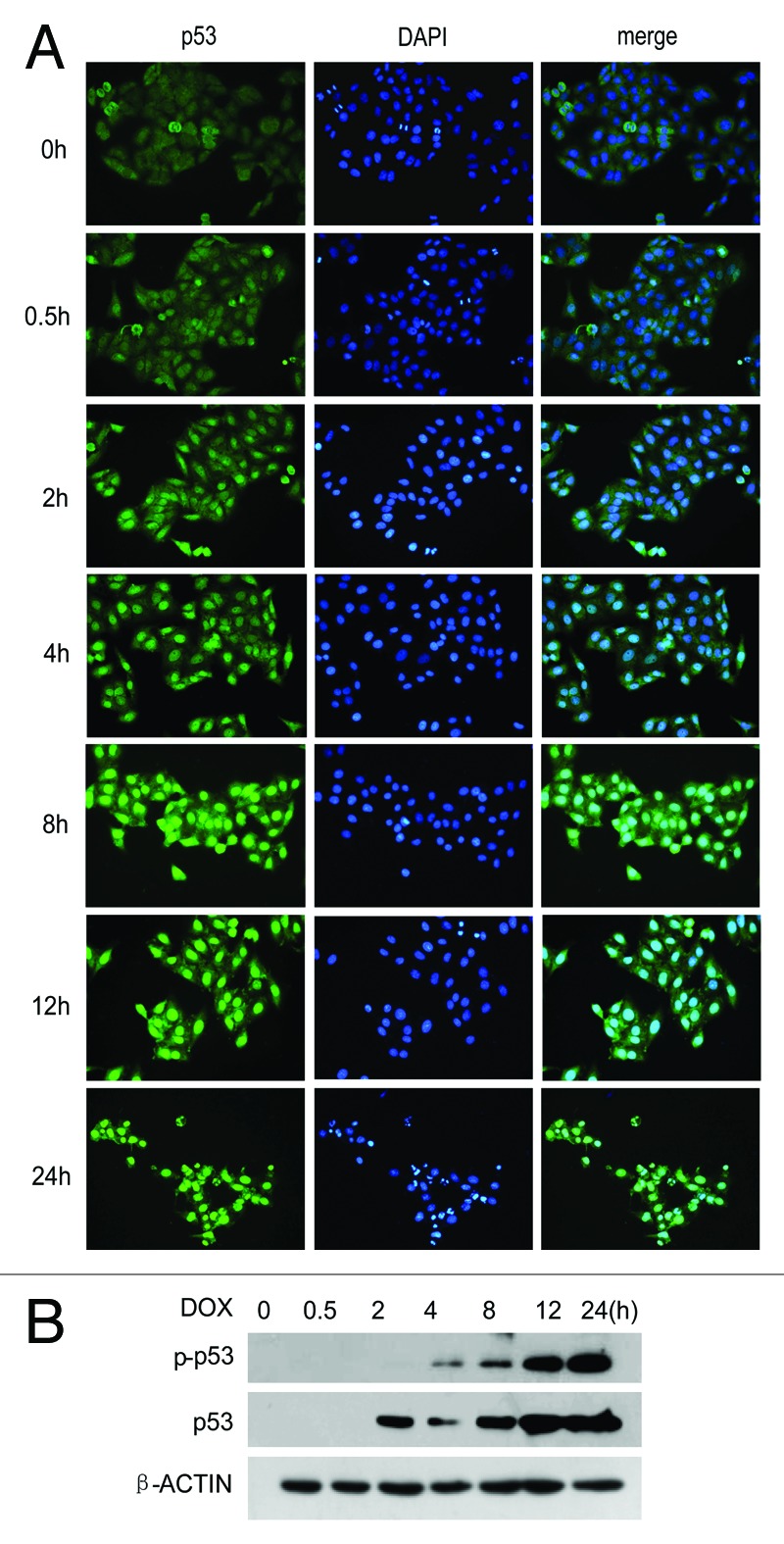
Figure 5. p53 is activated when treated with Doxorubicin. (A) HepG2 cells were treated with 0.7 μg/ml doxorubicin from 0 to 24 h; then cells were harvested for testing p53 by immunofluorescence (A) or testing p53 and p-p53 by western blots (B).
YAP improves apoptosis by binding to the p53 promoter to enhance p53 expression
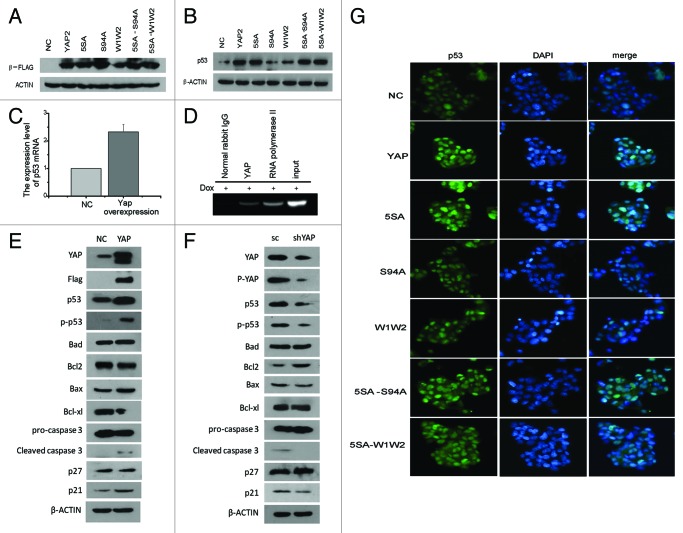
Since both YAP and p53 are activated, the relationship between them remains to be clarified. Are they involved in the same pathway or in paralleled pathways? To this end, we next transfected YAP and its mutants into HepG2 cells (Fig. 6A), and after 12 h treatment, the cells were harvested for western blots or stained for immnofluorescence (Fig. 6B and G). p53 was found to be increased when transfected with WT-YAP relative to empty vector controls. However, once TEAD binding domain and WW domain were destroyed, it would weaken the expression level of p53. In addition, in cells 5SA-S94A and 5SA-W1W2, their p53 was upregulated when compared with S94A or W1W2 cells (Fig. 6B and G). When we tested whether p53 is directly or indirectly regulated by YAP, we found that HepG2 cells transfected with WT-YAP expressed more p53 mRNA than cells containing empty vector (Fig. 6C). Since the exact binding site of YAP to the p53 promoter is unknown, primers were designed upstream of p53 promoter (0 to −2500 bp), with a pair of primers covering a region of 200 bp. Results of ChIP analysis confirmed that YAP did bind to the p53 promoter at a region from −2100 bp to −1926 bp (Fig. 6D). In addition, to investigate the role of YAP in p53 signaling, we test YAP's role downstream of DNA-damage induced apoptosis and cell-cycle regulation in YAP overexperession and YAP knockdown cells. The results turn out that YAP enhances the downstream proteins induced apoptosis and cycle inhibition, like p21, Bax and Caspase3. At the same time, YAP inhibits the downstream proteins against apoptosis like Bcl2 and Bcl-xl, but has no effects to p27 and Bad (Fig. 6E and F).Taken together, these results indicate that YAP and p53 are involved in the same pathway and that p53 is directly regulated by YAP.
Figure 6. YAP improves apoptosis by binding to the p53 promoter and enhances p53 expression. (A) HepG2 cells were transiently transfected with Flag-YAP or Flag-YAP mutants and subjected to western blots. After 48 h, the overexpression of Flag-YAP was confirmed by using flag antibody. (B) Western blot results of p53 in HepG2-Flag-YAP and HepG2-Flag-YAP mutants after treatment with 0.7 μg/ml doxorubicin for 24 h. (C) Real-time result of p53 in HepG2 cell transfected with empty vector control or WT-YAP plasmid and incubated with doxorubicin for 8 h. (D) ChIP was used to assay the binding of YAP to p53 promoter in HepG2 cells treated with doxorubicin for 8 h. (E and F) DNA-damage induced apoptosis and cell-cycle regulation proteins were examined. (G) Immunofluorescence of p53 (green) HepG2-Flag-YAP and HepG2-Flag-YAP mutants after treatment with doxorubicin for 24 h.
p53 can positively feedback YAP expression through binding to the YAP promoter
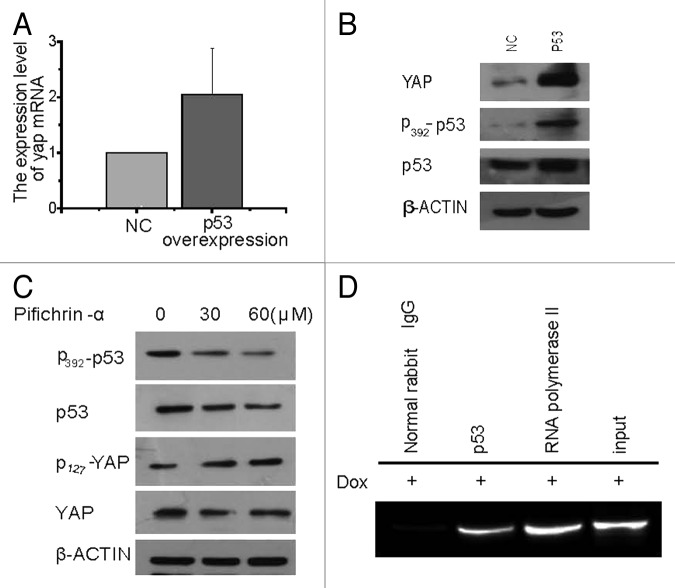
Initially, we assumed that p53 was also a transcription factor,22,23 which could positively feedback YAP expression. To prove this assumption, HepG2 cells were transfected with empty vector or p53-overexpression vector, treated with doxorubicin for 24 h and then RNAs and proteins harvested for testing. It was found that, compared with empty vector, cells with p53 overexpression vector had higher levels of both YAP protein and mRNA (Fig. 7A and B). When the same time, HepG2 cells were treated with or without p53 inhibitor pifichrin-α for 24 h, p-YAP increased. However, total YAP did not decrease significantly, which indicated that YAP was regulated by p53 (Fig. 7A–C). In order to assess whether this regulation was direct or indirect, a ChIP assay was performed. Since the exact binding site of p53 to YAP promoter is not known, primers were designed upstream of the YAP promoter covering 0 to −2500 bp, with each pair of primers covering a region of 200 bp. When HepG2 cells were treated doxorubicin for 24 h and then harvested for ChIP analysis, it turned out that p53 did regulate YAP by directly binding to the YAP promoter at the region from −2300 bp to −2105 bp (Fig. 7D).
Figure 7. p53 could positively feedback YAP expression through binding to YAP promoter. (A) Real-time RT-PCR and (B) western blot results of YAP in HepG2 cells transiently transfected with empty vector control or WT-p53 after treatment with doxorubicin for 24 h. (C) Western blot results of p53, YAP and their phosphorylation forms in HepG2 cells after 2 hours’ pretreatment with p53 inhibitor pifichrin-α and then 24 h treatment with doxorubicin in the presence of pifichrin-α. (D) HepG2 cells were treated with doxorubicin for 24 h and harvested for ChIP assay with normal rabbit IgG, p53 antibody or RNA polymerase II. PCR were done by using the primers of the YAP promoter.
Discussion
We report here that phosphorylated YAP protein is widely expressed in HCC samples. The same phenomenon was observed in HCC cell lines, indicating that YAP was regulated precisely. We also demonstrated that when cells were stimulated with chemotherapeutic drugs, YAP protein increased and was dephosphorylated and translocated to the nucleus. This phenomenon occurred at early time points, indicating that YAP was an incipient reaction factor once stimulated with chemotherapeutics.
YAP protein was reported to be an oncoprotein whose function is to improve proliferation of cells and development of organs as to be regulated by the hippo signaling pathway.24-26 However, our results suggest YAP to be a negative regulator of chemoresistance. We believe that the divergent results are not contradictory. Thus, since the signaling pathways regulation chemoresistance are abundant, they are not only regulated by signals of proliferation and anti-apoptosis,27,28 they also have a relationship with enzymes of the ceramide metabolism as well as changes in expression levels of drug targets or the inactivation of drugs.29-32 Consequently, there may be other signaling pathways existed which can regulate YAP without the hippo signaling pathway. Furthermore, the abundant transcription factors which can bind to YAP suggest various functions of YAP.33,34
M Yuan et al. first reported YAP as a tumor suppressor in breast cancer,35,36 proposing that YAP plays this role by regulating transcription factor p73, a member of p53 family. More recently, Ehsanian et al. reported that YAP dysregulation via phosphorylation promotes proliferation, survival and migration in head and neck cancer.17 Here, we demonstrated that YAP enhanced apoptosis and chemosensitivity when treated with chemotherapeutics. We then tried to find mechanisms responsible for this phenomenon. If one considers that p53 as a significant effect factor, which is activated once there is damage to DNA, there may also exist a relationship between YAP and p53. To prove this ratiocination, we selected HepG2 cell line which has wild type p53.37,38 There is some evidence that both, WW and TEAD binding domains, are required for YAP to perform its function.39,40 Thus, we transiently transfected WT-YAP and its mutants to HepG2 cells and then stimulated them with chemotherapeutic drugs, which resulted in YAP enhanced apoptosis via modulated p53. Once either WW or TEAD binding domains was destroyed, the chemosensitivity of HepG2 cells was partly lost. We found by ChIP analysis that YAP binds directly to the p53 promoter and thereby improves its expression. In addition, we found that p53 could positively regulate YAP expression through binding to the YAP promoter.
In summary, although our data predict a role played by YAP in chemosensitivity, the precise mechanisms of these events remains to be more clearly defined. We hope that further insights may be gained from our data which may help to design future sensitizers that can be used effectively in patients with hepatocellular carcinoma.
Materials and Methods
Cell culture
HL7702, L02 and HepG2 cells were cultured in high glucose DMEM supplemented with 10% FBS and 1mM penicillin and streptomycin. MHC97L and HCCLM3 cells were cultured in high glucose DMEM supplemented with 10% FBS medium, 1 mM nonessential amino acids and 1 mM penicillin and streptomycin.
Immunohistochemistry
Immunostaining was performed by standard protocols using antibodies to YAP (1.100; Cell Signaling); p-YAP (1.200; Cell Signaling), with secondary antibodies and detection kits from Santa Cruz Company.
Real–time RT-PCR of mRNAs
Total RNA was reverse transcribed by using the Takara cDNA synthesis kit. Reversed cDNA was used for PCR with the SYBR-Green Master PCR Mix in triplicate. The standard curve method was used to test the expression levels of samples. Data were normalized by endogenous β-actin.
Western blots
Western blots were performed by standard protocols using antibodies to YAP (1.1000; Cell Signaling); p-YAP (1.1000; Cell Signaling); p-p53 (1:200 Santa Cruz); p53 (1:1000; Cell Signaling), p21(1.1000; Cell Signaling); p27(1.1000; Cell Signaling); Apoptosis I Sample Kit (BD transduction laboratories), with secondary antibodies (1:5000) from Invitrogen Company.
Plasmids
pCMV-YAP plasmid and pCMV-mutant YAP plasmids are gifts from Kunliang Guan’s laboratory of the University of California San Diego.
Cell viability assay
Cells were transiently transfected with empty vector, WT-YAP or YAP-mutants, and cells were plated after 48 h into in 96-well-plates with 3000 cells/well. Once cells attached to the plates, medium was removed and new medium added containing 100 μl DDP or doxorubicin. This medium was produced by 1 mg/ml (stock concentration) DDP or doxorubicin doubling diluted. Cells in complete medium were regarded as control. Cells were incubated for 72 h, then 10 μl/100 μl WST-1/medium was added into each well, fallowed incubation for 2 h at 37°C. OD values were read at a wavelength of 600 nm. Data were normalized to the OD values of control.
Immunofluorescence
Cells were plated and grown in 24-well-plates and fixed with 4% paraformaldehyde for 30 min at room temperature. After rehydrating gradually with PBS, cells were washed 3 times with PBS and then blocked with 5% goat serum in PBS for 1 h. Samples were then incubated with primary antibody in 2% goat serum overnight at 4°C, washed and then incubated with secondary antibody and DAPI (1.5000) for 60 min in the dark.
ChIP assay
ChIP assay with HepG2 cells were performed by standard protocols of the chromatin immunoprecipitation kit from Millipore Company (#17–371). For all ChIP experiments, YAP and p53 monoclonal antibodies were used for immunoprecipitation, and PCR analyses were performed by using the TransFast Taq DNA polymerase PCR system (TransGen Biotech). RNA polymerase II, input as positive control and normal rabbit IgG as negative control. YAP promoter primers: F: 5′-AGA CAG AGT CTC GCT GTG TTG-3′; R: 5′-CCA AAA TGG TGA TAC CCT GTC-3′; PCR product 195 bp. P53 promoter primers: F: 5′-ATC ACC TGA GGT CAG GAG TTC-3′; R: 5′-CGA CTC ACT GTA ACC TCC ACC-3′; PCR product 174 bp.
Real time primers
p53 test primers
Forward primer: 5′-TGC GTG TGG AGT ATT TGG-3′
Reverse primer: 5′-GGA GTC TTC CAG TGT GAT GA-3′
YAP test primers
Forward primer: 5′-CAA ATC CCA CTC CCG ACA-3′
Reverse primer: 5′-TCT GAC CAG AAG ATG TCT TTG C-3′
YAP shRNA
CCA CCA AGC TAG ATA AAG A
Acknowledgments
This project was supported by grants from NSFC 30830096 (to R.X.) and Key Project of Tianjin Scientific and Technological Commission for China-Sweden Cooperation Research Program 09ZCZDSF04000 (to R.X.), National Science Foundation of China (NSFC) grant no.:91029734 (to Y. L.) and 81071711(to Y. L.).
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/24345
References
- 1.Trinchet JC, Beaugrand M. [Hepatocellular carcinoma: treatment with arterial chemo-embolization] Presse Med. 1994;23:831–3. [PubMed] [Google Scholar]
- 2.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–46. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 5.Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–41. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 6.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–41. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbelstein M, Strano S, Roth J, Blandino G. p73-induced apoptosis: a question of compartments and cooperation. Biochem Biophys Res Commun. 2005;331:688–93. doi: 10.1016/j.bbrc.2005.03.155. [DOI] [PubMed] [Google Scholar]
- 8.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 9.Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008;15:217–9. doi: 10.1038/sj.cdd.4402226. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Chan SW, Zhang X, Walsh M, Lim CJ, Hong W, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Zhao B, Wang P, Chen F, Dong Z, Yang H, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24:235–40. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347–58. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–9. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006;5:176–9. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 15.Webb C, Upadhyay A, Giuntini F, Eggleston I, Furutani-Seiki M, Ishima R, et al. Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry. 2011;50:3300–9. doi: 10.1021/bi2001888. [DOI] [PubMed] [Google Scholar]
- 16.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–41. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 17.Ehsanian R, Brown M, Lu H, Yang XP, Pattatheyil A, Yan B, et al. YAP dysregulation by phosphorylation or ΔNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29:6160–71. doi: 10.1038/onc.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–59. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki T, Nakagawara A. p53: the attractive tumor suppressor in the cancer research field. J Biomed Biotechnol. 2011;2011:603925. doi: 10.1155/2011/603925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol. 2011;2011:978312. doi: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011;52:9291–7. doi: 10.1167/iovs.11-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, He Y, Feng X, Huang J. Genome-wide studies of the transcriptional regulation by p53. Biochim Biophys Acta. 2012;1819:684–7. doi: 10.1016/j.bbagrm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu-Monette ZY, Medeiros LJ, Li Y, Orlowski RZ, Andreeff M, Bueso-Ramos CE, et al. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood. 2012;119:3668–83. doi: 10.1182/blood-2011-11-366062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalik MA, Saliba C, Pibiri M, Perra A, Ledda-Columbano GM, Sarotto I, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–96. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 26.Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11:1090–6. doi: 10.4161/cc.11.6.19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu LL, Dai N, Yu HG, Sun LM, Si JM. Akt associates with nuclear factor kappaB and plays an important role in chemoresistance of gastric cancer cells. Oncol Rep. 2010;24:113–9. doi: 10.3892/or_00000835. [DOI] [PubMed] [Google Scholar]
- 28.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–46. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knapp P, Baranowski M, Knapp M, Zabielski P, Błachnio-Zabielska AU, Górski J. Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat. 2010;92:62–6. doi: 10.1016/j.prostaglandins.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, et al. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8:809–20. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 31.Fu S, Hu W, Kavanagh JJ, Bast RC., Jr. Targeting Aurora kinases in ovarian cancer. Expert Opin Ther Targets. 2006;10:77–85. doi: 10.1517/14728222.10.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Bodó A, Bakos E, Szeri F, Váradi A, Sarkadi B. The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett. 2003;140-141:133–43. doi: 10.1016/S0378-4274(02)00497-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–92. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–9. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 36.Yuan M, Luong P, Hudson C, Gudmundsdottir K, Basu S. c-Abl phosphorylation of ΔNp63α is critical for cell viability. Cell Death Dis. 2010;1:e16. doi: 10.1038/cddis.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu ZF, Wang Q, Yang SJ. [The colocalized relationship between p53 mutants 249M, 273H and hsp70 in HepG2 cells] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:296–8. [PubMed] [Google Scholar]
- 38.Müller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, et al. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–13. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–98. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Grusche FA, Harvey KF. Control of tissue growth and cell transformation by the Salvador/Warts/Hippo pathway. PLoS One. 2012;7:e31994. doi: 10.1371/journal.pone.0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]