SUMMARY
Semi-fossorial ground squirrels face challenges to respiratory gas transport associated with the chronic hypoxia and hypercapnia of underground burrows, and such challenges are compounded in species that are native to high altitude. During hibernation, such species must also contend with vicissitudes of blood gas concentrations and plasma pH caused by episodic breathing. Here, we report an analysis of hemoglobin (Hb) function in six species of marmotine ground squirrels with different altitudinal distributions. Regardless of their native altitude, all species have high Hb–O2 affinities, mainly due to suppressed sensitivities to allosteric effectors [2,3-diphosphoglycerate (DPG) and chloride ions]. This suppressed anion sensitivity is surprising given that all canonical anion-binding sites are conserved. Two sciurid species, the golden-mantled and thirteen-lined ground squirrel, have Hb–O2 affinities that are characterized by high pH sensitivity and low thermal sensitivity relative to the Hbs of humans and other mammals. The pronounced Bohr effect is surprising in light of highly unusual amino acid substitutions at the C-termini that are known to abolish the Bohr effect in human HbA. Taken together, the high O2 affinity of sciurid Hbs suggests an enhanced capacity for pulmonary O2 loading under hypoxic and hypercapnic conditions, while the large Bohr effect should help to ensure efficient O2 unloading in tissue capillaries. In spite of the relatively low thermal sensitivities of the sciurid Hbs, our results indicate that the effect of hypothermia on Hb oxygenation is the main factor contributing to the increased blood–O2 affinity in hibernating ground squirrels.
KEY WORDS: adaptation, allostery, globin, hibernation, hypoxia
INTRODUCTION
Fossorial and semi-fossorial mammals face challenges to respiratory gas transport associated with the chronic hypoxia and hypercapnia of underground burrows (Boggs et al., 1984). At high altitude, the hypoxic conditions of underground burrows are further compounded by the reduced partial pressure of atmospheric O2 (PO2). In mountain ranges of western North America, burrow-dwelling rodents in the squirrel family (Sciuridae) are common denizens of the alpine and subalpine zones, most notably the golden-mantled ground squirrel (Callospermophilus lateralis) and the yellow-bellied marmot (Marmota flaviventris), which occur at the highest possible elevations in the contiguous United States (Armstrong, 1972; Chappell and Dlugosz, 2009). In addition to the hypoxic challenges associated with life underground and life at high altitude, many sciurid rodents face additional gas transport challenges associated with depressed ventilation and episodic breathing during winter hibernation (Milsom, 1992; Milsom and Jackson, 2011; Webb and Milsom, 1994). In hibernating ground squirrels, prolonged periods of apnea can result in wide fluctuations in arterial PO2 and plasma pH (Maginniss and Milsom, 1994; Malan et al., 1973; Musacchia and Volkert, 1971).
In mammals adapted to life underground or life at high altitude, an elevated blood–O2 affinity can enhance pulmonary O2 loading to compensate for the reduced PO2 of inspired air (Eaton, 1974; Eaton et al., 1974; Turek et al., 1973). Likewise, in hibernating mammals, a left-shifted blood–O2 equilibrium curve can help safeguard arterial O2 saturation during apneic periods and prevent unfavorable O2 release to tissues (Maginniss and Milsom, 1994; Milsom and Jackson, 2011; Revsbech et al., 2013). An increase in blood–O2 affinity can be achieved by changes in the intrinsic O2 affinity of hemoglobin (Hb), changes in the sensitivity of Hb to allosteric effectors and/or changes in the concentrations of allosteric effectors within the erythrocyte. In mammalian red cells, the main organic phosphate effector, 2,3-diphosphoglycerate (DPG), decreases Hb–O2 affinity by binding stereochemically at specific positively charged residues in the central cavity of deoxygenated Hb: β1Val, β2His, β82Lys and β143His (Arnone, 1972). The resultant stabilization of deoxy (T-state) Hb shifts the allosteric equilibrium between the low-affinity T-state and the high-affinity R-state in favor of the low-affinity quaternary structure (Perutz, 1970). Other factors that modulate Hb–O2 affinity are Cl− ions (Chiancone et al., 1972) and temperature (Antonini and Brunori, 1971), as well as protons and CO2, which also bind preferentially to deoxy-Hb and promote O2 unloading in the tissue capillaries via the Bohr effect (Perutz, 1983; Weber and Fago, 2004). Increases in blood–O2 affinity during winter hibernation have been documented in a number of mammals, and such changes are generally attributable to reductions in red cell DPG, increases in pH and/or decreased body temperature (Burlington and Whitten, 1971; Maginniss and Milsom, 1994; Musacchia and Volkert, 1971; Revsbech et al., 2013; Tempel and Musacchia, 1975). To assess the relative efficacy of different mechanisms for increasing blood–O2 affinity during hibernation, experiments on purified Hbs are required to evaluate the independent and joint effects of DPG, pH and temperature on Hb–O2 affinity under controlled conditions.
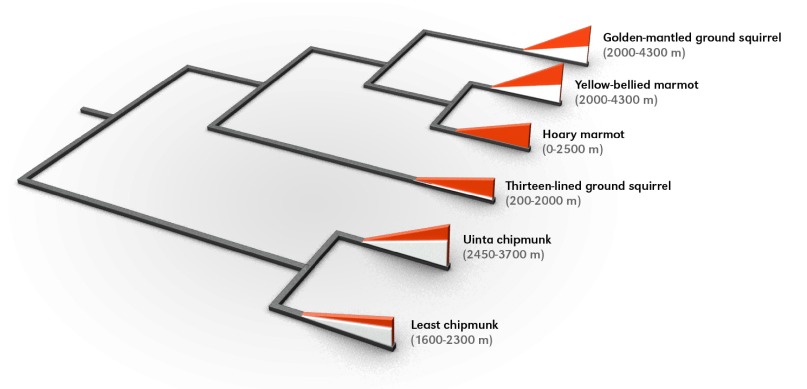
Here, we report an examination of the structural and functional properties of Hb in six species of marmotine ground squirrels (subfamily Xerinae, tribe Marmotini): golden-mantled ground squirrel (C. lateralis) (Say 1823), yellow-bellied marmot (M. flaviventris) (Audubon and Bachman 1841), hoary marmot (M. caligata) (Eschscholtz 1829), thirteen-lined ground squirrel (Ictidomys tridecemlineatus) (Mitchill 1821), Uinta chipmunk (Tamias umbrinus) (Allen 1890) and least chipmunk (T. minimus) (Bachman 1839). All six species are semi-fossorial, winter hibernators that are native to North America. These six species inhabit a range of different elevations (Fig. 1). For example, the yellow-bellied marmot is a strictly montane species that mainly occurs at elevations of 2000–4300 m in the Rocky Mountains, the Sierra Nevada, and other mountain ranges in western North America (Armstrong, 1972). By contrast, the closely related hoary marmot is mostly restricted to elevations <2500 m in Alaska and western Canada (Braun et al., 2011). The golden-mantled ground squirrel, Uinta chipmunk and least chipmunk are most common in the montane and subalpine zones, whereas the thirteen-lined ground squirrel is mainly restricted to low-elevation grassland (Fig. 1) (Armstrong, 1972; Streubel and Fitzgerald, 1978).
Fig. 1.
Phylogenetic relationships and elevational ranges of six marmotine ground squirrels included in the analysis of hemoglobin (Hb) function. Phylogenetic relationships are based on a consensus of previous studies (Harrison, 2003; Helgen et al., 2009; Herron et al., 2004). In each terminal branch, the height of the triangle is proportional to the elevational range limit of the corresponding species, and the demarcation between the red and white parts of the triangle denotes the lower range limit. Elevational ranges (from Armstrong, 1972; Braun et al., 2011; Streubel and Fitzgerald, 1978) depicted in this figure are specific to the geographic region in which each species was sampled.
The objectives of this study were (i) to test the hypothesis that the elevated blood–O2 affinities of burrow-dwelling, hibernating ground squirrels are attributable to genetically based changes in Hb function (i.e. an increase in intrinsic Hb–O2 affinity and/or a decrease in sensitivity to allosteric effectors), (ii) to evaluate the efficacy of different allosteric effectors in regulating Hb–O2 affinity, and (iii) to test whether interspecific variation in Hb–O2 affinity is associated with native altitude.
MATERIALS AND METHODS
Sampling
We collected blood samples from wild-caught specimens of each species in July–August 2010 and 2011. Golden-mantled ground squirrels (N=6) were collected from two localities in the Front Range of the southern Rocky Mountains: Boulder County, CO, USA (40°01′45″N, 105°50′77″W; 3103 m above sea level) and Grand County, CO, USA (38°84′37″N, 105°75′12″W; 2397 m). Yellow-bellied marmots (N=5) were collected from near the summit of Mount Evans, Clear Creek County, CO, USA (39°15′24″N, 106°10′54″W; 4300 m), and hoary marmots (N=6) were collected from two localities in central Alaska, viz., Eagle Summit, Yukon-Koyukuk County, AK, USA (65°29′8″N, 145°24′0″W; 1119 m) and Wickersham Dome, Fairbanks North Star County, AK, USA (65°12′40″N, 148°3′37″W; 915 m). Uinta chipmunks (N=2) and least chipmunks (N=6) were collected from Park County, CO, USA (39°34′71″N, 105°63′19″W; 3013 m) in addition to the same Grand County locality mentioned above. Thirteen-lined ground squirrels (N=2) were live-trapped in Lancaster County, NE, USA (40°52′12″N, 96°48′20″W; 430 m).
Animals were handled in accordance with guidelines approved by the University of Nebraska Institutional Animal Care and Use Committee (IACUC no. 07-07-030D and 519) and the National Institutes of Health (NIH publication no. 78-23). The thirteen-lined ground squirrels were anesthetized using isoflurane and blood was drawn from the femoral vein. Blood was collected in cryotubes, snap-frozen in liquid nitrogen, and stored at −80°C. Frozen blood samples were used as a source of Hb for experimental studies and reticulocytes served as a source of globin mRNA for cDNA sequencing. The animals were released at the site of capture after they had regained consciousness. In the remaining species, blood was collected via cardiac puncture immediately after each animal was killed and the blood samples were processed and stored as described above. Additionally, bone marrow was collected from the femurs of each animal as a source of globin mRNA. Bone marrow was preserved in RNAlater (Qiagen, Valencia, CA, USA) and stored at −80°C prior to RNA extraction. With the exception of the thirteen-lined ground squirrels, all animals were prepared as vouchered museum specimens and were deposited in the vertebrate collections of the Denver Museum of Nature and Science (Denver, CO, USA; catalog numbers 11959–11962, 11967–11969, 11979–11983, 11989–11990), the University of Alaska Museum of the North (Fairbanks, AK, USA; catalog numbers AF71385, AF71391–AF71395), and the University of Nebraska State Museum (Lincoln, NE, USA; catalog numbers NP1203–NP1207).
Globin sequencing
After annotating the α- and β-globin genes from the genome assembly of the thirteen-lined ground squirrel and other rodents (Hoffmann et al., 2008a; Hoffmann et al., 2008b; Opazo et al., 2008), we designed locus-specific primers for 5′ and 3′ RACE (rapid amplification of cDNA ends; Invitrogen Life Technologies, Carlsbad, CA, USA) to obtain cDNA sequence for the 5′ and 3′ untranslated regions (UTRs) of each adult-expressed globin gene. After designing paralog-specific PCR primers with annealing sites in the 5′ and 3′ UTRs, complete cDNAs were synthesized for each gene by reverse-transcriptase PCR (RT-PCR) using the OneStep RT-PCR kit (Qiagen). We performed RT-PCR on RNA template to amplify α- and β-globin cDNAs, which were then cloned and sequenced. Total RNA was extracted from reticulocytes or hematopoeitic bone marrow cells using the RNeasy Plus Mini Kit (Qiagen), and cDNA was generated from 1 μg RNA by reverse transcription. For RT-PCR, we used the SuperScript III Platinum One-Step RT-PCR system with Platinum Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA). PCR cycling conditions were as follows: one cycle at 50°C for 10 min, followed by 40 cycles at 94°C for 15 s, 55°C for 30 s, and 68°C for 2 min, and a final extension cycle at 68°C for 7 min. We then cloned gel-purified RT-PCR products into pCR4-TOPO vector using the TOPO TA cloning kit (Invitrogen). For each individual ground squirrel specimen, we sequenced a total of 20 clones containing products of paralog-specific RT-PCR (10 cloned α-globin cDNAs and 10 cloned β-globin cDNAs per individual). Thus, full-length inserts representing cDNAs of the adult-expressed α- and β-globin genes were sequenced at 10× coverage. PCR and RT-PCR primers for all species are available upon request. All DNA sequences were deposited in GenBank under the accession numbers KF010591–KF010606, KF153033–KF153050.
Analysis of Hb isoform composition
Hemolysates were prepared by adding a 3- to 5-fold volume of ice-cold 10 mmol l−1 Hepes pH 7.8 to frozen blood. The red supernatant was cleared by centrifugation and stripped from endogenous organic phosphates using a mixed-bed resin (MB-1, AG501-X8, Bio-Rad, Hercules, CA, USA) (Storz et al., 2012). The Hb isoform (isoHb) composition of hemolysates from each specimen was analyzed by isoelectric focusing (IEF, pH 5–8) using polyacrylamide gels (Phastgel, GE Healthcare Biosciences AB, Uppsala, Sweden). After separation of isoHbs by means of IEF, bands excised from each gel were digested with trypsin, and the resultant peptides were identified by means of tandem mass spectrometry (MS/MS). The peak lists of the MS/MS data were generated by Distiller (Matrix Science, London, UK) using the charge state recognition and de-isotoping with default parameters for quadrupole time-of-flight data. Database searches of the resultant MS/MS spectra were performed using Mascot v1.9.0 (Matrix Science). Specifically, peptide mass fingerprints derived from the MS/MS analysis were used to query a customized database of α- and β-globin amino acid sequences (based on conceptually translated DNA sequences) from each of the six sciurid species included in the study, including the full complement of pre- and post-natally expressed globin genes from the thirteen-lined ground squirrel genome assembly. The following search parameters were used: no restriction on protein molecular weight or isoelectric point, and methionine oxidation allowed as a variable peptide modification. Mass accuracy settings were 0.15 Da for peptide mass and 0.12 Da for fragment ion masses. We identified all significant protein hits that matched more than one peptide with P<0.05.
O2 equilibrium measurements
For all species, O2 equilibria were measured on 3 μl samples of purified hemolysate (0.2–0.3 mmol l−1 heme, 0.1 mol l−1 Hepes) at pH 7.4 and 37°C using a thermostatted thin-layer chamber technique (Sick and Gersonde, 1969; Weber, 1992). Curves were measured for each sample in the absence (stripped hemolysate) and presence of DPG (DPG/Hb tetramer ratio=2.0), Cl− ions (0.1 mol l−1 KCl) and of both anionic effectors as described previously (Storz et al., 2010a; Storz et al., 2012). As a measure of Hb–O2 affinity the PO2 at 50% heme saturation (P50) and the corresponding cooperativity coefficient (n50) were calculated by linear regression (r2≥0.99) from the zero intercept and slope, respectively, of Hill plots {log[Y/(1–Y)] versus log PO2, where Y is the fractional saturation} fitted to at least four saturation steps between 20 and 80%.
The sensitivity of Hb–O2 affinity to changes in pH (Bohr effect) and temperature were examined in the golden-mantled ground squirrel and in the thirteen-lined ground squirrel. These species were chosen to compare the functional consequences of unusual amino acid substitutions (see Results) found in the β-chain Hbs of the golden-mantled ground squirrel (a highland native) and the thirteen-lined ground squirrel (a lowland native). For the golden-mantled ground squirrel Hb, O2-binding curves were also measured in duplicate in the absence (stripped) and presence of DPG as described above at three different pH values (range 7.0–8.0, 37°C) (to assess the Bohr effect) and three different temperatures (range 23–37°C, pH 7.4; to measure the temperature effect). For thirteen-lined squirrels, only one of the two individual hemolysates was suitable for functional studies (N=1) and, because of limited sample availability, measures of pH and temperature sensitivity for the stripped Hbs of this species were based on two pH values (7.0 and 7.4) and two temperatures (23 and 37°C). The Bohr effect was quantified as φ=ΔlogP50/ΔpH (Bohr factor) by linear regression (r2≥0.99 for golden-mantled ground squirrel Hb) of the logP50 versus pH plot. The temperature dependence of O2 binding, expressed as the apparent heat of oxygenation (ΔH, kcal mol−1; 1 kcal=4.184 kJ, was derived by linear regression (r2≥0.99 for golden-mantled ground squirrel Hb) of the van't Hoff plot ΔH=2.303R(ΔlogP50)/Δ(1/T), where R is the gas constant (1.987 cal K−1 mol−1) and T is the absolute temperature (K). The ΔH values here reported were corrected for heat of O2 in solution (−3.0 kcal mol−1) (Antonini and Brunori, 1971).
RESULTS
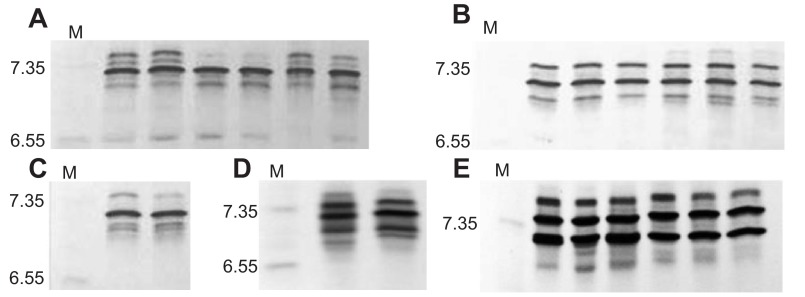
The IEF analysis revealed that each of the marmotine ground squirrels expressed one to three isoHbs, with one major isoHb (isoelectric point=7.2–7.4) that typically accounted for 50–65% of the total (Fig. 2). Combined results of the MS/MS analysis and cDNA sequence analysis revealed that isoHb heterogeneity is attributable to the expression of two to three structurally distinct β-globin chains (except for the Uinta chipmunk, which co-expresses two distinct α-chain isoforms) (Fig. 3). Multiple alignments of α- and β-globin amino acid sequences from each of the study species (Fig. 3) revealed unusual substitutions at highly conserved residue positions. Specifically, the golden-mantled ground squirrel, thirteen-lined ground squirrel, Uinta chipmunk and least chipmunk share an unusual substitution at the C-terminus of the α-chain, α141Arg→Cys, and the major β-chain of the golden-mantled ground squirrel has an unusual substitution at the C-terminus, β146His→Gln (Fig. 3). Both C-terminal residues are known to contribute to the pH sensitivity of Hb–O2 binding (the Bohr effect) in human Hb (Perutz, 1970; Weber and Fago, 2004; Perutz, 1983; Berenbrink, 2006; Fang et al., 1999; Lukin and Ho, 2004). The golden-mantled ground squirrel also has a highly unusual substitution at another highly conserved β-chain position, β55Met→Ile (Fig. 3). In each species, the α- and β-chain tryptic peptides of the major isoHb yielded highly significant matches to the translated cDNA sequences from the same individual specimens. The MS/MS analysis confirmed the presence of the unusual C-terminal residues mentioned above. The MS/MS analysis did not reveal the presence of any major subunit isoforms that were not already characterized at the DNA level.
Fig. 2.
Isoelectric focusing gels (pH 5–8) of native Hbs from specimens of marmotine ground squirrels. (A) Golden-mantled ground squirrel (Callospermophilus lateralis), (B) least chipmunk (Tamias minimus), (C) Uinta chipmunk (Tamias umbrinus), (D) thirteen-lined ground squirrel (Ictidomys tridecimlineatus), (E, first three lanes) yellow-bellied marmot (Marmota flaviventris) and (E, last three lanes) hoary marmot (Marmota caligata). In each panel, marker proteins are indicated by M and the corresponding isoelectric points are reported on the left side of the gels.
Fig. 3.
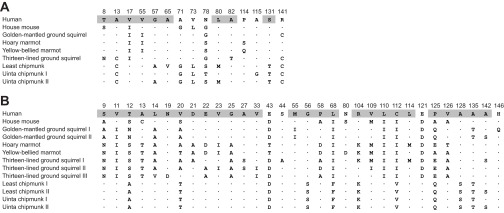
Variable residue positions in a multiple alignment of globin sequences for marmotine ground squirrels: golden-mantled ground squirrel (C. lateralis), hoary marmot (M. caligata), yellow-bellied marmot (M. flaviventris), thirteen-lined ground squirrel (I. tridecemlineatus), least chipmunk (T. minimus) and Uinta chipmunk (T. umbrinus), with orthologous sequences from human (Homo sapiens) HbA and mouse (Mus musculus) Hb included for comparison. Variable amino acid sites in the α- and β-globin sequences are shown in A and B, respectively. Sites in helical domains are shaded in the human reference sequence. Multiple sequences from the same species represent tandemly duplicated genes. For each species, αI and βI represent subunits of the major Hb isoform (isoHb; see Results for details).
The experimental measures of Hb function performed on the hemolysates under physiologically relevant conditions revealed that the six sciurid species have overall similar Hb–O2 affinities (i.e. P50 values) in the presence and absence of allosteric effectors (Table 1). Sensitivities to DPG and Cl− ions, as indexed by the difference in logP50 values for stripped samples in the presence both effectors, were in the range 0.13–0.35, with the exception of thirteen-lined squirrel Hb from a single individual, which showed a greater sensitivity of 0.45 (Table 1). Under similar experimental conditions, the measured anion sensitivity of human HbA was reported as ΔlogP50(DPG+KCl)–stripped≈0.4 (Weber, 1992). Thus, Hbs of the marmotine ground squirrels have generally low anion sensitivities relative to human HbA (see also Table 1). The Hill coefficients (n50) were in the range 1.8–2.9 under all conditions, indicating cooperative O2 binding and intact homotropic interactions (Table 1).
Table 1.
Mean Hb–O2 affinities, cofactor sensitivities and cooperativity coefficients of purified hemolysates from six sciurid species
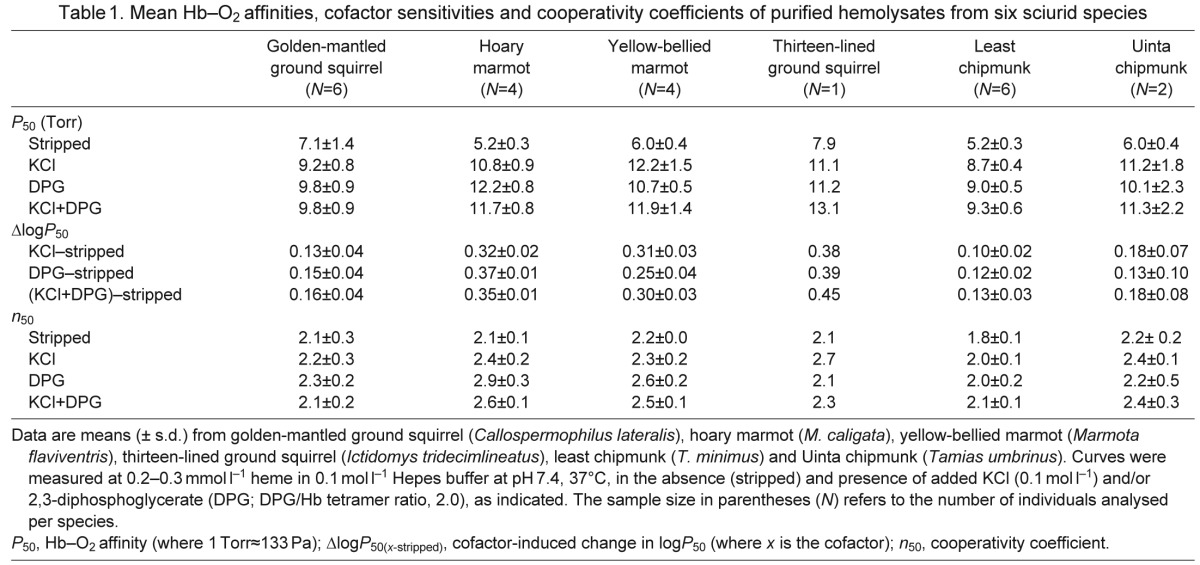
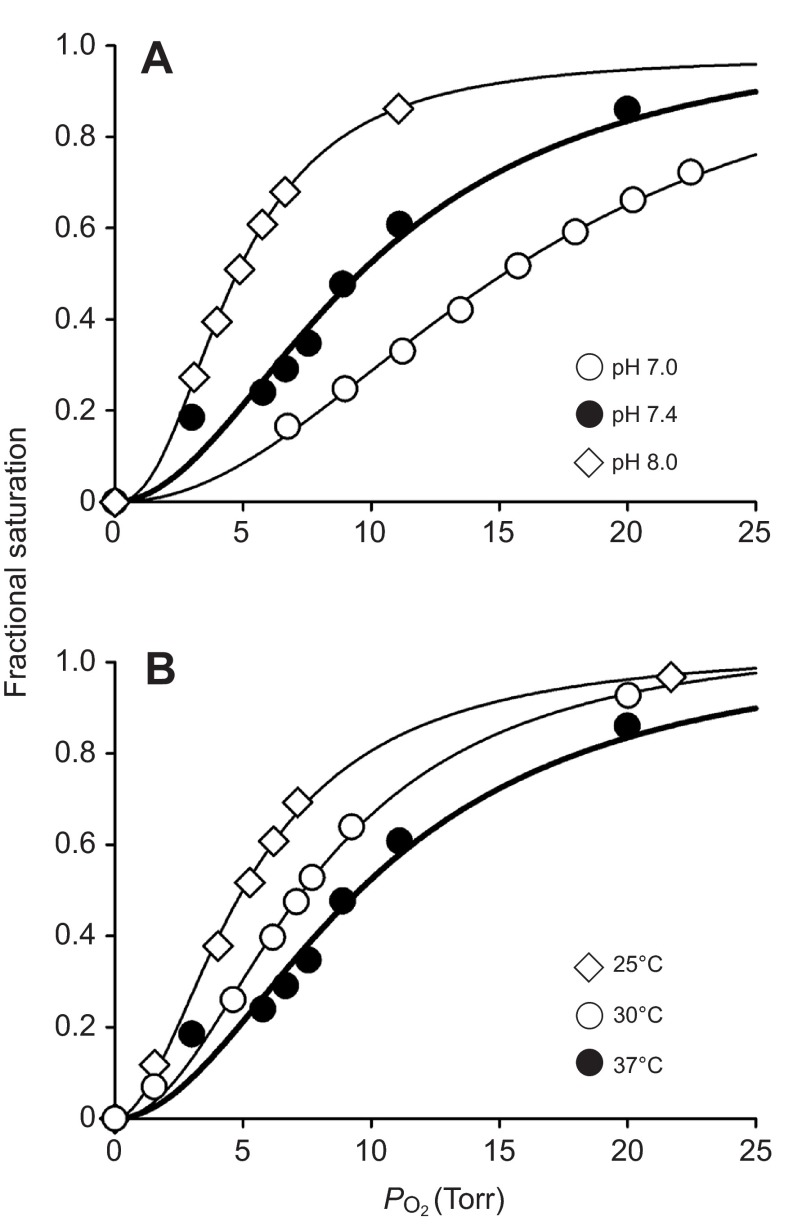
Measures of the pH sensitivity of Hb–O2 affinity revealed substantial Bohr effects for the Hbs of the golden-mantled and thirteen-lined ground squirrels, with Bohr factors (φ=ΔlogP50/ΔpH) of −0.5 and −0.8 (stripped) and −0.6 and −0.8 (with DPG added), respectively. Thus, in spite of the β146His→Gln substitution in the major isoHb of the golden-mantled ground squirrel, the measured Bohr coefficient was similar to that of the thirteen-lined ground squirrel Hb. The small changes in the Bohr factor observed upon addition of DPG are consistent with the suppressed anion sensitivity of Hb–O2 affinity in these species (Table 1). Because the Bohr factor represents the number of additional protons bound per O2 molecule (i.e. the number of additional protons released upon oxygenation if the Bohr factor is negative), these values indicate the presence of two to four O2-linked proton-binding sites per Hb tetramer in the T-state. For comparison, under similar experimental conditions, the Bohr coefficient for stripped human HbA has been reported to be −0.56 (Weber, 1992). The temperature sensitivity of stripped Hb oxygenation at pH 7.4 (data not shown), expressed as the heat of oxygenation (ΔH, i.e. enthalpy) was −6.8 kcal mol−1 in the golden-mantled ground squirrel Hb and −3.3 kcal mol−1 in the thirteen-lined ground squirrel Hb, whereas it is −14.3 kcal mol−1 in human HbA (Weber, 1992). In the golden-mantled ground squirrel, the enthalpy of Hb oxygenation decreased in magnitude to −4.4 kcal mol−1 in the presence of DPG, revealing the endothermic contribution of oxygenation-linked DPG dissociation. Representative O2-binding curves for golden-mantled ground squirrel Hb measured at different pH values and temperatures are shown in Fig. 4.
Fig. 4.
Representative O2-binding curves for golden-mantled ground squirrel Hb measured (A) at 37°C and pH 7.0, 7.4 and 8.0 in the presence of 2,3-diphosphoglycerate (DPG; DPG/Hb tetramer ratio 2.0) and 0.1 mol l−1 KCl, and (B) at pH 7.4 and 37, 30 and 23°C in the presence of DPG (DPG/Hb tetramer ratio 2.0) and 0.1 mol l−1 KCl. Fitting of data according to the sigmoidal Hill equation [Y=PO2 n50/(P50 n50+ PO2 n50)] is shown by solid lines. The same O2-binding curve measured at pH 7.4 and 37°C in the presence of DPG and KCl (filled circles, thick line) is shown in both panels to illustrate the effects of changes in pH (A) or temperature (B).
Although Hb–O2 affinities are often increased in mammals that are native to high altitude (Storz, 2007; Storz et al., 2010b; Weber, 2007), we found that the yellow-bellied marmot (a highland species) and hoary marmot (a predominantly lowland species) have Hbs with very similar O2 affinities (Table 1). For the six sciurid species that we examined, linear regressions revealed a suggestive but non-significant positive relationship between Hb–O2 affinity and native altitude (i.e. a negative relationship between P50 and altitude, measured under physiological conditions in the presence of KCl and DPG): P50(KCl+DPG) versus midpoint of elevational range, y=12.471–5.649x, r2=0.151 (P=0.446); P50(KCl+DPG) versus elevational range limit, y=12.150–3.035x, r2=0.050 (P=0.670). Linear regressions based on phylogenetically independent contrasts were also non-significant (data not shown).
DISCUSSION
Marmotine ground squirrels have markedly increased blood–O2 affinities in comparison with non-burrowing sciurids and other rodents (Hall, 1965; Hall, 1966; Bullard et al., 1966), and the results of our experiments suggest that these differences largely stem from genetically based changes in Hb function. In the presence of the physiological anionic effectors Cl− and DPG at pH 7.4, Hb–O2 affinities of the six sciurid species that we examined were appreciably higher than those of muroid rodents measured under identical experimental conditions. In the presence of allosteric effectors (DPG and Cl−), P50 values for the sciurid Hbs ranged from 9.3 to 11.9 Torr (where 1 Torr≈133 Pa), with thirteen-lined ground squirrel Hb from a single individual reaching 13.1 Torr (Table 1), whereas those for three species of house mice (Mus caroli, M. domesticus and M. musculus) ranged from 16.9 to 17.5 Torr (Runck et al., 2010; Storz et al., 2012;) and those for deer mice (Peromyscus maniculatus) ranged from 11.7 to 15.8 Torr (Storz et al., 2010a). Similarly, intrinsic Hb–O2 affinities, as indexed by P50 values for stripped hemolysates of the sciurids, ranged from 5.2 to 7.1 Torr (Table 1), whereas comparable measurements for house mice and deer mice were 7.6–8.8 Torr (Storz et al., 2012) and 7.4–7.9 Torr (Storz et al., 2010a), respectively. These comparisons suggest that the elevated Hb–O2 affinities of the marmotine ground squirrels are attributable to a generalized suppression of anion sensitivity combined with a high intrinsic Hb–O2 affinity (i.e. of the stripped Hb). In conjunction with results from other recent studies (Clementi et al., 2003; Natarajan et al., 2013; Storz et al., 2009; Storz et al., 2010a; Storz et al., 2012; Weber, 1992), the data summarized in Table 1 indicate that rodent Hbs are generally far less responsive to DPG than the Hbs of humans and many other mammals.
In hibernating golden-mantled ground squirrels and thirteen-lined ground squirrels, red cell DPG concentrations are reduced by nearly 50% compared with levels in non-hibernating individuals of the same species (Burlington and Whitten, 1971; Maginniss and Milsom, 1994). However, as the Hbs of these two species have such low anion sensitivities, the dramatic reductions in intracellular DPG may not have important direct effects on the regulation of Hb–O2 affinity during hibernation. Instead, the reduced DPG concentration may indirectly affect Hb–O2 affinity by altering the Donnan equilibrium of protons across the red cell membrane, as the reduced intracellular concentration of non-diffusible anions produces a corresponding reduction in proton concentration (Duhm, 1971). The resulting increase in intracellular pH at a given plasma pH would then increase Hb–O2 affinity via the pronounced Bohr effect of the sciurid Hbs (Fig. 4).
The elevated Hb–O2 affinities of the marmotine ground squirrels should enhance pulmonary O2 loading under hypoxia, but the trade-off is a reduced PO2 gradient for O2 diffusion from capillary blood to the perfused tissue. As suggested previously (Maginnis and Milsom, 1994), the strong Bohr effect should compensate for the high Hb–O2 affinity and promote O2 unloading in tissue capillary beds (where tissue pH is decreased) while simultaneously contributing to O2 loading at the pulmonary surfaces (where pH is increased). The large Bohr effect in the Hbs of marmotine ground squirrels may also play a role in pulmonary CO2 excretion, where Bohr protons liberated upon Hb oxygenation drive the formation of gaseous CO2 from plasma bicarbonate. Thus, a pronounced Bohr effect could be beneficial for tissue O2 delivery as well as pulmonary CO2 excretion not only at high altitudes but also in hypercapnic burrows and during hibernation when CO2 accumulates during prolonged apneic periods (Heldmaier et al., 2004; Milsom and Jackson, 2011). Overall blood PCO2 of the golden-mantled ground squirrel does not increase during hibernation, possibly due to effective CO2 excretion and/or high Hb buffer capacity, as suggested by high plasma bicarbonate (Maginniss and Milsom, 1994).
Similar to other mammals that are native to arctic or subarctic environments (Brix et al., 1989; De Rosa et al., 2004; Weber and Campbell, 2011), temperature changes have little effect on Hb–O2 affinity in the thirteen-lined and golden-mantled ground squirrels (Fig. 4). In regionally heterothermic organisms, this thermal insensitivity of Hb–O2 affinity helps to ensure O2 delivery to cold extremities while simultaneously reducing heat loss from the body (di Prisco et al., 1991; Coletta et al., 1992). In hibernating mammals, a low enthalpy of oxygenation would also reduce temperature-induced fluctuations in O2 delivery to tissues during periodic cycles of hypothermia and rapid rewarming upon arousal (Milsom and Jackson, 2011), thereby minimizing oxidative stress arising from mismatches between O2 supply and O2 consumption. Despite the remarkably low value of the oxygenation enthalpy in the golden-mantled ground squirrel Hb (−6.8 kcal mol−1) compared with that of human HbA (−14.3 kcal mol−1) (Weber, 1992), the strong decrease in body temperature of this hibernating species (>30°C) appears to be a major contributor to the left-shifted blood–O2 equilibrium curve (Maginniss and Milsom, 1994). This inference is supported by the fact that when subtracted for the heat of O2 solubilization (−3.0 kcal mol−1), ΔH values measured in the blood of hibernating and summer-active golden-mantled ground squirrels (−2.9 and −2.7 kcal mol−1, respectively) (Maginniss and Milsom, 1994) were similar to those measured here on purified Hb in the presence of DPG (−4.4 kcal mol−1). In the brown bear (Ursus arctos), by contrast, changes in temperature and DPG jointly modulate Hb oxygenation during hibernation to prevent inappropriate O2 delivery to tissues (Revsbech et al., 2013).
Given our current understanding of the structural basis of Hb allostery, the Hbs of marmotine ground squirrels are highly unusual in two respects: (i) they have markedly reduced anion sensitivities in spite of the fact that they retain each of the cationic residues that have been directly implicated in DPG and Cl− binding; and (ii) they have large Bohr effects in spite of substitutions that eliminate highly conserved C-terminal residues that mediate the Bohr effect in human HbA.
With respect to the Bohr effect, studies of human HbA have demonstrated that H+ ions mainly reduce Hb–O2 affinity by protonating the α-amino termini and the C-terminal β146His, which stabilizes the deoxy T-state through the formation of additional salt bridges within and between subunits (Berenbrink, 2006; Fang et al., 1999; Lukin and Ho, 2004; Perutz, 1970; Perutz, 1983; Weber and Fago, 2004). The C-terminal α141Arg does not directly contribute to the Bohr effect [the pKa (acid dissociation constant) of Arg is 12.0, so it is overwhelmingly protonated at pH 7.4], but it contributes indirectly to the Cl−-dependent Bohr effect by facilitating the Cl−-assisted charge stabilization of the free-NH2 terminus of α1Val. Accordingly, human HbA mutants that have the same residues as the golden-mantled ground squirrel (Hb Nunobiki, α141Arg→Cys and Hb Kodaira, β146His→Gln) are characterized by a marked reduction in the alkaline Bohr effect (Harano et al., 1992; Kwiatkowski and Noble, 1987; Shimasaki, 1985). In the case of the β-chain carboxy terminus, the positive charge on β146His is stabilized in the T-state by the negative charge of β94Asp and, at least in human HbA, it has been estimated that the oxygenation-linked deprotonation of β146His accounts for most of the Bohr effect (Fang et al., 1999; Lukin and Ho, 2004; Perutz, 1983; Perutz et al., 1985). In Hb Kodaira (and presumably in the major isoHb of the golden-mantled ground squirrel that also possesses β146Gln), replacement of the β94–β146 salt bridge by an unionizable H-bond would theoretically result in a diminished Bohr effect. The fact that the Bohr coefficient of golden-mantled ground squirrel Hb exceeds that of normal human HbA (with α141Arg and β146His) suggests that the C-terminal substitutions have different effects in the sequence context (or ‘genetic background’) of golden-mantled ground squirrel Hb than in human Hb. For example, the loss of the β146His Bohr group may be partly compensated for by the α113Leu→His substitution in marmotine ground squirrels, which results in the gain of two titratable His residues per tetramer relative to human HbA. The same α113Leu→His substitution was also suggested to contribute to a large Bohr effect in mole and shrew Hb (Campbell et al., 2012). Detailed mutagenesis and crystallographic studies will be required to illuminate the structure–function relationships of golden-mantled ground squirrel Hb. So far, our data support the view that the pH sensitivity of Hb–O2 affinity stems from many surface His residues with individually minor effects, rather than small numbers of key residues (e.g. β146His) with major effects.
The high Hb–O2 affinities of marmotine ground squirrels are consistent with data from other fossorial and semi-fossorial mammals (Hall, 1965; Hall, 1966; Jelkmann et al., 1981; Lechner, 1976; Campbell et al., 2010). Comparisons among the six species that we examined revealed a suggestive (but non-significant) positive relationship between Hb–O2 affinity and native altitude. However, the yellow-bellied marmot and hoary marmot exhibited no appreciable difference in Hb–O2 affinity in spite of their dramatically different elevational ranges. As ground squirrels are adapted to the respiratory gas transport challenges associated with semi-fossorial habits and winter hibernation, it may be that their ability to survive and function at high altitude does not require any additional modifications of Hb function. Consistent with this hypothesis, there are no appreciable differences in blood–O2 affinity between highland and lowland populations of burrowing mole rats (Cryptomys hottentotus mahali) (Broekman et al., 2006). Similarly, pocket gophers of the genus Thomomys have elevated blood–O2 affinities relative to non-fossorial rodents, but there are no apparent differences in Hb function between closely related pocket gopher species that are native to different elevational zones (Lechner, 1976).
CONCLUSION
The Hbs of marmotine ground squirrels are surprisingly insensitive to the modulating effects of DPG and Cl− ions, but are characterized by large Bohr coefficients and low thermal sensitivities relative to the Hbs of humans and other mammals. The suppressed anion sensitivity of the sciurid Hbs investigated here is unexpected in light of the fact that they retain all the canonical organic phosphate- and Cl−-binding sites in the central cavity, and the pronounced Bohr effect of golden-mantled ground squirrel Hb is surprising in light of two highly unusual substitutions, α141Arg→Cys and β146His→Gln, that are known to abolish the Bohr effect in human HbA. The elevated blood–O2 affinity of ground squirrels suggests enhanced capacities for pulmonary O2 loading under hypoxic and hypercapnic conditions, while the large Bohr effect should help to ensure efficient O2 unloading in tissue capillaries.
ACKNOWLEDGEMENTS
We thank G. Bachman, K. Bell, Z. Cheviron, A. Gunderson, L. Olson and A. Runck for assistance with fieldwork, J. Demboski and K. McCracken for logistical assistance in the field, J. Allison, A. Bang, M. B. Hemmingsen and K. Williams for assistance with lab work, and F. Hoffmann and C. Natarajan for assistance with gene annotation and sequence alignments.
LIST OF SYMBOLS AND ABBREVIATIONS
- DPG
2,3-diphosphoglycerate
- Hb
hemoglobin
- isoHb
hemoglobin isoform
- n50
cooperativity coefficient of Hb–oxygen binding
- P50
partial pressure of oxygen required to saturate Hb by 50%
- PO2
partial pressure of oxygen
- ΔH
enthalpy of Hb oxygenation
- φ
Bohr factor
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was funded by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute [grant numbers R01 HL087216, HL087216-S1]; the National Science Foundation [IOS-0949931]; the Danish Council for Independent Research, Natural Sciences [10-084565]; the Faculty of Science and Technology, Aarhus University; The American Society for Mammalogists, and a University of Nebraska Special Funds Research Grant. Deposited in PMC for release after 12 months.
REFERENCES
- Antonini E., Brunori M. (1971). Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam: North-Holland; [Google Scholar]
- Armstrong D. M. (1972). Distribution of mammals in Colorado. Monograph of the Museum of Natural History, University of Kansas 3, 1-415 [Google Scholar]
- Arnone A. (1972). X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237, 146-149 [DOI] [PubMed] [Google Scholar]
- Berenbrink M. (2006). Evolution of vertebrate haemoglobins: histidine side chains, specific buffer value and Bohr effect. Respir. Physiol. Neurobiol. 154, 165-184 [DOI] [PubMed] [Google Scholar]
- Boggs D. F., Kilgore D. L., Birchard G. F. (1984). Respiratory physiology of burrowing mammals and birds. Comp. Biochem. Physiol. 77A, 1-7 [Google Scholar]
- Braun J. K., Eaton T. S., Mares M. A. (2011). Marmota caligata (Rodentia: Sciuridae). Mamm. Species 43, 155-171 [Google Scholar]
- Brix O., Condò S. G., Lazzarino G., Clementi M. E., Scatena R., Giardina B. (1989). Arctic life adaptation – III. The function of whale (Balaenoptera acutorostrata) hemoglobin. Comp. Biochem. Physiol. 94B, 139-142 [DOI] [PubMed] [Google Scholar]
- Broekman M. S., Bennett N. C., Jackson C. R., Weber R. E. (2006). Does altitudinal difference modulate the respiratory properties in subterranean rodents' (Cryptomys hottentotus mahali) blood? Physiol. Behav. 88, 77-81 [DOI] [PubMed] [Google Scholar]
- Bullard R. W., Broumand C., Meyer F. R. (1966). Blood characteristics and volume in two rodents native to high altitude. J. Appl. Physiol. 21, 994-998 [DOI] [PubMed] [Google Scholar]
- Burlington R. F., Whitten B. K. (1971). Red cell 2,3-diphosphoglycerate in hibernating ground squirrels. Comp. Biochem. Physiol. 38A, 469-471 [DOI] [PubMed] [Google Scholar]
- Campbell K. L., Storz J. F., Signore A. V., Moriyama H., Catania K. C., Payson A. P., Bonaventura J., Stetefeld J., Weber R. E. (2010). Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol. Biol. 10, 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. L., Signore A. V., Harada M., Weber R. E. (2012). Molecular and physicochemical characterization of hemoglobin from the high-altitude Taiwanese brown-toothed shrew (Episoriculus fumidus). J. Comp. Physiol. B 182, 821-829 [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Dlugosz E. M. (2009). Aerobic capacity and running performance across a 1.6 km altitude difference in two sciurid rodents. J. Exp. Biol. 212, 610-619 [DOI] [PubMed] [Google Scholar]
- Chiancone E., Norne J. E., Forsén S., Antonini E., Wyman J. (1972). Nuclear magnetic resonance quadrupole relaxation studies of chloride binding to human oxy- and deoxyhaemoglobin. J. Mol. Biol. 70, 675-688 [DOI] [PubMed] [Google Scholar]
- Clementi M. E., Petruzzelli R., Filippucci M. G., Capo C., Misiti F., Giardina B. (2003). Molecular adaptation to hibernation: the hemoglobin of Dryomys nitedula. Pflugers Archiv. 446, 46-51 [DOI] [PubMed] [Google Scholar]
- Coletta M., Clementi M. E., Ascenzi P., Petruzzelli R., Condò S. G., Giardina B. (1992). A comparative study of the temperature dependence of the oxygen-binding properties of mammalian hemoglobins. Eur. J. Biochem. 204, 1155-1157 [DOI] [PubMed] [Google Scholar]
- De Rosa M. C., Castagnola M., Bertonati C., Galtieri A., Giardina B. (2004). From the Arctic to fetal life: physiological importance and structural basis of an ‘additional’ chloride-binding site in haemoglobin. Biochem. J. 380, 889-896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Prisco G., Condò S. G., Tamburrini M., Giardina B. (1991). Oxygen transport in extreme environments. Trends Biochem. Sci. 16, 471-474 [DOI] [PubMed] [Google Scholar]
- Duhm J. (1971). Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on oxygen affinity and intracellular pH of human erythrocytes. Pflugers Archiv. 326, 341-356 [DOI] [PubMed] [Google Scholar]
- Eaton J. W. (1974). Oxygen affinity and environmental adaptation. Ann. New York Acad. Sci. 241, 491-497 [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Berger E. (1974). Survival at extreme altitude: protective effect of increased hemoglobin-oxygen affinity. Science 183, 743-744 [DOI] [PubMed] [Google Scholar]
- Fang T. Y., Zou M., Simplaceanu V., Ho N. T., Ho C. (1999). Assessment of roles of surface histidyl residues in the molecular basis of the Bohr effect and of β 143 histidine in the binding of 2,3-bisphosphoglycerate in human normal adult hemoglobin. Biochemistry 38, 13423-13432 [DOI] [PubMed] [Google Scholar]
- Hall F. G. (1965). Hemoglobin and oxygen: affinities in seven species of sciuridae. Science 148, 1350-1351 [DOI] [PubMed] [Google Scholar]
- Hall F. G. (1966). Minimal utilizable oxygen and oxygen dissociation curve of blood of rodents. J. Appl. Physiol. (1985) 21, 375-378 [DOI] [PubMed] [Google Scholar]
- Harano T., Harano K., Kushida Y., Imai K., Nishinakamura R., Matsunaga T. (1992). Hb Kodaira [beta 146(HC3)His→Gln]: a new beta chain variant with an amino acid substitution at the C-terminus. Hemoglobin 16, 85-91 [DOI] [PubMed] [Google Scholar]
- Harrison R. G. (2003). Phylogeny and evolutionary history history of the ground squirrels (Rodentia: Marmotinae). J. Mamm. Evol. 10, 249-276 [Google Scholar]
- Heldmaier G., Ortmann S., Elvert R. (2004). Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317-329 [DOI] [PubMed] [Google Scholar]
- Helgen K. M., Cole F. R., Helgen L. E., Wilson D. E. (2009). Generic revision in the Holarctic ground squirrel genus Spermophilus. J. Mammal. 90, 270-305 [Google Scholar]
- Herron M. D., Castoe T. A., Parkinson C. L. (2004). Sciurid phylogeny and the paraphyly of Holarctic ground squirrels (Spermophilus). Mol. Phylogenet. Evol. 31, 1015-1030 [DOI] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C., Storz J. F. (2008a). New genes originated via multiple recombinational pathways in the β-globin gene family of rodents. Mol. Biol. Evol. 25, 2589-2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C., Storz J. F. (2008b). Rapid rates of lineage-specific gene duplication and deletion in the α-globin gene family. Mol. Biol. Evol. 25, 591-602 [DOI] [PubMed] [Google Scholar]
- Jelkmann W., Oberthür W., Kleinschmidt T., Braunitzer G. (1981). Adaptation of hemoglobin function to subterranean life in the mole, Talpa europaea. Respir. Physiol. 46, 7-16 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski L. D., Noble R. W. (1987). Contribution of arginine (HC3) 141 α to the Bohr effect of the fourth binding step in the reaction of ligand with human hemoglobin. Proteins 2, 72-77 [DOI] [PubMed] [Google Scholar]
- Lechner A. J. (1976). Respiratory adaptations in burrowing pocket gophers from sea level and high altitude. J. Appl. Physiol. 41, 168-173 [DOI] [PubMed] [Google Scholar]
- Lukin J. A., Ho C. (2004). The structure–function relationship of hemoglobin in solution at atomic resolution. Chem. Rev. 104, 1219-1230 [DOI] [PubMed] [Google Scholar]
- Maginniss L. A., Milsom W. K. (1994). Effects of hibernation on blood oxygen transport in the golden-mantled ground squirrel. Respir. Physiol. 95, 195-208 [DOI] [PubMed] [Google Scholar]
- Malan A., Arens H., Waechter A. (1973). Pulmonary respiration and acid-base state in hibernating marmots and hamsters. Respir. Physiol. 17, 45-61 [DOI] [PubMed] [Google Scholar]
- Milsom W. K. (1992). Control of breathing in hibernating mammals. In Lung Biology in Health and Disease: Strategies of Physiological Adaptation, Vol. 56, pp.119-148 New York, NY: Marcel Dekker, Inc. [Google Scholar]
- Milsom W. K., Jackson D. C. (2011). Hibernation and gas exchange. Comp. Physiol. 1, 397-420 [DOI] [PubMed] [Google Scholar]
- Musacchia X. J., Volkert W. A. (1971). Blood gases in hibernating and active ground squirrels: HbO2 affinity at 6 and 38 C. Am. J. Physiol. 221, 128-130 [DOI] [PubMed] [Google Scholar]
- Natarajan C., Inoguchi N., Weber R. E., Fago A., Moriyama H., Storz J. F. (2013). Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G., Storz J. F. (2008). Differential loss of embryonic globin genes during the radiation of placental mammals. Proc. Natl. Acad. Sci. USA 105, 12950-12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. (1970). Stereochemistry of cooperative effects in haemoglobin. Nature 228, 726-734 [DOI] [PubMed] [Google Scholar]
- Perutz M. F. (1983). Species adaptation in a protein molecule. Mol. Biol. Evol. 1, 1-28 [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Gronenborn A. M., Clore G. M., Shib D. T., Craescu C. T. (1985). Comparison of histidine proton magnetic resonances of human carbonmonoxyhaemoglobin in different buffers. J. Mol. Biol. 186, 471-473 [DOI] [PubMed] [Google Scholar]
- Revsbech I. G., Malte H., Fröbert O., Evans A., Blanc S., Josefsson J., Fago A. (2013). Decrease in the red cell cofactor 2,3-diphosphoglycerate increases hemoglobin oxygen affinity in the hibernating brown bear Ursus arctos. Am. J. Physiol. 304, R43-R49 [DOI] [PubMed] [Google Scholar]
- Runck A. M., Weber R. E., Fago A., Storz J. F. (2010). Evolutionary and functional properties of a two-locus β-globin polymorphism in Indian house mice. Genetics 184, 1121-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S. (1985). A new hemoglobin variant, hemoglobin Nunobiki [α 141 (HC3) Arg→Cys]. Notable influence of the carboxy-terminal cysteine upon various physicochemical characteristics of hemoglobin. J. Clin. Invest. 75, 695-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick H., Gersonde K. (1969). Method for continuous registration of O2-binding curves of hemoproteins by means of a diffusion chamber. Anal. Biochem. 32, 362-376 [DOI] [PubMed] [Google Scholar]
- Storz J. F. (2007). Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 88, 24-31 [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., Moriyama H., Weber R. E., Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106, 14450-14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E., Fago A. (2010a). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Scott G. R., Cheviron Z. A. (2010b). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125-4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Weber R. E., Fago A. (2012). Oxygenation properties and oxidation rates of mouse hemoglobins that differ in reactive cysteine content. Comp. Biochem. Physiol. 161A, 265-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel D. P., Fitzgerald J. B. (1978). Spermophilus tridecemlineatus. Mamm. Species 103, 1-5 [Google Scholar]
- Tempel G. E., Musacchia X. J. (1975). Erythrocyte 2,3-diphosphoglycerate concentrations in hibernating, hypothermic, and rewarming hamsters. Proc. Soc. Exp. Biol. Med. 148, 588-592 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Hoofd L. J. C. (1973). Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflugers Arch. 342, 185-197 [DOI] [PubMed] [Google Scholar]
- Webb C. L., Milsom W. K. (1994). Ventilatory responses to acute and chronic hypoxic hypercapnia in the ground squirrel. Respir. Physiol. 98, 137-152 [DOI] [PubMed] [Google Scholar]
- Weber R. E. (1992). Use of ionic and zwitterionic (Tris, BisTris and HEPES) buffers in studies on hemoglobin function. J. Appl. Physiol. 72, 1611-1615 [DOI] [PubMed] [Google Scholar]
- Weber R. E. (2007). High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 158, 132-142 [DOI] [PubMed] [Google Scholar]
- Weber R. E., Campbell K. L. (2011). Temperature dependence of haemoglobin-oxygen affinity in heterothermic vertebrates: mechanisms and biological significance. Acta Physiol. 202, 549-562 [DOI] [PubMed] [Google Scholar]
- Weber R. E., Fago A. (2004). Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir. Physiol. Neurobiol. 144, 141-159 [DOI] [PubMed] [Google Scholar]